Abstract
Objective
To evaluate if biomarkers reflecting left ventricular/vascular extracellular matrix remodeling are associated with cardiovascular disease (CVD) and death in the community.
Methods and Results
In 922 Framingham Study participants (mean age 58 years; 56% women), we related circulating concentrations of matrix metalloproteinase-9 (MMP-9; binary variable, detectable versus undetectable), tissue inhibitor of matrix metalloproteinase-1 (log-TIMP-1) and procollagen type-III amino-terminal peptide (log-PIIINP), to incident CVD and death.
On follow-up (mean 9.9 years), 51 deaths and 81 CVD events occurred. Each standard deviation increment of log-TIMP-1 and log-PIIINP was associated multivariable-adjusted hazards ratios (HR) of 1.72 (95% confidence interval [CI] 1.30–2.27) and 1.47 (1.11–1.96) for mortality risk, respectively. Log-PIIINP concentrations were also associated with CVD risk (HR [CI] per SD, 1.35 [1.05–1.74]). Death and CVD incidence rates were two-fold higher in participants with both biomarkers above the median (corresponding HR [CI] of 2.78 [1.43–5.40] and 1.77 [1.04–3.03], respectively) compared to those with either/both below the median. Inclusion of both biomarkers improved the C-statistic (for predicting mortality) from 0.78 to 0.82 (P=0.03). MMP-9 was unrelated to either outcome.
Conclusions
Higher circulating TIMP-1 and PIIINP concentrations are associated with mortality, and higher PIIINP with incident CVD in the community.
Keywords: Extracellular matrix, remodeling, left ventricle, cardiovascular disease, matrix metalloproteinase-9, tissue inhibitor of matrix metalloproteinase-1, procollagen type III amino-terminal peptide
Left ventricular1 (LV) and systemic vascular2 remodeling precede and predict incident cardiovascular disease (CVD). Circulating biomarkers of ventricular and vascular remodeling may therefore aid prediction and stratification of CVD risk. Extracellular matrix (ECM) turnover is an integral component of cardiovascular remodeling. Consequently, three classes of proteins reflecting ECM synthesis and degradation, i.e., the matrix metalloproteinases (MMPs), their tissue inhibitors (TIMPs) and the byproducts of collagen turnover (procollagen terminal peptides) have received considerable attention in recent years.3 Several previous investigations reported associations of markers of ECM remodeling with cardiovascular risk factors,4-6 progression to hypertension,7 and with atherosclerotic coronary artery3 and cerebrovascular disease8 as well as with risk of death in patients with known CVD.9-11 However, it is unclear whether these biomarkers are associated with incident CVD events and all-cause mortality in people free of overt CVD in the community.
Accordingly, we evaluated the relations of circulating concentrations of MMP-9, TIMP-1 and procollagen type III amino-terminal peptide (PIIINP) to the incidence of CVD and all-cause mortality in a community-based sample. Of several possible biomarkers of ECM turnover, we chose these three because of previously demonstrated associations with ventricular and vascular remodeling.12-14 We hypothesized that elevated circulating levels of the aforementioned biomarkers indicate active remodeling/turnover, and will be associated with increased incidence of CVD and death. We further posited that these markers will improve risk prediction when added to statistical models with conventional cardiovascular risk factors.
Methods
Study Sample and Design
The details regarding the selection and sampling of the Framingham Heart Study offspring cohort have been published previously.15 Briefly, 5124 participants who are the children of the Framingham Original cohort, and the spouses of these children, were enrolled in 1971 and have been evaluated approximately every four years. Of these, 3532 participants who attended examination cycle 6 (1995 – 1998) were eligible for the present investigation.
Given the relative novelty of the ECM biomarkers under consideration, we measured them only in a sub-sample of examination cycle 6 attendees to conserve non-renewable precious serological resources and yet maximize statistical efficiency. The description of, and rationale for our sampling strategy have been published previously,6,7,16 and were determined by our primary objective of relating these ECM biomarkers to echocardiographic indices of LV remodeling. In brief, we identified candidates for ECM biomarker measurement by examining the distributions of echocardiographically measured LV wall thickness and LV end-diastolic dimension (LVWT and LVEDD respectively), and sampled participants with both LVEDD and LVWT below the 50th percentile and either above the 90th percentile of the distributions. Thus, plasma MMP-9, TIMP-1 and PIIINP were measured in 700, 1032 and 944 participants respectively. Of these, we excluded participants with prevalent CVD (see definition of CVD below). Relations of MMP-9, TIMP-1 and PIIINP to incident CVD and death were evaluated in the remaining 607, 922 and 840 participants respectively. All participants provided written informed consent and the study protocol was approved by the Institutional Review Board of Boston Medical Center.
Measurement of Biomarkers
Plasma samples for biomarker measurement were drawn after overnight fast, typically between 8 AM and 9 AM and stored at 80° C without any freeze-thaw cycles. We measured the biomarkers in duplicate, using two-site sandwich ELISA assays (Amersham Pharmacia Biotech) for MMP-9 and TIMP-1 and a radioimmunoassay (Orion Diagnostica) for PIIINP. Plasma total MMP-9 assay measured MMP-9, ProMMP-9 and Pro-MMP-9/TIMP-1 complex. Plasma total TIMP-1 assays measured free TIMP-1 and complexes of TIMP-1 with various MMPs. Intra-assay coefficients of variation were: <18% for MMP-9, <5% for TIMP-1, and 6% for PIIINP.
Echocardiographic Methods
We measured LVEDD and the end-diastolic thicknesses of the inter-ventricular septum (IVST) and posterior wall (PWT) using American Society of Echocardiography recommended leading-edge technique17 and calculated LV mass (LVM) and LVWT as follows:
Participants with both LVEDD and LVWT less than 50th percentile of distribution were classified as the “referent” group and those with either of these measures greater than 90th percentile as “remodeled” group (we termed these groups “LV sampling groups”).7
Assessment of Outcomes
A panel of three Heart Study investigators reviewed all cardiovascular events and validated them according to pre-specified criteria. A neurologist examined participants with suspected stroke and a separate panel that included a neurologist validated all cerebrovascular events. The two end points for this investigation are (a) all-cause mortality and (b) all fatal or non-fatal CVD events. CVD included recognized and unrecognized myocardial infarction, coronary insufficiency (unstable angina), angina pectoris, non-hemorrhagic stroke, transient ischemic attack, intermittent claudication and heart failure. The Framingham Heart Study criteria for validating these events have been published previously.18
Statistical Analysis
Owing to low rates of MMP-9 detection,16 we modeled this biomarker as a dichotomous variable (detectable vs. undetectable) only. We natural-logarithmically transformed (to account for skewed distributions) TIMP-1 and PIIINP. We evaluated correlations between the latter two biomarkers by estimating age- and sex-adjusted Pearson correlation coefficients.
To examine the associations of biomarkers with the endpoints of interest, we estimated Cox proportional hazards regression models (after confirming the assumption of proportionality of hazards) and related MMP-9, log-TIMP-1 and log-PIIINP individually to all-cause mortality and incident CVD (separate models for each outcome) in age- and sex-adjusted models and in multivariable models additionally adjusting for BMI, systolic blood pressure, hypertension treatment, diabetes, total cholesterol/high-density lipoprotein cholesterol ratio, current smoking, LVM and LV sampling group. We also evaluated the relations of the biomarkers to both endpoints, separately in the referent and remodeled LV sampling groups.
We evaluated the relative contributions of TIMP-1 and PIIINP to mortality and CVD risk by including both biomarkers together in multivariable models and relating them to each endpoint separately. To determine the incremental value of ECM markers over clinical factors in predicting death and incident CVD, we calculated the c-statistics for models with and without biomarkers and tested if the inclusion of biomarkers significantly improved the c-statistic. We also evaluated net reclassification improvement (NRI) and integrated discrimination improvement (IDI) using the methods described by Pencina et al.19
In additional analyses, we grouped participants into those with biomarkers levels at or below versus above the median value and constructed cumulative mortality incidence curves for each group. Also, we evaluated the hazards for mortality and CVD in those with both biomarkers above median, compared to participants with either biomarker at or below median, and evaluated if hazards for increment in the risk of death or CVD (when both biomarkers are elevated) are additive or synergistic by including the product of median TIMP-1 and median PIIINP as an interaction term in the model. Additionally, we evaluated whether biomarker-outcome relations varied according to LV sampling group by including first order interaction terms (biomarker × LV sampling group) in the multivariable models relating each biomarker separately to two endpoints, death and CVD. Finally, since B-type natriuretic peptide (BNP) and C - reactive protein (CRP) have been consistently reported to predict death and CVD, we added them to the multivariable models to evaluate if ECM markers predicted either outcome independent of these established biomarkers. All analyses were performed using SAS version 8.2 and a two-sided p-value <0.05 denoted statistical significance.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
The baseline clinical, echocardiographic and ECM biomarker characteristics of the participants are presented in Table 1. The overall sample of 922 individuals included 572 and 350 participants in the referent and remodeled groups respectively. Over follow-up of up to 12.7 years (mean 9.9 years), a total of 51 deaths and 81 first CVD events occurred. The age- and sex-adjusted Pearson correlation coefficient between log-TIMP-1 and log-PIIINP was 0.18 (P < 0.0001).
Table 1.
Baseline characteristics of study participants.
| Mean (SD) or % | Whole Sample N = 922 | Referent Group N = 572 | Remodeled Group N = 350 |
|---|---|---|---|
| Age, yrs | 58 (10) | 55 (9) | 59 (10) |
| Women, % | 58 | 57 | 61 |
| Body mass index, kg/m2 | 27.1 (5.0) | 25.2 (3.7) | 29.6 (5.5) |
| Systolic blood pressure, mmHg | 126 (19) | 121 (17) | 134 (21) |
| ACE inhibitors, % | 10 | 5 | 17 |
| Beta-blockers, % | 7 | 5 | 11 |
| Hypertension treatment | |||
| Calcium channel blockers, % | 7 | 3 | 13 |
| Diuretics, % | 7 | 3 | 13 |
| Lipid modifying treatment, % | 9 | 5 | 16 |
| Diabetes, % | 10 | 3 | 14 |
| Total/HDL cholesterol ratio | 4.3 (1.4) | 4.2 (1.5) | 4.4 (1.4) |
| Current smoking, % | 15 | 16 | 12 |
| Estimated glomerular filtration rate, ml/min/1.73 m2 | 92 (50) | 93 (58) | 90 (35) |
| Echocardiographic Characteristics | |||
| LV mass, gm | 159 (55) | 125 (26) | 203 (48) |
| LV wall thickness, cm | 1.89 (0.31) | 1.72 (0.14) | 2.13 (0.34) |
| LV end-diastolic dimension, cm | 4.74 (0.61) | 4.46 (0.35) | 5.06 (0.60) |
| Biomarkers Characteristics | |||
| MMP-9 (% detectable) | 20 | 14 | 24 |
| TIMP-1, ng/ml, (N = 922) | 20.0 (4.0) | 19.2 (3.2) | 20.5 (4.2) |
| PIIINP, ng/ml, (N = 840) | 4.0 (3.8) | 4.0 (4.1) | 4.0 (3.6) |
SD = standard deviation; ACE = angiotensin converting enzyme; HDL = high density lipoprotein; LV = left ventricular; MMP-9 = matrix metalloproteinase-9; TIMP-1 = tissue inhibitor of matrix metalloproteinase; PIIINP = procollagen type III amino-terminal peptide.
Relations of Biomarkers to Mortality Risk
In age- and sex-adjusted models, we observed that each standard deviation increment of log-TIMP-1 and log-PIIINP levels were associated with a 97% and 48% increased risk of death respectively (Table 2). Adjustment for other covariates only slightly attenuated the risk associated with log-TIMP-1, but did not alter the estimates for log-PIIINP (Table 2); further adjustment for BNP and CRP did not substantially alter these results (data not shown). MMP-9 was not significantly associated with mortality risk.
Table 2.
Relations of ECM biomarkers to CVD and mortality.
| CVD | Death | |||
|---|---|---|---|---|
| Adjusted HR (CI) | P-value | Adjusted HR (CI) | P-value | |
| A. MMP-9 as binary covariate (n=607; 64 CVD events and 41 deaths) * | ||||
| Age- and sex-adjusted | 1.50 (0.87 – 2.58) | 0.15 | 1.24 (0.62 – 2.49) | 0.54 |
| Multivariable-adjusted | 1.18 (0.63 – 2.20) | 0.60 | 0.76 (0.35 – 1.63) | 0.48 |
| B. log-TIMP-1 (n=922; 81 CVD events and 51 deaths) † | ||||
| Age- and sex-adjusted | 1.36 (1.09 – 1.71) | 0.007 | 1.97 (1.53 – 2.53) | <0.0001 |
| Multivariable-adjusted | 1.10 (0.86 – 1.39) | 0.46 | 1.72 (1.30 – 2.27) | 0.0002 |
| C. log-PIIINP (n=840; 75 CVD events and 46 deaths) † | ||||
| Age- and sex-adjusted | 1.31 (1.05 – 1.63) | 0.01 | 1.48 (1.13 – 1.93) | 0.004 |
| Multivariable-adjusted | 1.35 (1.05 – 1.74) | 0.02 | 1.47 (1.11 – 1.96) | 0.008 |
HR = hazards ratio; CI = confidence interval; MMP-9= matrix metalloproteinase-9; TIMP-1 = tissue inhibitor of matrix metalloproteinase; PIIINP = procollagen type III amino-terminal peptide.
Multivariable model included age, sex, BMI, systolic blood pressure, hypertension treatment, diabetes, total/HDL cholesterol ratio, current smoking, LV mass and LV sampling group.
HR indicates hazards in those with detectable MMP-9 compared to those without.
HR per standard deviation change in biomarker levels.
Relations of Biomarkers to Incidence of CVD
We observed that higher PIIINP levels were associated with a 31% increased risk of CVD per standard deviation increment log-marker in age- and sex-adjusted models (Table 2). Multivariable-adjustment did not attenuate these findings, but relations of PIIINP to CVD were no longer statistically significant after additional adjustment for BNP and CRP. Relations of log-TIMP-1 to CVD were statistically significant in age- and sex-adjusted models but not in multivariable models (Table 2). MMP-9 was not associated with CVD risk.
Conjoint Relations of PIIINP and TIMP-1 to Risk of Mortality and CVD
In multivariable models that included log-TIMP-1 and log-PIIINP together, we noted that both markers were significantly related to mortality; each standard deviation increase of log-TIMP-1 and log-PIIINP were associated with a 60% and 41% increase in risk of mortality, respectively (Table 3). In multivariable analyses relating both biomarkers together to CVD risk, log-TIMP-1 and log-PIIINP were associated with a 4% and 35% increase in hazards, but the relations of log-TIMP-1 were not statistically significant (P = 0.77; Table 3).
Table 3.
Conjoint relations of TIMP-1 and PIIINP to the risk of death and CVD.
| Adjusted HR* (CI) | p-value | |
|---|---|---|
| A. Death (n = 808; 44 events) | ||
| Log-TIMP-1 | 1.60 (1.19 – 2.15) | 0.002 |
| Log-PIIINP | 1.41 (1.03 – 1.92) | 0.03 |
| B. CVD (n = 808; 64 events) | ||
| Log-TIMP-1 | 1.04 (0.79 – 1.37) | 0.77 |
| Log-PIIINP | 1.35 (1.05 – 1.74) | 0.02 |
HR = hazards ratio; CI = confidence interval; TIMP-1 = tissue inhibitor of matrix metalloproteinase; PIIINP = procollagen type III amino-terminal peptide.
HR per standard deviation change in biomarker levels, adjusted for age, sex, BMI, systolic blood pressure, hypertension treatment, diabetes, total/HDL cholesterol ratio, current smoking, LV mass and LV sampling group.
Inclusion of both biomarkers in multivariable models evaluating risk of death significantly improved the c-statistic from 0.78 to 0.82 (P = 0.03). For prediction of CVD, inclusion of biomarkers did not improve the model c-statistic. Inclusion of TIMP-1 and PIIINP to the model with clinical covariates did not improve net reclassification for prediction of either mortality or CVD. However, inclusion of both biomarkers showed integrated discrimination improvement that was of borderline significance for predicting death (IDI statistic = 0.18; p = 0.07) and statistically significant for predicting CVD (IDI statistic = 0.009; p = 0.03).
Additional Analyses
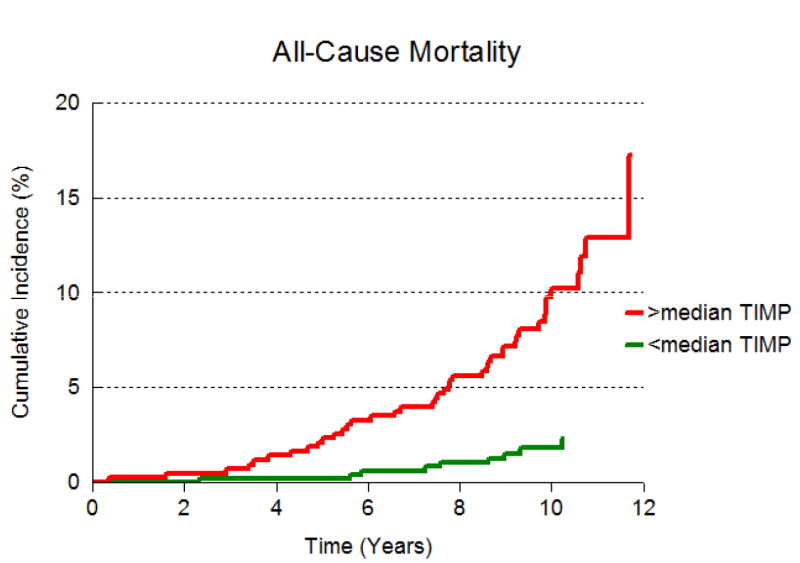
The cumulative incidence of death by median PIINP and median TIMP-1 are presented in Figures 1A and 1B respectively. Participants with both biomarkers above median experienced mortality and CVD at rates that were twice as high compared to those with either below (Figure 2 and Table 4). However, the interaction term for the product of median TIMP-1 and median PIIINP was not significant, suggesting that the hazards for death and CVD portended by elevation of both biomarkers are additive, not synergistic.
Figure 1.
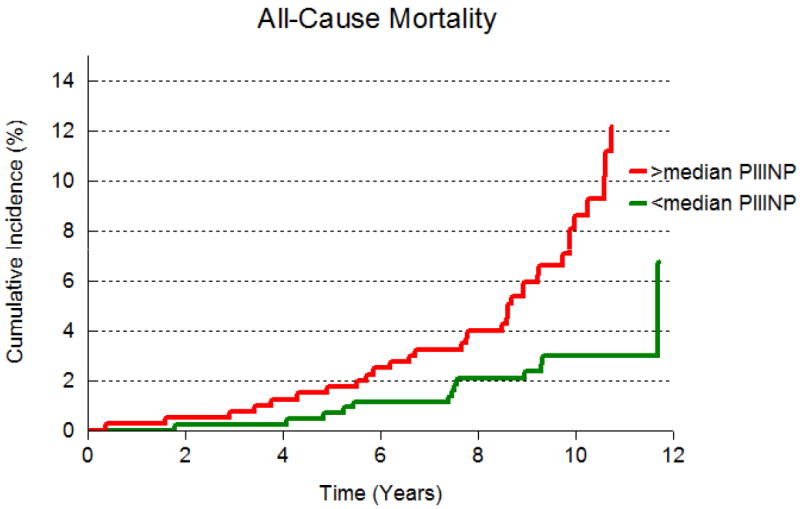
A: Cumulative incidence of all-cause mortality in participants with PIIINP above median and at or below median.
B: Cumulative incidence of all-cause mortality in participants with TIMP-1 above median and at or below median.
Figure 2.
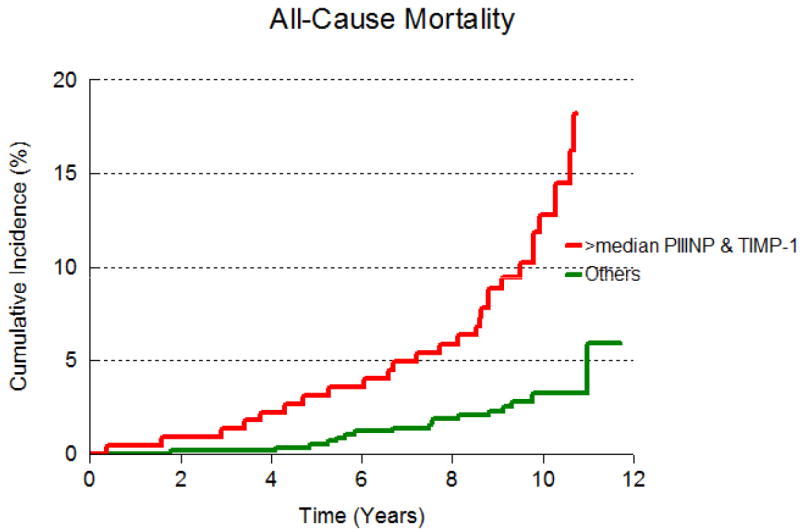
Cumulative incidence of all-cause mortality in participants with both TIMP-1 and PIIINP above median and either or both biomarkers at or below median.
Table 4.
Event rates and hazards in participants with both biomarker levels above median.
| Either at or Below Median | Both Above Median | p-value | |
|---|---|---|---|
| A. Death | |||
| Event proportions* | 17/585 (2.9) | 27/223 (12.1) | N/A |
| Event Rates† | 7.4 (0.0 – 14.2) | 14.1 (5.7 – 21.4) | N/A |
| Hazards ratios‡ (CI) | Referent | 2.78 (1.43 – 5.40) | 0.003 |
| B. CVD | |||
| Event proportions* | 29/585 (5.0) | 35/223 (15.7) | N/A |
| Event Rates† | 7.8 (3.3 -11.8) | 13.6 (7.3 -19.2) | N/A |
| Hazards ratios‡ (CI) | Referent | 1.77 (1.04 – 3.03) | 0.04 |
CI = confidence interval; TIMP-1 = tissue inhibitor of matrix metalloproteinase; PIIINP = procollagen type III amino-terminal peptide.
Number of events/number at risk (%).
Age- and sex-adjusted event rates per 100 person-years of follow-up.
Hazards ratios for participants with BOTH biomarker levels above median, compared to those with either at or below (referent), adjusted for age, sex, BMI, systolic blood pressure, hypertension treatment, diabetes, total/HDL cholesterol ratio, current smoking, LV mass and LV sampling group.
Results of analyses relating TIMP-1 and PIIINP to all-cause mortality separately in the referent and remodeled groups were consistent with those from the overall sample (Supplementary Tables 1 and 2). With respect to incident CVD, results for both markers in the remodeled group paralleled those from the combined sample (Supplementary Table 2). In the referent group, TIMP-1 and PIIINP were not associated with CVD (Supplementary Table 1). In addition, none of the interaction terms in the models evaluating if relations of biomarkers to outcomes varied by LV sampling group were statistically significant (p values for all interactions exceeded 0.05).
Discussion
Principal Findings
In a community-based sample free of baseline CVD, we observed that higher circulating levels of TIMP-1 and PIIINP were associated with increased risk of death, and higher PIIINP concentrations were associated with incident CVD events. Although ECM biomarkers previously have been reported to be associated with LV and vascular remodeling,6,16 we noted that the associations of these markers with death and CVD were maintained even after adjustment for LVM and LV remodeling, and their relations to death endured after additional adjustment for BNP and CRP. Not surprisingly, PIIINP (a direct measure of tissue turnover) had the strongest and most consistent associations with death and CVD in all models. The results of analyses stratified by LV sampling group were not significantly different from our main results (for the total sample) and none of the interaction terms were statistically significant; however, given the modest event density in our sample, we may be unable to completely rule out heterogeneity in association of matrix markers with outcomes according to LV group. To our knowledge, ours is one of the first investigations that demonstrated the association of these biomarkers with incident CVD and all-cause mortality in a community-based sample.
Relations of ECM Remodeling Markers to CVD – Insights from Previous Reports
As noted above, several previous investigations reported the associations of ECM remodeling markers with CVD risk factors.4-7 Prior reports also described the associations of ECM biomarkers with LV structural change,20 LV remodeling after MI,21,22 measures of carotid atherosclerosis,23 hypertension-related systolic and diastolic heart failure,24 functional status measures in those with overt heart failure25 and progression of heart failure due to volume overload.26 Other investigators have explored the quantitative differences in circulating ECM marker levels between normal individuals, those with risk factor substrate for heart failure, and those with clinical heart failure.27 One report identified the utility of these markers in the diagnosis of diastolic dysfunction and heart failure with preserved ejection.28 However, previous investigations did not evaluate the association of ECM biomarkers with CVD risk in those free of prevalent disease.
Our investigation is relatively novel in three respects. First, we demonstrate the independent relations of ECM biomarkers to death and incident CVD in a population free of pre-existing CVD. Second, we report the incremental value of these biomarkers over conventional cardiovascular risk factors in predicting risk of death as evidenced by a significant improvement in model c-statistic. Third, we observe that these markers are related to death and CVD even after adjusting for echocardiographic indices of LV remodeling suggesting that the adverse prognosis related to matrix remodeling may not be fully captured by imaging measures.
Potential Mechanisms
Cardiovascular remodeling is an active process consisting of adaptive and maladaptive changes in response to pressure or volume overload, and antedates the development of overt CVD.1,29 In addition to hemodynamic load, several biological processes, notably inflammation, oxidative stress, a pro-thrombotic state or activation of the renin-angiotensin-aldosterone (RAAS) and natriuretic peptide systems are associated with both ventricular and vascular remodeling, and the subsequent risk for cardiovascular disease. As injury from any or all cardiovascular risk factors leads to remodeling, ECM turnover can potentially be viewed as a final common process that identifies the cumulative effects of several risk factors and pathways.
Thus, there are several potential explanations for our results. First, elevated levels of ECM turnover markers in people without clinically apparent CVD could reflect subclinical disease, and thus are identifying those with greater risk. Second, ECM markers are related to several conventional CVD risk factors, but in our study were related to CVD risk above and beyond these factors. Third, ventricular remodeling (as measured by changes in LV structure via echocardiography) is independently related to risk of death and CVD,30-32 and therefore the biomarkers (reflecting this process) are related to mortality and CVD risk. However, we observed that ECM markers are associated with mortality and CVD risk after adjusting for remodeling indices. Fourth, apart from structural change, other process like inflammation, oxidative stress and RAAS activation are associated with CVD risk; ECM markers are associated with markers of these pathways,33 and thereby may be capturing the risk associated with them; indeed, relations of PIIINP to CVD were no longer significant after adjustment for BNP and CRP. Lastly, conventional CVD risk factors do not completely explain CVD risk, and ECM markers may be identifying the residual risk.
Although the results of our investigation draw attention to several pathophysiological mechanisms involved in cardiovascular events as noted above, the low-risk clinical profile of our sample and the low event-density suggest caution in generalizing these finding to clinical settings. However, our findings do imply that ECM biomarkers merit further investigation for their role in risk prediction in individuals with average or higher CVD risk.
Limitations
From a large family of markers of ECM turnover, we measured only three biomarkers; other markers may be equally or more informative. We did not measure the biomarkers in a sample that included the entire range of LVWT and LVEDD, and therefore our results may not be generalized to people with LVEDD and LVWT in the intermediate range. We were hampered in the evaluation of the relations of MMP-9 to CVD and death due to low rates of detectability. Pre-analytical factors related to degradation of MMP-9 in frozen specimens may have contributed to the low detectability.34 Owing to modest numbers of mortality and CVD events, we did not have adequate power to detect relations of biomarkers to individual CVD event types (e.g. CHD, HF, etc), and separately to risk of cardiovascular versus non-cardiovascular death. Statistical modeling cannot completely account for the impact of comorbidities and there may be residual confounding not accounted for by the covariates in our models. Lastly, our sample is comprised of middle-aged white individuals of European descent and our results cannot be generalized to people of other age groups or ethnicities.
Conclusions
In a free-living cohort of middle-aged individuals we demonstrate that circulating biomarkers of ECM turnover, specifically TIMP-1 and PIIINP predict incident CVD and death. Participants with both markers above median are at markedly higher risk of death and CVD. Our findings confirm and extend previous research that demonstrated that ventricular and vascular remodeling antedates clinical CVD. Additional research is needed to delineate the utility of these measures for screening, in estimating risk of individual CVD events and for use in clinical settings.
Supplementary Material
Acknowledgments
None
Sources of Funding: This work was supported by the National Heart, Lung and Blood Institute (contract No. N01-HC-25195), NIH grant HL080124 (R.S. Vasan) and the Swedish Research Council grant 2007–5942 and the Swedish Heart Lung Foundation grant 20041151 (Johan Sundström).
Footnotes
Conflicts of Interests: None to disclose
Reference List
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–1438. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 3.Sundstrom J, Vasan RS. Circulating biomarkers of extracellular matrix remodeling and risk of atherosclerotic events. Curr Opin Lipidol. 2006;17:45–53. doi: 10.1097/01.mol.0000203891.34890.b5. [DOI] [PubMed] [Google Scholar]
- 4.Quilliot D, Alla F, Bohme P, Bruntz JF, Hammadi M, Dousset B, Ziegler O, Zannad F. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int J Obes (Lond) 2005;29:1321–1328. doi: 10.1038/sj.ijo.0803022. [DOI] [PubMed] [Google Scholar]
- 5.Tayebjee MH, Nadar S, Blann AD, Gareth BD, MacFadyen RJ, Lip GY. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in hypertension and their relationship to cardiovascular risk and treatment: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) Am J Hypertens. 2004;17:764–769. doi: 10.1016/j.amjhyper.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Wilson PW, Vasan RS. Relations of plasma total TIMP-1 levels to cardiovascular risk factors and echocardiographic measures: the Framingham heart study. Eur Heart J. 2004;25:1509–1516. doi: 10.1016/j.ehj.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Dhingra R, Pencina MJ, Schrader P, Wang TJ, Levy D, Pencina K, Siwik DA, Colucci WS, Benjamin EJ, Vasan RS. Relations of matrix remodeling biomarkers to blood pressure progression and incidence of hypertension in the community. Circulation. 2009;119:1101–1107. doi: 10.1161/CIRCULATIONAHA.108.821769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero JR, Vasan RS, Beiser AS, Au R, Benjamin EJ, Decarli C, Wolf PA, Seshadri S. Association of matrix metalloproteinases with MRI indices of brain ischemia and aging. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blankenberg S, Rupprecht HJ, Poirier O, Bickel C, Smieja M, Hafner G, Meyer J, Cambien F, Tiret L. Plasma concentrations and genetic variation of matrix metalloproteinase 9 and prognosis of patients with cardiovascular disease. Circulation. 2003;107:1579–1585. doi: 10.1161/01.CIR.0000058700.41738.12. [DOI] [PubMed] [Google Scholar]
- 10.Cavusoglu E, Ruwende C, Chopra V, Yanamadala S, Eng C, Clark LT, Pinsky DJ, Marmur JD. Tissue inhibitor of metalloproteinase-1 (TIMP-1) is an independent predictor of all-cause mortality, cardiac mortality, and myocardial infarction. Am Heart J. 2006;151:1101–1108. doi: 10.1016/j.ahj.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 11.Lubos E, Schnabel R, Rupprecht HJ, Bickel C, Messow CM, Prigge S, Cambien F, Tiret L, Munzel T, Blankenberg S. Prognostic value of tissue inhibitor of metalloproteinase-1 for cardiovascular death among patients with cardiovascular disease: results from the AtheroGene study. Eur Heart J. 2006;27:150–156. doi: 10.1093/eurheartj/ehi582. [DOI] [PubMed] [Google Scholar]
- 12.Lindsay MM, Maxwell P, Dunn FG. TIMP-1: a marker of left ventricular diastolic dysfunction and fibrosis in hypertension. Hypertension. 2002;40:136–141. doi: 10.1161/01.hyp.0000024573.17293.23. [DOI] [PubMed] [Google Scholar]
- 13.Li YY, Feldman AM, Sun Y, McTiernan CF. Differential expression of tissue inhibitors of metalloproteinases in the failing human heart. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 14.Diez J, Laviades C, Mayor G, Gil MJ, Monreal I. Increased serum concentrations of procollagen peptides in essential hypertension. Relation to cardiac alterations. Circulation. 1995;91:1450–1456. doi: 10.1161/01.cir.91.5.1450. [DOI] [PubMed] [Google Scholar]
- 15.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 16.Sundstrom J, Evans JC, Benjamin EJ, Levy D, Larson MG, Sawyer DB, Siwik DA, Colucci WS, Sutherland P, Wilson PW, Vasan RS. Relations of plasma matrix metalloproteinase-9 to clinical cardiovascular risk factors and echocardiographic left ventricular measures: the Framingham Heart Study. Circulation. 2004;109:2850–2856. doi: 10.1161/01.CIR.0000129318.79570.84. [DOI] [PubMed] [Google Scholar]
- 17.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 18.Kannel WB, Wolf PA, Garrison RJ, editors. The Framingham Heart Study: 30 Year Follow-Up. National Institute of Health; Bethesda, MD: 1987. Some Risk Factors Related to the Annual Incidence of Cardiovascular Disease and Death in Pooled Repeated Biennial Measurements. Section 34. NIH publication 87-2703. [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Sr, D’Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 20.Hansson J, Lind L, Hulthe J, Sundstrom J. Relations of serum MMP-9 and TIMP-1 levels to left ventricular measures and cardiovascular risk factors: a population-based study. Eur J Cardiovasc Prev Rehabil. 2009;16:297–303. doi: 10.1097/HJR.0b013e3283213108. [DOI] [PubMed] [Google Scholar]
- 21.Iraqi W, Rossignol P, Angioi M, Fay R, Nuee J, Ketelslegers JM, Vincent J, Pitt B, Zannad F. Extracellular cardiac matrix biomarkers in patients with acute myocardial infarction complicated by left ventricular dysfunction and heart failure: insights from the Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) study. Circulation. 2009;119:2471–2479. doi: 10.1161/CIRCULATIONAHA.108.809194. [DOI] [PubMed] [Google Scholar]
- 22.Rohde LE, Aikawa M, Cheng GC, Sukhova G, Solomon SD, Libby P, Pfeffer J, Pfeffer MA, Lee RT. Echocardiography-derived left ventricular end-systolic regional wall stress and matrix remodeling after experimental myocardial infarction. J Am Coll Cardiol. 1999;33:835–842. doi: 10.1016/s0735-1097(98)00602-0. [DOI] [PubMed] [Google Scholar]
- 23.Romero JR, Vasan RS, Beiser AS, Polak JF, Benjamin EJ, Wolf PA, Seshadri S. Association of carotid artery atherosclerosis with circulating biomarkers of extracellular matrix remodeling: the Framingham Offspring Study. J Stroke Cerebrovasc Dis. 2008;17:412–417. doi: 10.1016/j.jstrokecerebrovasdis.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez B, Gonzalez A, Querejeta R, Larman M, Diez J. Alterations in the pattern of collagen deposition may contribute to the deterioration of systolic function in hypertensive patients with heart failure. J Am Coll Cardiol. 2006;48:89–96. doi: 10.1016/j.jacc.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 25.Radauceanu A, Ducki C, Virion JM, Rossignol P, Mallat Z, McMurray J, Van Veldhuisen DJ, Tavazzi L, Mann DL, Capiaumont-Vin J, Li M, Hanriot D, Zannad F. Extracellular matrix turnover and inflammatory markers independently predict functional status and outcome in chronic heart failure. J Card Fail. 2008;14:467–474. doi: 10.1016/j.cardfail.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson KR, Stewart JA, Jr, Lucchesi PA. Extracellular matrix remodeling during the progression of volume overload-induced heart failure. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alla F, Kearney-Schwartz A, Radauceanu A, Das DS, Dousset B, Zannad F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur J Heart Fail. 2006;8:147–153. doi: 10.1016/j.ejheart.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Martos R, Baugh J, Ledwidge M, O’Loughlin C, Murphy NF, Conlon C, Patle A, Donnelly SC, McDonald K. Diagnosis of heart failure with preserved ejection fraction: improved accuracy with the use of markers of collagen turnover. Eur J Heart Fail. 2009;11:191–197. doi: 10.1093/eurjhf/hfn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opie LH, Commerford PJ, Gersh BJ, Pfeffer MA. Controversies in ventricular remodelling. Lancet. 2006;367:356–367. doi: 10.1016/S0140-6736(06)68074-4. [DOI] [PubMed] [Google Scholar]
- 30.Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32:1454–1459. doi: 10.1016/s0735-1097(98)00407-0. [DOI] [PubMed] [Google Scholar]
- 31.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 32.Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Left ventricular mass and incidence of coronary heart disease in an elderly cohort. The Framingham Heart Study. Ann Intern Med. 1989;110:101–107. doi: 10.7326/0003-4819-110-2-101. [DOI] [PubMed] [Google Scholar]
- 33.Joseph J, Pencina MJ, Wang TJ, Hayes L, Tofler GH, Jacques P, Selhub J, Levy D, D’Agostino RB, Sr, Benjamin EJ, Vasan RS. Cross-sectional relations of multiple biomarkers representing distinct biological pathways to plasma markers of collagen metabolism in the community. J Hypertens. 2009;27:1317–1324. doi: 10.1097/HJH.0b013e328329fc20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rouy D, Ernens I, Jeanty C, Wagner DR. Plasma storage at -80 degrees C does not protect matrix metalloproteinase-9 from degradation. Anal Biochem. 2005;338:294–298. doi: 10.1016/j.ab.2004.10.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


