Abstract
Sharks are representatives of the earliest vertebrates that possess an immune system utilizing V(D)J recombination to generate antigen receptors. Their antibody repertoire diversity is based in part on a somatic hypermutation process that introduces adjacent nucleotide substitutions of 2-5 bp. We have isolated mutant nonfunctional immunoglobulin rearrangements and intronic flank sequences in order to characterize the non-selected, intrinsic properties of this phenomenon; changes unique to shark were observed. Duplications and deletions were associated with N additions, suggesting participation of a DNA polymerase with some degree of template independence during the repair of DNA breaks initiated by activation-induced cytidine deaminase (AID). Other mutations were consistent with some in vitro activities of mammalian translesion DNA polymerase eta: tandem base substitutions, strand slippage, small insertions/deletions. The nature of substitution patterns shows that DNA lesions at shark immunoglobulin genes recruit DNA repair factors with a species-specific repertoire of activities. We speculate that the tandem mutations are introduced by direct sequential misinsertions and that, in shark B cells, the mispairs tend to be extended rather than proofread. Despite extensive changes undergone by some mutants, the physical range of mutational activity remained restricted to VDJ and within the first 2 kb portion of the 6.8 kb J-C intron, perhaps a self-regulating aspect of AID action that is conserved in evolution.
INTRODUCTION
Adaptive immunity in vertebrates is based on lymphocytes and their diverse antigen receptors. The antigen combining site of immunoglobulin (Ig) or T cell receptor (TCR) is generated by V(D)J rearrangement, a somatic recombination mechanism entailing combinatorial joining of multiple, tandemly duplicated gene segments [1]. During the recombination process the flanks of the V, (D) or J gene segments are cleaved and undergo modifications such as nucleotide deletion as well as nontemplated additions by terminal deoxynucleotidyl transferase (TdT). This process generates diverse sequence and sequence length differences at the joining sites, which encode loops forming the antigen-combining site. Additional post-rearrangement mechanisms as gene conversion and somatic hypermutation (SHM) further diversify the antigen receptor repertoire; and depending upon the animal species, the repertoire is expanded in immature B lymphocytes and/or in antigen-specific cells, honing antibody affinity during an immune response [2]. Both mechanisms are initiated by activation-induced cytidine deaminase (AID), which converts cytidine to uracil in the Ig gene [3, 4, 5]. An AID homolog has been isolated in every jawed vertebrate class including cartilaginous fishes [6].
In tetrapods almost all the changes resulting from SHM are point mutations. Repair of the uridine/guanosine mismatch results in mutational DNA synthesis, at the position or nearby [recently reviewed in 7, 8]. Base excision repair (BER) generates an abasic site, across which REV1 inserts an untemplated nucleotide (nt) during DNA synthesis. BER and mismatch repair both can give rise to a single-stranded gap that is filled in with low-fidelity by translesion DNA synthesis polymerases such as polymerase eta (Pol η) and others. The polymerases each have a mutational “signature” but altogether produce a composite mutational pattern where substitutions from G/C and A/T basepairs occur about equally, with a bias of ≥ 50% frequency of transition changes. In the past decade the pathways acting in SHM have been variously deduced by determining the shift in mutational spectra in mice deficient in the target gene or combination of genes. Although major components have been identified, roles for other candidate enzymes or pathways have yet to be elucidated [reviewed in 9, 10].
Very little is known of SHM in systems outside mouse or human, but of those that have been studied, the amphibian Xenopus and the shark present very different substitution patterns, showing that the contributions by low-fidelity repair polymerases vary among species. The mutations in Xenopus Ig consist of 90% substitutions from G/C basepairs with overall 62% transition changes [11]. Based on the current model for SHM in mouse, the majority of changes in Xenopus could have been generated due to the retention of uracil during DNA replication, causing G/C transition mutations, or by excision of the uracil, recruiting Rev1 to insert across the abasic site and generating some transversion mutations. The 10% A/T changes could arise from gap repair involving Pol η, which preferentially targets A/T basepairs and tends to generate transition mutations (T to C or the complement A to G). In Xenopus the balance of the various pathways is different from mouse, perhaps with BER out-competing mismatch repair [12].
In the shark, a representative of the earliest vertebrates with rearranging Ig/TCR gene systems, SHM in Ig and Ig-like serum proteins includes a unique feature: half of the changes consist of side-by-side tandem substitutions [13, 14]. The shark point mutation patterns resemble those in mouse and human Ig, possibly coincidental but indicating the participation of BER and mismatch repair pathways. The tandem substitutions of 2-5 bp appear to be generated by another polymerase/pathway that does not favor transition mutations although they, too, are AID-initiated because of the common bias for RGYW/WRCY hotspots. Gene conversion would seem the most likely mechanism for simultaneous adjacent substitutions, but no sequence donation was apparent among the six genes of the L chain isotype examined [14]. However, these earlier studies involved only productive rearrangements, expressed Ig that may have undergone antigenic selection. To avoid biases imposed by selective pressures and discover the intrinsic features of SHM, Storb [15] and Neuberger [16] analyzed changes occurring in passenger L chain transgenes while Dörner and Lipsky [17-19] examined out-of-frame V regions isolated from single human B cells. In this study we devised a novel strategy for amplifying nonproductive V(D)J rearrangements from nurse shark mRNA. Mutated out-of-frame V regions would in effect serve as non-selected bystanders, or reporters, for SHM that occurred in shark B cells activated by environmental antigens or deliberate immunization.
All germline genes encoding the IgM H chain and the NS5 L chain isotype have been defined in the nurse shark [20, 21, 22]. For these studies we isolated rearrangements from one single copy IgL gene and two single copy IgH genes (Fig. 1 and legend). Mutations in VJ, VDJ and the IgH intronic flanking region were analyzed. We assessed the impact of selection on shark Ig sequences by comparing in- and out-of-frame sequences and examined whether potential mechanisms such as gene conversion or DNA slippage could be participating in shark SHM. From the novel mutations obtained in the study, we conclude that changes were consistent with a transient misalignment process as well as error patterns generated by translesion polymerases like Pol η during in vitro DNA synthesis.
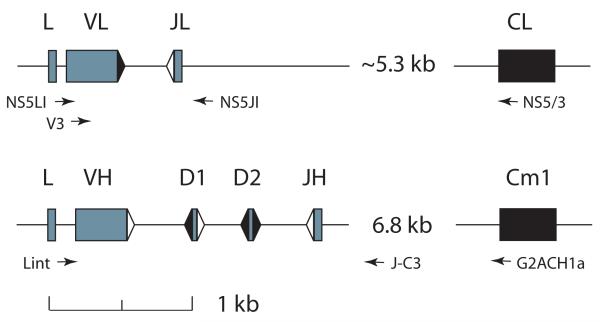
Figure 1.
Organization of the Ig L and H chain genes used in this study. Elasmobranch IgL and IgH genes are organized as miniloci that rearrange independently. The NS5-2 L chains and the G2A and G2B H chains are encoded by single copy genes. Top, IgL NS5-2 gene. There are four L chain isotypes in the nurse shark: kappa, lambda, sigma, NS5. Of the two functional, rearranging genes of the NS5 L chain isotype, one is preferentially amplified in these studies [22]. Leader, V and J gene segments, and C exon of L chain gene represented by filled blocks. Recombination signal sequences (RSS) with 12 bp spacer shown as black triangle, 23 bp spacer as white triangle. The distance from J to C is approximate, as the entire sequence could not be determined. Bottom, IgH G2A gene. There are 9-12 functional IgH genes, grouped into subfamilies: G1, G2 (A, B), G3, G4 (A-G, variable number of members), and G5 [21]. Every animal so far examined carries G2A and G2B as single-copy genes [21, 23, 24]. Leader, V, D1, D2 and JH gene segments shown as gray blocks, the first Cmu exon as black block. The J-C intron varies 6-10 kb among IgH genes. Relative distances between V gene segments according to the scale. Forward and reverse PCR primers (arrows) are described in Methods.
MATERIALS AND METHODS
Animals
Nurse sharks (Ginglymostoma cirratum) were captured off the coast of the Florida Keys and bled from the caudal sinus into heparinized syringes. Some animals were sacrificed after capture (shark-33) and others had been immunized (shark-Y, shark-J and shark-GR) [21, 24]. cDNA libraries were constructed from shark-33 epigonal organ and were screened for Ig sequences as previously described [23]. PCR was performed on genomic DNA and total RNA extracted separately from the peripheral blood leukocytes (PBL) of shark-J [22]. Surface Ig-positive PBL (sIg+) were isolated from shark-GR using anti-shark Ig monoclonal antibodies, and genomic DNA extracted from this sample was previously used for genomic Southern blotting [24].
Probes
Probes to the V and C regions of L chain NS5-2 [22] were used to screen cDNA libraries of shark-33. Probes to the VH gene segment of G2 (vh) and the intersegmental region between D2 and JH (dj) were described previously [24].
PCR
For L chain: oligonucleotide primers specific to the NS5 leader intron (NS5LI: 5′ CTGGCAAAGTCACTCAAAGTG 3′) and the NS5-2 intronic region 3′ of J gene segment (NS5JI: 5′ AGAGAACAGAATCGACACTGG 3′) were used to amplify sequences from PBL genomic DNA. For RT-PCR, forward primers in the leader intron (NS5LI) or in FR1 (V3: 5′ GTACATTGGCCGTTCCTG 3′) were used together with a primer in the NS5-2 C region (NS5/3: 5′ AGGACAGATGGTTTCCG 3′), after first strand priming with oligo dT (Roche). Accession numbers for SPH-156, SPH 2-2, SPH4 and JNS5-38,-39,-46,-61,-80, and -83 are HM171572-HM171581.
For H chain: genomic VDJ and the 3′ intron sequence, about 1.5 kb, was amplified by a two-step PCR protocol. Genomic DNA was extracted from sIg+ shark-GR PBL, and 50-200 ng DNA was added to 50 μl reactants (1× PCR, 200 μM dNTP, 5 units Taq per 200 μl reaction, primers in the leader intron (“Lint”, Fig.1) and J-C intron (LintC (5′-TTCTTATCCTGTGAGTCT-3′) and G2JCR2 (5′-ACCTTCAGTTTCTGCATT-3′, resp.)) and subjected to 20 cycles of PCR (95°C 1 min., 46°C 2 min, 72°C 2 min; last cycle 72°C 15 min). The PCR products were treated with EXOSAP-IT (USB) and 1 μl was added to 50 μl reaction mix and subjected to a second round of PCR (15 or 20 cycles of 94°C 1 min, 46°C 0.5 min, 72°C 1.5 min, last cycle 72°C 15 min) with nested primers (LintD (5′-TTCCCCAGTGTTAGTCMC-3′) and G2JCR3 (5′-GCATTAAGTCGGTTGTCAG-3′)). The background Taq mutation in this two-stage protocol was 0.15% (15/10,000 bp).
Long template PCR (Roche) was performed according to manufacturer’s instructions for System 3, for a total of 35 cycles, and optimized for the amount of input genomic DNA. Primers LintA (5′-ATTCAGCAATCAGATAAT-3′) and J-C3 (5′-ATGGTTAACTGCGATAC-3′) were used to raise rearranged products of about 5.8 kb (G2A) and 5.1 kb (G2B). LintD and G2ACH1a (5′-GAGACGAGACCATAAAGC-3′) were used to obtain the region from the G2A VH to the first 38 bp of the first C region exon, about 7.4 kb for the rearranged configuration.
PCR products were cloned into pGEM (Promega) and plasmid samples were sequenced by Genewiz, Inc., South Plainfield, NJ. Cloned PCR products were screened by colony hybridization and selected by expected size. Amplified genomic non-rearranged L chain was anticipated to be 880 bp, distinguishable from recombined VJ of about 486 bp; the latter was isolated by size fractionation. Genomic non-rearranged H chain was distinguished by hybridization of dj probe to the intrasegmental D2-JH region by colony hybridization assays whereas rearranged VDJ hybridized only with the vh probe. Accession numbers for S1, S4, S22, S23, S37, S21, S25, S57, S122, A2, A3, C9 are HM029142-HM029150 and HM171582-HM171584.
Germline genes in outbred animals
Two IgH and one IgL genes were analyzed in this study; they are single-copy genes each with one V, one J and one C region [21-24, Fig. 1]. All rearranged H chain sequences were obtained from shark-GR, whose IgH germline genes were previously cloned for genomic single-cell studies [24]. The shark-GR G2A and G2B IgH genes (accession numbers EU719631, EU719632) were identical to G2 germline sequences from other individuals [21, 23]. The rearranged NS5-2 L chain sequences were obtained from three animals, shark-Y, -J, -33. The germline NS5-2 was first isolated from a shark-Y cosmid library [22, AY720853] and it was the same as germline NS5-2 clones amplified from shark-J [22]. Both were identical to germline NS5-2 clones isolated from shark-33 erythrocyte DNA (not shown).
Cloned rearrangements either were non-mutated or contained substitutions. In every batch of cloned sequences there were hardly any shared substitutions among the variants unless they also shared a common CDR3 sequence. The absence of consistently shared changes demonstrated that the substitutions were independently acquired and somatic in origin (see Supplemental Fig. 1).
RESULTS
Experimental Design
In this report, rearrangements of one L chain gene (NS5-2) and two H chain genes (G2A, G2B) were examined for mutational changes such as substitutions, duplications, insertions and deletions. A strategy was devised to amplify nonproductive VJ preferentially from cDNA in order to obtain sequences that had mutated without selection pressure. The nature of substitions from in- and out-of-frame VJ was analyzed, and the efficacy of the PCR approach evaluated. A second source of non-selected sequence was the intronic region flanking mutated H chain rearrangements, cloned from genomic DNA of sIg+ PBL. The nature and extent of mutation in the 6.8 kb J-C intron was investigated and the 3′ boundary of SHM at shark IgH determined.
Isolating nonproductive Ig rearrangements
The first murine L chain sequence containing a nonproductive rearrangement also carried an unspliced leader intron [25]. This observation suggested out-of-frame V regions could be preferentially amplified by using a forward primer targeting the leader intron, and the single-copy NS5-2 gene [22] was selected to test this idea. Table I shows L chain sequences generated using a forward primer in the leader intron compared with those amplified with a primer in framework 1 (Fig. 1, NS5LI and V3, respectively). With the latter, V3, the VJ are mostly in-frame (25/31, 81%, Table I). Of 52 sequences raised with the leader intron primer, 39 (75%) contained nonproductive rearrangements; 24/39 clones contained somatic mutations and six mutants carried insertions or deletions (indel). The association of indels only with mutants is statistically significant among the 126 clones shown in Table I (0 indel in 54 nonmutants, 10 indel in 72 mutants, P= 0.005, Fisher’s exact test). These changes are examined in a separate section below.
Table I.
L chain rearrangements amplified from shark-J PBL DNA and RNA
| Source | Primer pair | total | Productive |
Non-productive |
series | ||||
|---|---|---|---|---|---|---|---|---|---|
| total clones |
mutants | In/del | total clones |
mutants | In/del | ||||
| cDNA | Intron-CL | 52 | 13 (25%) | 5 (38%) | 1a | 39 (75%) | 24 (62%) | 6a | JNS5 |
| cDNA | FR1-CL | 31 | 25 (81%) | 24 (96%) | 0 | 6 (19%) | 3 (50%) | 0 | V3 |
| genomic | Intron-JL | 43 | 14 (33%) | 5 (36%) | 0 | 29 (67%) | 11 (38%) | 3a | SPH |
| 34 | 1 | 38 | 9 | ||||||
In/del occurred in mutant sequences JNS5-83 (in-frame); JNS5-38,39,46,48,61,80 and SPH2,4,156 (nonproductive). Tally is: 0 in/del in 54 nonmutant sequences, 10 in/del in 72 mutant sequences (p = 0.005, Fisher’s exact test). 0/36 in/del in nonproductive nonmutants compared to 8/37 nonproductive mutants. 0/18 in/del in productive nonmutants compared to 1/35 productive mutants.
To get a better idea of the rearrangements present in the B cell population, VJ were amplified from genomic DNA. Of the 43 SPH sequences, only 35% were in frame and overall 37% were mutated. Thus, nonproductive L chain rearrangements are targeted bystanders in lymphocytes undergoing somatic hypermutation at transcribing Ig genes.
Selection in shark Ig
In a previous study [14] we could not show that shark L chain mutants undergo selection according to the criteria traditionally applied in mammalian systems, RS values. Nucleotide changes in mouse Ig mutants are scored according to a ratio of replacement (R) to synonymous (S) changes calculated for the framework (FR) and complementarity-determining regions (CDR). Having in hand two distinct Ig sequence groups, one clearly not functional, the shark Ig mutation patterns were reassessed.
In Table II, 24 in-frame V3 sequences with 183 mutations are compared with 18 nonproductive JNS5 sequences with 169 mutations. The selected sequences do not contain indels. Because the PCR primer targets the 5′ area of FR1 in the V3 series this is also left out of the JNS5 group. To avoid the frameshift complication, only the first six codons of CDR3 are used. The RS values for nonproductive cDNA are 4.8 for FR and 5.3 for CDR and for in-frame cDNA are 1.9 for FR and 4.2 for the CDR. These values are consistent with the nature of the rearrangements: in nonfunctional mutants FR and CDR values are similar, whereas in functional mutants there is greater disparity. Region by region, the extent of change is at the same frequency in both groups except in FR2, where the clear difference in the productive (0.8%) versus the nonproductive group (5.2%), demonstrates selection against changes in FR2 of functional Ig sequences.
Table II.
Comparing substitution patterns between in-frame and nonproductive V regions
| FR1 21 codons |
CDR1 13 codons |
FR2 15 codons |
CDR2 11 codons |
FR3 34 codons |
CDR3 6 codons |
||
|---|---|---|---|---|---|---|---|
| scored codons | scored codons | scored codons | scored codons | scored codons | scored codons | totals | |
| IN (24 sequences)a | |||||||
| tallied: | 504 | 312 | 360 | 264 | 816 | 144 | 2400 |
| affected: | 10 (2.0%) | 32 (10.3%) | 3 (0.8%) | 17 (6.4%) | 34 (4.2%) | 17 (11.8%) | 113 |
| mean | 0.42 ± 0.93 | 1.33 ± 1.61 | 0.13 ± 0.34 | 0.71 ± 1.12 | 1.42 ± 1.74 | 0.71 ± 0.81 | |
| Replacements | 7 | 23 | 2 | 15 | 22 | 15 | 84 |
| Replacement:Synonymous | 2.3 | 2.5 | 2 | 7.5 | 1.8 | 7.5 | |
| OUT (18 sequences)a | |||||||
| tallied: | 378 | 234 | 270 | 198 | 612 | 108 | 1800 |
| affected: | 6 (1.6%) | 23 (9.8%) | 14 (5.2%) | 11 (5.5%) | 32 (5.2%) | 16 (14.8%) | 102 |
| mean | 0.33 ± 0.77 | 1.28 ± 1.71 | 0.78 ± 1.22 | 0.61 ± 1.24 | 1.78 ± 1.80 | 0.89 ± 1.18 | |
| Replacements | 5 | 18 | 10 | 9 | 28 | 15 | 85 |
| Replacement:Synonymous | 5 | 3.6 | 2.5 | 4.5 | 7 | 15 |
Nature of L chain substitutions
In Table III substitutions from four groups of L chain mutants are characterized. The first set consists of 29 sequences from shark-Y PBL obtained by RT-PCR and from shark-33 epigonal cDNA library [23]. The second and third sets are respectively from in-frame V3 mutants and from nonproductive JNS5/SPH mutants from shark-J. Sequences with indels are not included. As noted previously [14] there is a bias for mutated positions associated with the RGYW/WRCY motif [26]. The motifs make up 81 bp (19.5%) out of the 415 bp of the leader, VL and JL. In 15 cDNA sequences isolated from the shark-33 library there were 141 substitutions of which 65 (46%) were in or partly in the motif.
Table III.
Nature of substitutions in shark L chaina
| In-frame shark-Y/shark-33 mutantsb | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All substitutions |
tandem substitutions |
single base substitutions |
||||||||||||||
| From | G | A | T | C | From | G | A | T | C | From | G | A | T | C | ||
| to G | - | 25 | 20 | 27 | to G | - | 15 | 16 | 13 | 44 | to G | - | 10 | 4 | 14 | 28 |
| A | 30 | - | 7 | 14 | A | 6 | - | 3 | 10 | 19 | A | 24 | - | 4 | 4 | 32 |
| T | 12 | 18 | - | 35 | T | 5 | 10 | - | 13 | 28 | T | 7 | 8 | - | 22 | 37 |
| C | 23 | 13 | 25 | - | C | 11 | 11 | 13 | - | 35 | C | 12 | 2 | 12 | - | 26 |
| Totals | 65 | 56 | 52 | 76 | 22 | 36 | 32 | 36 | 43 | 20 | 20 | 40 | ||||
| Total: 249 changes | Total: 126 changes (55 groups) | Total: 123 changes | ||||||||||||||
| Transitions: 46% | Transitions: 37% (21/26) | Transitions: 55% (34/34) | ||||||||||||||
| In-frame shark-J mutants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All substitutions |
tandem substitutions |
single base substitutions |
||||||||||||||
| From | G | A | T | C | From | G | A | T | C | From | G | A | T | C | ||
| to G | - | 25 | 8 | 20 | to G | - | 15 | 6 | 6 | 27 | to G | - | 10 | 2 | 14 | 26 |
| A | 22 | - | 9 | 11 | A | 7 | - | 4 | 6 | 17 | A | 15 | - | 5 | 5 | 25 |
| T | 10 | 11 | - | 21 | T | 4 | 8 | - | 5 | 17 | T | 6 | 3 | - | 16 | 25 |
| C | 13 | 17 | 16 | - | C | 6 | 12 | 8 | - | 26 | C | 7 | 5 | 8 | - | 20 |
| Totals | 45 | 53 | 33 | 52 | 17 | 35 | 18 | 17 | 28 | 18 | 15 | 35 | ||||
| Total: 183 changes | Total: 87 changes (36 groups) | Total: 96 changes | ||||||||||||||
| Transitions: 46% | Transitions: 40% (22/13) | Transitions: 51% (25/24) | ||||||||||||||
| Non-productive shark-J mutants | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All substitutions |
tandem substitutions |
single base substitutions |
||||||||||||||
| From | G | A | T | C | From | G | A | T | C | From | G | A | T | C | ||
| to G | - | 22 | 25 | 23 | to G | - | 10 | 20 | 16 | 46 | to G | - | 12 | 5 | 7 | 24 |
| A | 20 | - | 22 | 6 | A | 10 | - | 16 | 2 | 28 | A | 10 | - | 6 | 4 | 20 |
| T | 17 | 15 | - | 30 | T | 11 | 9 | - | 11 | 31 | T | 6 | 6 | - | 19 | 31 |
| C | 43 | 22 | 37 | - | C | 30 | 17 | 16 | - | 63 | C | 13 | 5 | 21 | - | 39 |
| Totals | 80 | 59 | 84 | 59 | 51 | 36 | 52 | 29 | 29 | 23 | 32 | 30 | ||||
| Total: 282 changes | Total: 168 changes (71 groups) | Total: 114 changes | ||||||||||||||
| Transitions: 39% | Transitions: 28% (20/27) | Transitions: 54% (22/41) | ||||||||||||||
V and J gene segments nucleotide content: 101 G, 78 A, 94 T, 96 C, 369 total. To normalize GC and AT basepair ratios, the derived ratio is multiplied by 0.87. For example, in the top set of point mutations the GC/AT is 2.08 (83 changes from GC/40 changes from AT in shark-Y/33). This is multiplied by 172 (A+T)/197 (G+C), or 0.87. The result, 1.81, is the normalized GC and AT ratio.
Total 249 changes, 159 from RT-PCR of shark-Y PBL, 90 from shark-33 cDNA library
For point mutations all three sets show a transition frequency of greater than 50%, both for G/C and A/T basepairs. The transition bias in A/T could reflect activity of Pol η, which tends to incorporate Gs opposite Ts, leading to T to C or A to G transition changes [27]. Tandem mutations usually occurred as di- or trinucleotide substitutions. In the nonproductive sequences the transition frequency in tandem changes is even lower, 28%, and is closer to the expected randomly acquired changes, 30%. At this point we asked whether the frequencies of transitions among tandem mutations in the first two sets, 37% and 40%, might have been skewed upwards by selection. We extended this study to H chain flanking intronic sequence because the J-C intron of NS5-2 L chain gene contains sequencing blockages.
Hypermutation at IgH
A stock of genomic DNA extracted from shark-GR sIg+ PBL was characterized previously [22]. In preliminary studies using this DNA 21 G2 VDJ sequences of 500 bp were cloned (not shown). Ten sequences were nonproductive and five carried 3 to 52 substitutions; 11 sequences were in-frame and one mutant carried two changes. Most of the clones (18/21) were G2A although the primers also detect the G2B isotype. Similar results were obtained in the three experiments described below, so that the mutants tallied in this study are almost all nonproductively rearranged G2A sequences.
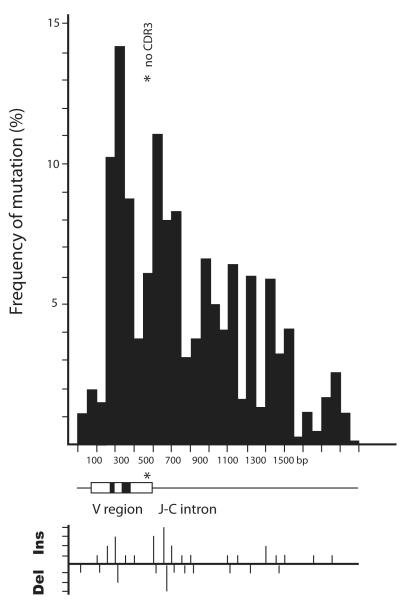
In the first experiment, the VDJ and 3′ flanking region of 1.1 kb were amplified and cloned. G2A/B genes were amplified equally, although G2B tended to be in germline or partially rearranged configuration (screening results not shown). The 5% nucleotide and indel differences in the two isotypes allowed us to evaluate the extent of in vitro crossing over, if any. Fig. 2 shows the frequency of mutation over the VDJ and 1 kb of flanking sequence and generally resembles that published by Lebecque and Gearhart [28] for mouse mutants. Multiple indels were present, as depicted below the drawing of the VDJ gene, but these were tallied separately from substitutions. The indel pattern parallels that of the substitutions and so is part of the same process.
Figure 2.
Frequency of mutation distributed over rearranged VDJ and 3′ flanking sequence. Fifty-six sequences were analyzed, of which 18 were mutants. Mutations in the 16 G2A sequences were tallied, excluding CDR3, and their distribution over the V region and downstream flank calculated as the number of substitutions in 50 bp segments divided by 50, divided by the number of sequences [28]. A diagram of the H chain gene is shown beneath the graph and the asterisk indicates the position of CDR3, which has been omitted. A second graph underneath depicts the number of insertions and deletions per 50 bp segment in increments of 1. The changes are mostly represented by nonfunctional sequences (13/16) which also contained more indels.
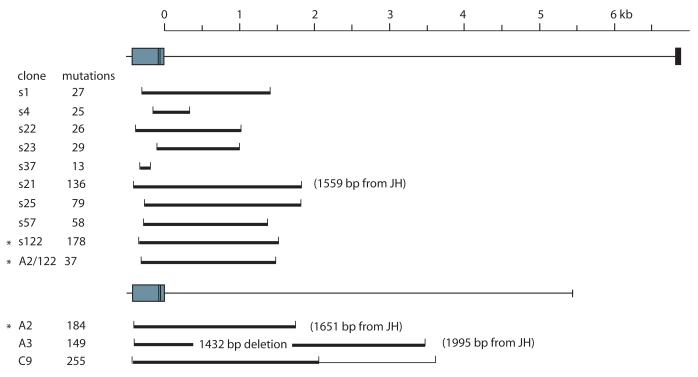
Two sets of clones with longer 3′ flank sequence were obtained and the extent of their tandem mutations shown in Fig. 3. Occasional point mutations were found scattered throughout the intronic region, and some are probably in vitro changes (Fig. 3 legend). We thus prefer to distinguish the area where tandem substitutions occurred since they are the distinctive feature of shark hypermutation. The brackets encompass the 5′-most and 3′-most tandem groups (doublets or triplets) of the clone; all substitutions are tallied at the left hand side. The mutations in clone A3 at the 3.5 kb region of the reference sequence are actually located 1995 bp from its own JH, the consequence of the large 1.4 kb deletion decreasing distance from the VH promoter. In clone C9 255 changes occurred in the first part of the gene (heavy brackets) but an isolated group of seven substitutions appeared downstream over an area of 400 bp (light brackets).
Figure 3.
The boundaries of mutation in the shark H chain gene. The fully rearranged VDDJ is shown, together with a scale marking distance from the JH gene segment to the first C exon (6.8 kb). Genomic G2A rearrangements cloned with 5357 bp (C9, A2, A3) or 6848 bp (s series) of 3′ flanking sequence were scored for total substitutions (left column). The extent of the 5′-most and 3′-most tandem changes is depicted as a bracket for each clone. The lighter bracket in C9 includes an isolated group of seven mutations distributed in the 3′-most 400 bp. Some of the mutants contained extensive deletion, and the true distance from the JH border is indicated in parentheses if there is a discrepancy of >100 bp. For example, the 3′-most doublet in A3 is at position 3496 bp according to the J-C intron reference sequence, but it is located 1995 bp from its JH border. Asterisks mark the related clones A2 and s122; the hypothetical ancestral mutant “A2/122” is discussed in the text. The error for the Expand Long Template enzyme mix (Roche) is 0.11% (4 substitutions/3696 bp) as scored in nonrearranged clones.
The “s” series in Fig. 3 contain the ~7.4 kb of sequence from the leader intron to the first C exon of G2A. 103 VDJ were sequenced, of which 34 were mutants and represented 15 unique rearrangements. Nine clones in Fig. 3 confirm that hypermutation mostly takes place within a 2.5 region encompassing the VDJ. In clone s1 the 27 mutations occurred over the same area as the 58 mutations in s57 or the 178 in s122.
Comparison of mutants from all PCR sets showed that A2 and s122 shared ancestry. Although the CDR3 were mutated, there are common N region additions and multiple shared mutations. In Fig. 4 this pair of nonfunctional VDJ from long-diverged daughter clones demonstrates one point clearly, that the early (shared) mutations occurred in the same region as the later ones. There are 184 mutations in A2 and 178 in s122, not counting nucleotides in insertions/duplications. Of these, 37 shared mutations (dark shading, Fig. 4) are distributed over an area of 1.7 kb that overlap with the subsequent 147/141 changes. This is diagrammed in Fig. 3 (asterisked s122, A2 and the ancestral A2/122).
Figure 4.
Comparison of related IgH mutants. The reference germline (GL) gene G2A is shown without intergenic sequence in VH, D1, D2 and JH gene segments (labeled in boldface, gaps indicated by slashes). The start of the VH gene segment is indicated over the splice acceptor site and is the divided into framework (FR) and complementarity-determining (CDR) regions. The beginning of the J-C intron is indicated over the splice donor site. Clones A2 and s122 are aligned with respect to the GL and the PCR primers are italicized. Differences from the GL are highlighted in blue and those shared between the mutants are highlighted in purple. Changes in CDR3 are not marked. Although the rearrangement is in-frame, the less mutated A2 CDR3 contains two stops. The VDJ was probably never functional, and its first mutations (in CDR1, AT to GA generated TGA) were deleterious. Dashes indicate gaps. Duplicated sequences are highlighted and their template underlined. Lower case n is placed where point deletion was introduced during PCR slippage. Asterisks show sites of inserted nt, crosshatches deletions. The numbering of the reference sequence includes leader intron and coding VH gene segments (1-474) and 3′ flank (475-2289).
The distribution of changes in the ancestral clone as well as in A2 and s122 suggests that there are roughly two regions of about 1-1.1 kb, with the one proximal to the promoter experiencing more changes. The decrease in mutation frequency is apparent in the distal 1 kb; the difference is not due to any disproportionate distribution of hotspots (not shown). Although there is considerable difference in AT to GC content in the VH gene segment (1.04) compared to 2 kb of the J-C intron (1.967), this does not explain the mutation pattern. The first 700 bp of the J-C intron, which is included in the proximal 1.1 kb, is heavily mutated and this has the same nucleotide representation as the downstream portion.
Nature of changes in the J-C intron
Nucleotide substitutions in the J-C intron were scored in s1, s4, s22, s23, s37, s25 and s57 because the substitutions are sufficiently sparse for most to be deemed independent events. The overall pattern of nucl IV) is similar to that in L chain mutants, about half the changes were in tandem groups (77/150) and the frequency of transition changes is higher for point mutations (56%) compared to tandem mutations (36%).
Short (1-3 bp) insertions or 1-bp deletions were frequent in mutated J-C intron sequence but were not scored in Table IV. Some examples are marked with asterisk and crosshatch in Fig. 4. In Fig. 5 three kinds of insertions are shown in the top sequence (Fig. 5A, clone G20-88). One is a 1-bp deletion that is replaced by 2-bp mutation, the second is a 7-bp insertion that appears to be a (mutated) inverted repeat of the adjacent 5′ flank, and the third is a 2-bp AG insertion. The 7-bp insertion could be “T nucleotides” which are inserts that are direct or inverted repeats of flank sequence [29] and coupled to repair after a double-strand DNA break (DSB).
Table IV.
Shark-GR J-C intron mutationsa
| All substitutions |
All tandem substitutions |
All single base substitutions |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From | G | A | T | C | From | G | A | T | C | From | G | A | T | C | |||
| to G | - | 21 | 25 | 16 | 62 | to G | - | 10 | 17 | 4 | 31 | to G | - | 11 | 8 | 12 | 31 |
| A | 9 | - | 10 | 2 | 21 | A | 0 | - | 6 | 2 | 8 | A | 9 | - | 4 | 0 | 13 |
| T | 3 | 10 | - | 13 | 26 | T | 2 | 8 | - | 4 | 14 | T | 1 | 2 | - | 9 | 12 |
| C | 9 | 6 | 26 | - | 41 | C | 7 | 3 | 14 | - | 24 | C | 2 | 3 | 12 | - | 17 |
| Totals | 21 | 37 | 61 | 31 | 9 | 21 | 37 | 10 | 12 | 16 | 24 | 21 | |||||
| Total: 150 changes | Total: 77 changes | Total: 73 changes | |||||||||||||||
| Transitions: 46% | Transitions: 36% | Transitions: 56% | |||||||||||||||
Data from clones s1,s4,s22,s23,s25,s37,s57
Nucleotide composition of first 2 kb J-C intron: 19.1% G, 28.3% A, 38% T, 14.6% C. To normalize GC/AT, multiply ratio by 1.97. The composition differs considerably from the VH gene segment, which is 27% G, 27% A, 24% T, 22% C out of 308 bp.
Figure 5.
Insertions and deletions in Ig sequence. A. Portion of the J-C intron from clone G20-88 containing 3 different insertion events. B-K. Ten L chain sequences with indels from experiments shown in Table I. B. JNS5-38, nonproductive, insertion in CDR1. C. JNS5-39, nonproductive, insertion in JL. The letter “i” over T indicates insertion in inversely duplicated sequence D. JNS5-46, nonproductive, deletion in FR3. E. JNS5-48, nonproductive, deletion in CDR1. F. JNS5-61, nonproductive, deletion in CDR1; deletion and incomplete duplication in FR3. G. JNS5-80, nonproductive, deletion in CDR3. H. JNS5-83, in-frame, insertion in CDR3. I. SPH-156, in-frame but lacking structural TTC in JL, duplication in FR3; insertion in CDR3. The distance between insert and upstream template is 46 bp. J. SPH2-2, nonproductive, insertion in CDR2. K. SPH4, nonproductive, duplication in CDR2; insertion in FR3. The sequences are compared with the J-C intron or NS5 reference sequence, identities indicated by vertical bars. Mutations are shown in lower case. Duplicated nt are shaded and the template sequence is underlined. In those instances that could involve T nucleotides, both insert and flank template are marked with arrows indicating their relative orientation.
Indels and T/N nucleotides
The duplications and deletions in shark Ig are often accompanied by nucleotides of unknown origin. The single instance involving an in-frame VJ (JNS5-83, Fig. 5H) was a nondisruptive 3 bp insertion in a loop region, CDR3. Many events shown in Fig. 5 could have resulted from a staggered double strand break (DSB) from nearby lesions on either strand. Depending on the nature of the breaks, deletion from DNA end resection or duplication from filling in may result. There are simple deletion events, as in JNS5-46 or JNS5-61(1), but in others sequence was added. In JNS5-80 (Fig. 5G) the CGCC appears to be nontemplated. Similarly, some duplications are simple (SPH2-2, Fig. 5J) but in others there are accompanying additions (JNS5-61, GGGG; SPH-156, AG; SPH4(1), G). The insertions in duplications and deletions tend to be GC-rich, although not in all cases.
The events designated with arrows in Fig. 5 are interpreted as T nucleotides because the presumed template is in complementary orientation to the insert or removed from the mutated site by 2 to 49 bp (Fig. 5H, JNS5-83, I(2) SPH-156, K(2) SPH4). The T nucleotide copies tend to contain mismatches, including indels, like in Fig. 5C (JNS5-39, marked by “i”) [30]. However some complex events like the incomplete duplication (missing final A) in JNS5-61 (Fig. 5F) and the extra nucleotide at one end of the duplication in SPH4 (Fig. 5K(1)) cannot easily be explained by a staggered DSB and fill-in.
Mutation by strand slippage?
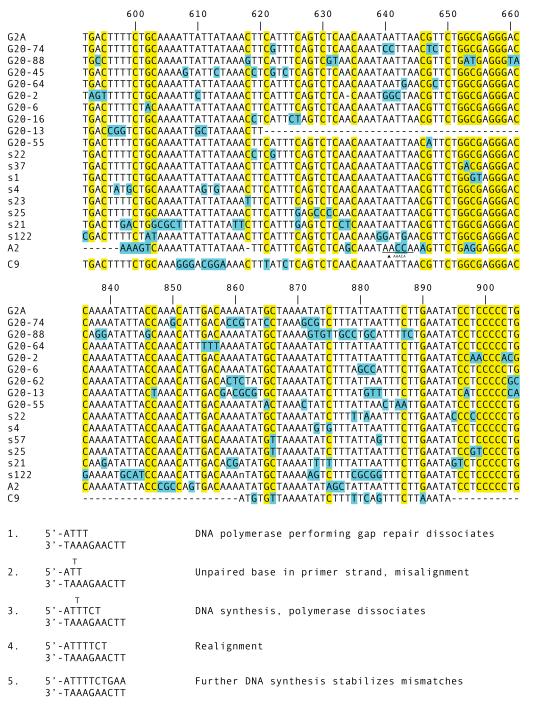
Besides possessing some degree of template-independence [31] Pol μ is prone to misalignment [32, 33], leading us to look for evidence of strand slippage. A pathway like transient misalignment [34] would give a templated origin to tandem substitutions and explain a transition frequency closer to the anticipated random one. We inspected the J-C intron, expecting that slippage could occur more frequently in a region of relatively repetitive DNA [35]. In Fig. 6 the TC tandem mutation at positions 887-888 in clone G20-74 could have resulted from a misalignment occurring when an upstream T on the primer strand assumed an extrahelical position (Fig. 6, bottom, step 2). After synthesis of TCT the error-prone polymerase dissociates, and the primer strand realigns with the template, shifting the 3′-most T to position 889 from position 888 (Fig. 6, bottom, step 4). There are other substitutions possibly generated this way, via transient misalignments of the primer or the template strand with 1-3 unpaired bases, but it does not explain many other changes. If tandem substitutions arose from transient misalignment events, we would expect to find mostly A to T and T to A changes in the A/T –rich regions, but this is generally not the case.
Figure 6.
Changes in A/T-rich areas in the J-C intron. Top. Clones with substitutions in two regions of the J-C intron of the G2A gene are shown, with numbering according to the reference sequence in Fig. 4. G/C nucleotides are highlighted in yellow and mutations in blue. An inserted duplication (AAACA) is shown for clone A2 at position 640. Dashes indicate deleted sequence. Lower case n is placed where point deletion was introduced during PCR slippage. Bottom. Hypothetical pathway by which CT at position 887-888 in clone G20-88 are mutated to TC through transient misalignment during gap repair. Step 1: Dissociation of DNA polymerase causes fraying, followed by Step 2: strand misalignment. Step 3: Distributive DNA synthesis. Polymerase dissociates, with attendant fraying. Step 4: Primer and template realign. Step 5: Extension of primer stabilizes mismatches. Tandem mutations CT to TC established.
In Fig. 6, top, the germline G/C are highlighted, and these are potential sites for AID action on one of the strands. Substitutions can take place at or at least 1-6 nt away from these sites. The maximal distance is derived from the longest stretch of A/T at positions 604-617, where point and tandem changes occur six nt from the nearest cytosine in clones G20-2 and G20-13. Taking clone G20-6 as an example, the GCC substitution at positions 880-882 is isolated and unlikely to be the result of more than one AID-initiated event, so that the lesion could have been generated at positions 875 or 887 or 890. A single-stranded gap is created, extending at least 7 nt downstream of position 875 or 7 nt upstream of position 887 or on the complementary strand 10 nt downstream of position 890. Subsequent gap repair with error-prone polymerases generate 3 mismatching nt GCC.
The region depicted in Fig. 6 is within 500 bp of the 3′ flank of JH of G2A. We inspected the same region of five other IgH genes G2B, G1, G3, G4A, and G5, looking for donor templates in a gene conversion mechanism for tandem mutations. There were none for the homologous positions (not shown). For instance, the GCC mutation of clone 20-6 would have to be a 3 nt gene conversion tract copied from upstream or downstream sequence or from a nonhomologous position in another IgH or is a longer tract derived from an unknown source.
DISCUSSION
The Ig genes in shark and other chondrichthyan fishes have an early alternative organization of multiple miniloci [36] where each one consists of a few gene segments individually undergoing intralocus V(D)J recombination (see Fig. 1 and legend) and SHM in B cells [21, 24]. AID has been isolated from nurse shark and found in adult lymphoid tissue by RT-PCR (E. Hsu, unpublished results), and the biased occurrence of all mutations at RYGW hotspots suggests their common initiation by AID [14]. The unique pattern of adjacent mutations in shark Ig and Ig-like genes was first observed in the IgNAR [37] and fully characterized in traditional Ig L chains [14]. In this study we isolated nonproductive Ig rearrangements and 3′ flanking sequence to define the intrinsic properties of this unusual hypermutation process. We first compared in- and out-of-frame L chain VJ mutants to reassess the standard criter selection. These ratios showed that the nonfunctional rearrangements were unselected but, at the same time, the low differential in FR/CDR RS values for in-frame mutants suggests that antigenic selection in shark L chains is not as strong as in mammals. For example, data from an unselected human B cell population [18] show a greater differential between nonproductive (2.1 for FR versus 2.5 for CDR) and productive (1.4 FR versus 7.8 CDR) rearrangements in the kappa Vκ12/O2 genes. Only the relative absence of replacement mutations in FR2 indicated that expressed mutants were selected at least on the basis of structural integrity.
Germinal centers do not exist in cartilaginous fishes or in the amphibian Xenopus [reviewed in 39], but affinity maturation has been demonstrated in their humoral responses [40, reviewed in 41]. Dooley and Flajnik [40] studied the nurse shark antibody response to hen egg lysozyme and found that, of the 7S and 19S IgM present in serum, it was the monomeric species that increased in affinity. They speculated that cells producing the monomer are from a T cell-dependent lineage that is responsible for the specificity of the response. SHM in sharks is an antigen-driven process [42, 14, 43]; there are no H or L chain mutants in neonates. However, antigenic selection may not need to be comparably as effective as in mammals [41], and variant sequences resulting from the remarkably efficient shark SHM could also serve to diversify the existing repertoire [44].
Boundaries of hypermutation at shark Ig genes
The boundaries of hypermutation have been studied extensively at murine IgH and IgL loci and in a variety of transgene constructs where the relative positions of the cis-acting elements were shifted. The upstream boundary is characterized as an abrupt increase in mutation frequency 180 bp after the transcription start site [45, 46], as if determined by a fixed point from the promoter. That correlation between hypermutation and transcription was further demonstrated by Peters and Storb [47] who inserted the Vκpromoter 5′ of the Cκ exon and elicited hypermutation over a similar distance downstream as the endogenous promoter. In contrast, the downstream boundary is not clear-cut one, and the area targeted by SHM is characterized as extending 1-2 kb [45] or rarely beyond 1.5-2 kb [46] from the promoter or occurring “with a high frequency for almost 1 kb before tapering off” [48]. These descriptions also fit observations in shark IgH.
We interpret the shark IgH mutation distribution curve as consisting of two distinct regions, of high and low substitution frequency. Any place within ~2 kb stretch can be mutated, at any time, although the frequency is higher for the upstream 1.1 kb. The related clones A2 and s122 give us three snapshots: the deduced ancestor with 37 mutations and its two descendants with 150 subsequent changes. The mutation spread is the same in all three and suggests, as in the murine system [46], there exists a limiting element no matter what the mutation load. Models for AID-targeting couple the mutator with transcription; for example, one idea is that the mutator is associated with transcription initiation/elongation factors (“mutasome”), slides along the DNA, and initiates the mutation event with greater probability at the beginning of the V segment [49]. What restricts the area affected could be the half-life of the mutator, which becomes exhausted or disengages from the transcription complex [49, 47].
In contrast, a larger area is affected by SHM in the switch regions (4-5 kb [50, 51]) and a smaller one in an in vitro transcription-based assay system of AID activity (500 bp before “declining precipitously” [52]). The differences from in vivo SHM at the VDJ may be respectively attributed to a different content of the mutasome at the I exon promoters [50] and suboptimal input in vitro components.
Consideration of TdT and/or Pol μ
Duplication and deletion occur during SHM in 43% of out-of-frame and 4% of in-frame human VDJ [53], but there are no examples of nontemplated additions longer than 1 bp or any inserts that are inverted repeats like those shown in Fig. 5. The only instance where DSB repair in Ig was accompanied by >1 bp insertions was from experiments in Ramos Burkitt, which constitutively mutates its Ig genes, after transfection with a construct with TdT under a beta-globin promoter and IgH enhancer [54]. In that case, duplications and deletions, usually simple ones when the Ig sequences are isolated from organ tissue [53, 55], acquired G/C-rich nontemplated additions.
We investigated whether the nurse shark Ig insertions could have been introduced by TdT, looking for evidence of TdT expression outside of the thymus and bone marrow equivalent (epigonal organ). From a battery of organ samples TdT RT-PCR signal consistently coincided with RAG2 and only sometimes with AID or RAG1 (C. Zhu and E. Hsu, unpublished observations). At this point, the probable explanation is that TdT and RAG2 signals in non-primary lymphoid tissue originated from newly mature B cells in the process of downregulating both. It would have been surprising to find TdT at a stage well beyond the completion of V(D)J rearrangement, although TdT expression in activated B cells does not appear to affect lymphocyte viability in TdT-transgenic mice [56, 57]. Pol μ, on the other hand, is more likely to be present at all times; the zebrafish homolog is expressed in all tissues examined [58], increased in lymphoid tissues, like in mouse and human [59]. It is difficult to distinguish a duplication with N addition from a T insertion, but some of the mutations in shark indels can be explained as inverted repeats or repeats of nearby but non-abutting flank sequence, which could have involved a polymerase tending to slippage, like Pol μ [30]. What is clear is that some of the duplications in shark Ig copied mutated nt (Fig. 5F, 5K), showing that DNA breakage and repair took place during or after SHM events; the strict correlation of indels with mutants in Table I (footnote) suggests breakage was AID-initiated. In conclusion, the polymerases/pathways available for DSB repair are different for shark and human B cells during SHM.
TdT and Pol μ are members of family × polymerases that contribute to BER and nonhomologous end joining [60]. Although overexpressed Pol μ in the Ramos cell line introduced mutations that can be explained by a transient misalignment process [61], Pol μ and Pol λ-deficient mice display no effects on SHM [62] and a role of family × polymerases in this process has yet to be established [9]. This however does not rule out such a function existing in B lymphocytes of species other than mouse or human beings.
Candidate polymerases and pathways
Following deamination of cytidine by AID, the resultant uracil is removed and a single-stranded gap may be generated, perhaps extending ≤29 nt upstream and/or ≤18 nt downstream of the lesion in mouse systems [63]. In shark, the gap is at least 6 nt, and perhaps the process supporting nuclease accessibility leads to some DNA fraying. We hypothesize that several error-prone polymerases are available in shark B cells, some of which can add 2 or more mismatching nucleotides before dissociating. The extension efficiency depends on the nature of the terminus mispairing, and repair may switch to another polymerase [64] or one specifically targeting and extending aberrant primer-terminal base pairs [65, 66]. Once the gap is filled, strand ligation completes the repair.
The detection of an unexpected DNA polymerase activity during DSB repair raises some speculation as to its involvement in generating tandem mutations, which are unique to sharks or elasmobranchs. If we hypothesize that a template-independent polymerase adds nt, both this model and one for transient misalignment [34, 67] require some unwinding of the primer terminus. While there is no direct evidence for this occurring during processing of the uracil, models for AID-initiated gene conversion postulate single-strand invasion into the homologous duplex DNA template [68, 69]; and a single-stranded gap or nick has been demonstrated as the intermediate [70, 71].
An alternative idea is that the low-fidelity polymerases consecutively misincorporate 2 or more nucleotides. Gene conversion may appear energetically more palatable, but as observed in the Results section, the conversion tract would have to be very short and initiated with minimum homology. Moreover, the donor sequences would be randomly acquired since identical and identically positioned tandem mutations have seldom been observed outside of clonally unrelated sequences. Although it seems unlikely one mismatch would be extended with another [72], in vitro experiments monitoring base-substitution fidelity of mammalian Pol η and yeast Pol ζ showed that these polymerases are capable of direct tandem misinsertions [27, 73].
Pol η can produce adjacent substitutions
Whereas a role of Pol ζ in SHM has not been fully elucidated, that of Pol η was demonstrated in humans or mice deficient for the enzyme [74, 75]. In the classical studies that established the specificity preference of human and murine Pol η for placing G opposite T, a high frequency of 2- and 3-bp substitutions was also observed [27]. These tandem mutations occurred at a rate 10% of point mutations. However, the substitution spectrum is different from shark. The human Pol η in vitro mutations, both single and tandem, are strongly A and T biased (normalized GC/AT ratio 0.2 for tandem substitiutions, Table V) with an overwhelming preference for T to C changes; the single and tandem changes roughly parallel. In contrast, the shark tandem mutations display some A/T bias (GC/AT ratio 0.7, Table III) and no transition preference. Inasmuch as the shark point mutations from A/T have an overall >50% transition frequency (Table III) that suggests a Pol η-like activity is present, this would not by itself be responsible for the tandem substitutions. At least another polymerase is involved in tandem mutations from A/T and the large numbers of changes from G/C.
Table V.
Human Pol η in vitro synthesis mutationsa
| All substitutions |
All tandem substitutions |
All single base substitutions |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| From | G | A | T | C | From | G | A | T | C | From | G | A | T | C | |||
| to G | - | 116 | 53 | 14 | 183 | to G | - | 12 | 8 | 1 | 21 | to G | - | 104 | 45 | 13 | 162 |
| A | 140 | - | 78 | 43 | 261 | A | 4 | - | 18 | 4 | 26 | A | 136 | - | 60 | 39 | 235 |
| T | 50 | 202 | - | 119 | 371 | T | 11 | 38 | - | 9 | 58 | T | 39 | 164 | - | 110 | 313 |
| C | 51 | 141 | 700 | - | 892 | C | 5 | 24 | 49 | - | 78 | C | 46 | 117 | 651 | - | 814 |
| Totals | 241 | 459 | 831 | 176 | 20 | 74 | 75 | 14 | 221 | 385 | 756 | 162 | |||||
| Total: 1707 changes | Total: 183 changes | Total: 1524 changes | |||||||||||||||
One strand only, data from Ref 27. Nucleotide composition: 23.5% G, 24% A, 22.1% T, 30.3% C.
Why have tandem substitutions not been observed in mammalian systems? Our previous randomization trials using κ passenger transgene sequences showed that, surprisingly, side-by-side mutations actually occurred more frequently in the mouse than expected [14]. Although murine Pol η has the capability to extend a mismatch with a mismatch, it is thought that the greater DNA helix distortion created by tandem mismatches target them for removal in vivo [76]. This proofreading capacity could be suspended in shark B cells, where the mispair formations are extended rather than eliminated.
We propose that the unique tandem mutations in shark Ig arise from error-prone DNA polymerase activities that involve sequential insertions as well as primer or template slippage/realignment. As yet there are no error specificity data for elasmobranch polymerases, and from the unusual mutations observed in these studies we anticipate species-specific characteristics in the translesion DNA synthesis polymerases in the shark.
Supplementary Material
ACKNOWLEDGMENTS
The authors wish to thank Grace Chan for her contributions to this study and Drs. Thomas Kunkel, Dale Ramsden, and Hirota Kouji for their very helpful comments.
3 Abbreviations used in this paper
- AID
activation-induced cytidine deaminase
- DSB
double-stranded break
- SHM
somatic hypermutation
- BER
base excision repair
- RSS
recombination signal sequence
- FR
framework
- CDR
complementarity-determining regions
- sIg+
surface Ig positive
- nt
nucleotides
Footnotes
This research was supported by grants from the National Institutes of Health (R01-GM068095).
REFERENCES
- 1.Schatz DG, Spanopoulou E. Biochemistry of V(D)J recombination. Curr. Top. Microbiol. Immunol. 2005;290:49–85. doi: 10.1007/3-540-26363-2_4. [DOI] [PubMed] [Google Scholar]; Argon Y, Burrone OR, Milstein C. Molecular characterization of a nonsecreting myeloma mutant. Eur. J. Immunol. 1983;13:301–305. doi: 10.1002/eji.1830130406. [DOI] [PubMed] [Google Scholar]
- 2.Du Pasquier L, Wilson M, Greenberg AS, Flajnik MF. Somatic mutation in ectothermic vertebrates: musings on selection and origins. Curr. Top. Microbiol. Immunol. 1998;229:199–216. doi: 10.1007/978-3-642-71984-4_14. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Arakawa H, Hauschild J, Buerstedde JM. Requirement of the activation-induced deaminase (AID) gene for immunoglobulin gene conversion. Science. 2002;295:1301–1306. doi: 10.1126/science.1067308. [DOI] [PubMed] [Google Scholar]
- 5.Harris RS, Sale JE, Petersen-Mahrt SK, Neuberger MS. AID is essential for immunoglobulin V gene conversion in a cultured B cell line. Curr. Biol. 2002;12:435–8. doi: 10.1016/s0960-9822(02)00717-0. [DOI] [PubMed] [Google Scholar]
- 6.Conticello SG, Thomas CJ, Petersen-Mahrt SK, Neuberger MS. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol. Biol. Evol. 2005;22:367–377. doi: 10.1093/molbev/msi026. [DOI] [PubMed] [Google Scholar]
- 7.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annu. Rev. Biochem. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 8.Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, Scharff MD. The biochemistry of somatic hypermutation. Annu. Rev. Immunol. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 9.Weill JC, Reynaud CA. DNA polymerases in adaptive immunity. Nat. Rev. Immunol. 2008;8:302–312. doi: 10.1038/nri2281. [DOI] [PubMed] [Google Scholar]
- 10.Saribasak H, Rajagopal D, Maul RW, Gearhart PJ. Hijacked DNA repair proteins and unchained DNA polymerases. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:605–611. doi: 10.1098/rstb.2008.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson M, Hsu E, Marcuz A, Courtet M, Du Pasquier L, Steinberg C. What limits affinity maturation of antibodies in Xenopus - the rate of somatic mutation or the ability to select mutants? EMBO J. 1992;11:4337–4347. doi: 10.1002/j.1460-2075.1992.tb05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao Z, Ray M, Jiang C, Clark AB, Rogozin IB, Diaz M. Known components of the immunoglobulin A:T mutational machinery are intact in Burkitt lymphoma cell lines with G:C bias. Mol. Immunol. 2007;44:2659–2666. doi: 10.1016/j.molimm.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diaz M, Velez J, Singh M, Cerny J, Flajnik MF. Mutational pattern of the nurse shark antigen receptor gene (NAR) is similar to that of mammalian Ig genes and to spontaneous mutations in evolution: the translesion synthesis model of somatic hypermutation. Int. Immunol. 1999;11:825–833. doi: 10.1093/intimm/11.5.825. [DOI] [PubMed] [Google Scholar]
- 14.Lee SS, Tranchina DS, Ohta Y, Flajnik MF, Hsu E. Hypermutation in shark immunoglobulin light chain genes results in contiguous substitutions. Immunity. 2002;16:571. doi: 10.1016/s1074-7613(02)00300-x. [DOI] [PubMed] [Google Scholar]
- 15.Hackett J, Jr., Rogerson BJ, O’Brien RL, Storb U. Analysis of somatic mutations in κ transgenes. J. Exp. Med. 1990;172:131–137. doi: 10.1084/jem.172.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betz AG, Rada C, Pannell R, Milstein C, Neuberger MS. Passenger transgenes reveal intrinsic specificity of the antibody hypermutation mechanism: clustering, polarity, and specific hot spots. Proc. Natl. Acad. Sci. USA. 1993;90:2385–2388. doi: 10.1073/pnas.90.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dörner T, Brezinschek H-P, Brezinschek RI, Forster SJ, Domiati-Saad R, Lipsky PE. Analysis of the frequency and pattern of somatic mutations within nonproductively rearranged human variable heavy chain genes. J. Immunol. 1997;158:2779–2789. [PubMed] [Google Scholar]
- 18.Foster SJ, Dörner T, Lipsky PE. Targeting and subsequent selection of somatic hypermutations in the human Vκ repertoire. Eur. J. Immunol. 1999;29:3122–3132. doi: 10.1002/(SICI)1521-4141(199910)29:10<3122::AID-IMMU3122>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 19.Monson NL, Dörner T, Lipsky PE. Targeting and selection of mutations in human Vλ rearrangements. Eur. J. Immunol. 2000;30:1597–1605. doi: 10.1002/1521-4141(200006)30:6<1597::AID-IMMU1597>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Hsu E, Pulham N, Rumfelt L, Flajnik MF. The plasticity of immunoglobulin gene systems in evolution. Immunol. Rev. 2006;210:8–26. doi: 10.1111/j.0105-2896.2006.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee V, Huang JL, Lui MF, Malecek K, Ohta Y, Mooers A, Hsu E. The evolution of multiple isotypic IgM heavy chains in the shark. J. Immunol. 2008;180:7461–7470. doi: 10.4049/jimmunol.180.11.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fleurant M, Changchien L, Chen CT, Flajnik MF, Hsu E. Shark immunoglobulin light chain junctions are as diverse as in heavy chains. J. Immunol. 2004;173:5574–5582. doi: 10.4049/jimmunol.173.9.5574. [DOI] [PubMed] [Google Scholar]
- 23.Malecek K, Brandman J, Brodsky JE, Ohta Y, Flajnik MF, Hsu E. Somatic hypermutation and junctional diversification at immunoglobulin heavy chain loci in the nurse shark. J. Immunol. 2005;175:8105–8115. doi: 10.4049/jimmunol.175.12.8105. [DOI] [PubMed] [Google Scholar]
- 24.Malecek K, Lee V, Feng W, Huang JL, Flajnik MF, Ohta Y, Hsu E. Immunoglobulin heavy chain exclusion in the shark. PLoS Biol. 2008;6:e157. doi: 10.1371/journal.pbio.0060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Argon Y, Burrone OR, Milstein C. Molecular characterization of a nonsecreting myeloma mutant. Eur. J. Immunol. 1983;13:301–305. doi: 10.1002/eji.1830130406. [DOI] [PubMed] [Google Scholar]
- 26.Rogozin IB, Kolchanov NA. Somatic hypermutation in immunoglobulin genes. II. Influence of neighboring base sequences on mutagenesis. Biochim. Biophys. Acta. 1992;1171:11–18. doi: 10.1016/0167-4781(92)90134-l. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda T, Bebenek K, Masutani C, Rogozin IB, Hanaoka F, Kunkel TA. Error rate and specificity of human and murine DNA Polymerase η. J. Mol. Biol. 2001;312:335–46. doi: 10.1006/jmbi.2001.4937. [DOI] [PubMed] [Google Scholar]
- 28.Lebecque SG, Gearhart PJ. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5′ boundary is near the promoter, and 3′ boundary is approximately 1 kb from V(D)J gene. J. Exp. Med. 1990;172:1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jäger U, Böcskör S, Le T, Mitterbauer G, Bolz I, Chott A, Kneba M, Mannhalter C, Nadel B. Follicular lymphomas’ BCL-2/IgH junctions contain templated nucleotide insertions: novel insights into the mechanism of t(14;18) translocation. Blood. 2000;95:3520–3529. [PubMed] [Google Scholar]
- 30.Marculescu R, Vanura K, Montpellier B, Roulland S, Le T, Navarro JM, Jager U, McBlane F, Nadel B. Recombinase, chromosomal translocations and lymphoid neoplasia: targeting mistakes and repair failures. DNA Repair. 2006;33:342–344. doi: 10.1016/j.dnarep.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Domínguez O, Ruiz JF, Laín de Lera T, García-Díaz M, González MA, Kirchhoff T, Martínez-A C, Bernad A, Blanco L. DNA polymerase mu (Pol μ), homologous to TdT, could act as DNA mutator in eukaryotic cells. EMBO J. 2000;19:1731–1742. doi: 10.1093/emboj/19.7.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Wu X, Yuan F, Xie Z, Wang Z. Highly frequent frameshift DNA synthesis by human DNA polymerase μ. Mol. Cell. Biol. 2001;21:7995–8006. doi: 10.1128/MCB.21.23.7995-8006.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tippin B, Kobayashi S, Bertram JG, Goodman MF. To slip or skip, visualizing frameshift mutation dynamics for error-prone DNA polymerases. J. Biol. Chem. 2004;279:45360–45368. doi: 10.1074/jbc.M408600200. [DOI] [PubMed] [Google Scholar]
- 34.Kunkel TA, Soni A. Mutagenesis by transient misalignment. J. Biol. Chem. 1988;263:14784–14789. [PubMed] [Google Scholar]
- 35.Garcia-Diaz M, Kunkel TA. Mechanism of a genetic glissando: structural biology of indel mutations. Trends Biochem. Sci. 2006;31:206–214. doi: 10.1016/j.tibs.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Hinds KR, Litman GW. Major reorganization of immunoglobulin VH segmental elements during vertebrate evolution. Nature. 1986;320:546–549. doi: 10.1038/320546a0. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg AS, Avila D, Hughes M, Hughes A, McKinney EC, Flajnik MF. A new antigen receptor gene family that undergoes rearrangement and extensive somatic diversification in sharks. Nature. 1995;374:168–173. doi: 10.1038/374168a0. [DOI] [PubMed] [Google Scholar]
- 38.Dörner T, Foster SJ, Brezinschek HP, Lipsky PE. Analysis of the targeting of the hypermutational machinery and the impact of subsequent selection on the distribution of nucleotide changes in human VHDJH rearrangements. Immunol. Rev. 1998;162:161–171. doi: 10.1111/j.1600-065x.1998.tb01439.x. [DOI] [PubMed] [Google Scholar]
- 39.Zapata A, Amemiya CT. Phylogeny of lower vertebrates and their immunological structures. Curr. Top. Microbiol. Immunol. 2000;248:67–107. doi: 10.1007/978-3-642-59674-2_5. [DOI] [PubMed] [Google Scholar]
- 40.Dooley H, Flajnik MF. Shark immunity bites back: affinity maturation and memory response in the nurse shark, Ginglymostoma cirratum. Eur. J. Immunol. 2005;35:935–945. doi: 10.1002/eji.200425760. [DOI] [PubMed] [Google Scholar]
- 41.Hsu E. Mutation, selection, and memory in B lymphocytes of exothermic vertebrates. Immunol. Rev. 1998;162:25–36. doi: 10.1111/j.1600-065x.1998.tb01426.x. [DOI] [PubMed] [Google Scholar]
- 42.Diaz M, Greenberg AS, Flajnik MF. Somatic hypermutation of the new antigen receptor (NAR) in the nurse shark does not generate the repertoire: possible role in antigen-driven reactions in the absence of germinal centers. Proc. Natl. Acad. Sci. USA. 1998;95:4343–14348. doi: 10.1073/pnas.95.24.14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dooley H, Stanfield RL, Brady RA, Flajnik MF. First molecular and biochemical analysis of in vivo affinity maturation in an ectothermic vertebrate. Proc. Natl. Acad. Sci. USA. 2006;103:1846–1851. doi: 10.1073/pnas.0508341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longo NS, Lipsky PE. Why do B cells mutate their immunoglobulin receptors? Trends Immunol. 2006;27:374–380. doi: 10.1016/j.it.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Rada C, Yélamos J, Dean W, Milstein C. The 5′ hypermutation boundary of χ chains is independent of local and neighbouring sequences and related to the distance from the initiation of transcription. Eur. J. Immunol. 1997;27:3115–3120. doi: 10.1002/eji.1830271206. [DOI] [PubMed] [Google Scholar]
- 46.Longerich S, Tanaka A, Bozek G, Nicolae D, Storb U. The very 5′ end and the constant region of Ig genes are spared from somatic mutation because AID does not access these regions. J. Exp. Med. 2005;202:1443–54. doi: 10.1084/jem.20051604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peters A, Storb U. Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity. 1996;4:57–65. doi: 10.1016/s1074-7613(00)80298-8. [DOI] [PubMed] [Google Scholar]
- 48.Winter DB, Gearhart PJ. Dual enigma of somatic hypermutation of immunoglobulin variable genes: targeting and mechanism. Immunol. Rev. 1998;162:89–96. doi: 10.1111/j.1600-065x.1998.tb01432.x. [DOI] [PubMed] [Google Scholar]
- 49.Rada C, Milstein C. The intrinsic hypermutability of antibody heavy and light chain genes decays exponentially. EMBO J. 2001;20:4570–4576. doi: 10.1093/emboj/20.16.4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xue K, Rada C, Neuberger MS. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2-/-ung-/- mice. J. Exp. Med. 2006;203:2085–2094. doi: 10.1084/jem.20061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rajagopal D, Maul RW, Ghosh A, Chakraborty T, Khamlichi AA, Sen R, Gearhart PJ. Immunoglobulin switch μ sequence causes RNA polymerase II accumulation and reduces dA hypermutation. J. Exp. Med. 2009;206:1237–1244. doi: 10.1084/jem.20082514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Besmer E, Market E, Papavasiliou FN. The transcription elongation complex directs activation-induced cytidine deaminase-mediated DNA deamination. Mol. Cell Biol. 2006;26:4378–4385. doi: 10.1128/MCB.02375-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goosens T, Klein U, Küppers R. Frequent occurrence of deletions and duplications during somatic hypermutations: implications for oncogene translocations and heavy chain disease. Proc. Natl. Acad. Sci. USA. 1998;95:2463–2468. doi: 10.1073/pnas.95.5.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sale JE, Neuberger MS. TdT-accessible breaks are scattered over the immunoglobulin V domain in a constitutively hypermutating B cell line. Immunity. 1998;9:859–869. doi: 10.1016/s1074-7613(00)80651-2. [DOI] [PubMed] [Google Scholar]
- 55.Wilson PC, de Bouteiller O, Liu YJ, Potter K, Banchereau J, Capra JD, Pascual V. Somatic hypermutation introduces insertions and deletions into immunoglobulin V genes. J. Exp. Med. 1998;187:59–70. doi: 10.1084/jem.187.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bentolila LA, Wu GE, Nourrit F, Fanton d’Andon M, Rougeon F, Doyen N. Constitutive expression of terminal deoxynucleotidyl transferase in transgenic mice is sufficient for N region diversity to occur at any Ig locus throughout B cell differentiation. J. Immunol. 1997;158:715. [PubMed] [Google Scholar]
- 57.Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/s1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- 58.Beetz S, Diekhoff D, Steiner LA. Characterization of terminal deoxynucleotidyl transferase and polymerase mu in zebrafish. Immunogenet. 2007;59:735–744. doi: 10.1007/s00251-007-0241-7. [DOI] [PubMed] [Google Scholar]
- 59.Aoufouchi S, Flatter E, Dahan A, Failli A, Bertocci B, Storck S, Delbos F, Cocea L, Gupta N, Weill J-C, Reynaud C-A. Two novel human and mouse DNA polymerases of the polX family. Nuc. Acids Res. 2000;28:3684–3693. doi: 10.1093/nar/28.18.3684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nick McElhinny SA, Havener JM, Garcia-Diaz M, Juárez R, Bebenek K, Kee BL, Blanco L, Kunkel TA, Ramsden DA. A gradient of template dependence defines distinct biological roles for family × polymerases in nonhomologous end joining. Mol. Cell. 2005;19:357–366. doi: 10.1016/j.molcel.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 61.Ruiz JF, Lucas D, García-Palomero E, Saez AI, González MA, Piris MA, Bernad A, Blanco L. Overexpression of human DNA polymerase μ (Pol μ) in a Burkitt’s lymphoma cell line affects the somatic hypermutation rate. Nucleic Acids Res. 2004;32:5861–5873. doi: 10.1093/nar/gkh929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bertocci B, De Smet A, Flatter E, Dahan A, Bories JC, Landreau C, Weill JC, Reynaud CA. Cutting edge: DNA polymerases mu and lambda are dispensable for Ig gene hypermutation. J. Immunol. 2002;168:3702–3706. doi: 10.4049/jimmunol.168.8.3702. [DOI] [PubMed] [Google Scholar]
- 63.Unniraman S, Schatz DG. Strand-based spreading of mutations during somatic hypermutation. Science. 2007;317:1227–1230. doi: 10.1126/science.1145065. [DOI] [PubMed] [Google Scholar]
- 64.Masuda K, Ouchida R, Hikida M, Kurosaki T, Yokoi M, Masutani C, Seki M, Wood RD, Hanaoka F, O-Wang J. DNA Polymerases η and θ function in the same genetic pathway to generate mutations at A/T during somatic hypermutation of Ig genes. J. Biol. Chem. 2007;282:13787–13794. doi: 10.1074/jbc.M611849200. [DOI] [PubMed] [Google Scholar]
- 65.Prakash S, Prakash L. Translesion DNA synthesis in eukaryotes: A one- or two-polymerase affair. Genes Dev. 2002;16:1872–1883. doi: 10.1101/gad.1009802. [DOI] [PubMed] [Google Scholar]
- 66.Carlson KD, Johnson RE, Prakash L, Prakash S, Washington MT. Human DNA polymerase κ forms nonproductive complexes with matched primer termini but not with mismatched primer termini. Proc. Natl. Acad. Sci. USA. 2006;103:15776–15781. doi: 10.1073/pnas.0605785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kunkel TA. DNA replication fidelity. J. Biol. Chem. 2004;279:16895–16898. doi: 10.1074/jbc.R400006200. [DOI] [PubMed] [Google Scholar]
- 68.Thompson CB. New insights into V(D)J recombination and its role in the evolution of the immune system. Immunity. 1995;3:531–539. doi: 10.1016/1074-7613(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 69.Ratcliffe MJH. Antibodies, immunoglobulin genes and the bursa of Fabricius in chicken B cell development. Dev. Comp. Immunol. 2006;30:101–118. doi: 10.1016/j.dci.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 70.Nakahara M, Sonoda E, Nojima K, Sale JE, Takenaka K, Kikuchi K, Taniguchi Y, Nakamura K, Sumimoto Y, Bree RT, Lowndes NF, Takeda S. Genetic evidence for single-strand lesions initiating Nbs1-dependent homologous recombination in diversification of Ig V in chicken B lymphocytes. PloS Genet. 2009;5:e1000356. doi: 10.1371/journal.pgen.1000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ordinario EC, Yabuki M, Larson RP, Maizels N. Temporal regulation of Ig gene diversification revealed by single-cell imaging. J. Immunol. 2009;183:4545–4553. doi: 10.4049/jimmunol.0900673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendelman L, Petruska J, Goodman MF. Base mispair extension kinetics. Comparison of DNA polymerase α and reverse transcriptase. J. Biol. Chem. 1990;265:2338–2346. [PubMed] [Google Scholar]
- 73.Zhong X, Garg P, Stith CM, McElhinny SAN, Kissling KGE, Burgers PMJ, Kunkel TA. The fidelity of DNA synthesis by yeast DNA polymerase zeta alone and with accessory proteins. Nucleic Acids Res. 2006;34:4731–4742. doi: 10.1093/nar/gkl465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zeng X, Winter DB, Kasmer C, Kraemer KH, Lehmann AR, Gearhart PJ. DNA polymerase eta is an A-T mutator in somatic hypermutation of immunoglobulin variable genes. Nat. Immunol. 2001;2:537–541. doi: 10.1038/88740. [DOI] [PubMed] [Google Scholar]
- 75.Delbos F, Aoufouchi S, Faili A, Weill JC, Reynaud CA. DNA polymerase eta is the sole contributor of A/T modifications during immunoglobulin gene hypermutation in the mouse. J. Exp. Med. 2007;204:17–23. doi: 10.1084/jem.20062131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Winter DB, Phung QH, Umar A, Baker SM, Tarone RE, Tanaka K, Liskay RM, Kunkel TA, Bohr VA, Gearhart PJ. Altered spectra of hypermutation in antibodies from mice deficient for the DNA mismatch repair protein PMS2. Proc. Natl. Acad. Sci. USA. 1998;95:6953–6958. doi: 10.1073/pnas.95.12.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.