Introduction
Knee osteoarthritis (OA) is the most common joint disorder among adults in the US and severity of knee radiographic OA (ROA) is strongly associated with knee pain1. Pain from knee OA accounts for a large proportion of limitations with common activities of daily living2, and it is also the main reason for total knee replacement3. Because of the impact of symptoms from knee OA, people with symptomatic knee OA may be more motivated to take steps to prevent disease progression than those without ROA. Thus, investigators and persons with knee OA are particularly interested in risk factors that are associated with ROA progression because such knowledge will provide insightful guidance for secondary prevention4.
Over the past few decades, many observational studies have examined risk factors for the occurrence (i.e., incident) and worsening (i.e., progressive) of knee OA. Several risk factors (i.e., female gender, obesity, high bone mineral density (BMD), joint injury, repetitive occupational stress on joints, and certain sports) have been found to be strongly associated with an increased risk for incident knee ROA5, 6. In contrast, findings on risk factors for ROA progression have been inconclusive. Except for the level of serum hyaluronic acid and generalized OA, no other risk factor has been consistently associated with the risk of ROA progression7. Interestingly, some risk factors (e.g., high BMD, low vitamin C) increase the risk of incident knee ROA but are not associated with, or even decrease, the risk of ROA progression8-11.
This paradoxical phenomenon is not limited to studies of ROA. Many studies have shown that overweight and obesity increase the risk of cardiovascular disease; however, a number of studies have also reported that among subjects with preexisting cardiovascular disease, those who are overweight or moderately obese have an improved survival and lower risk of major cardiovascular events compared with subjects with normal weight12. These findings have been termed the “obesity paradox”. Similarly, while low body mass index (BMI) is associated with increased incident chronic obstructive pulmonary disease13, it does not, however, increase the risk of recurrent exacerbations of the disease14. While the risk factors for incident events may be biologically different from those for secondary events, there are also compelling methodological explanations behind such discrepancies.
In this paper, we provide several explanations that may underlie the discrepancy between findings for knee ROA progression and those for knee ROA incidence. To be consistent with most studies, we consider knees eligible for studies of ROA progression being those that have preexisting mild (K/L=2) or moderate (K/L=3) ROA at baseline. We discuss how study design, study implementation, and outcome measures in studies of ROA progression can potentially bias the effect estimates of risk factors of interest using causal diagrams15. We also present findings from several large prospective studies of ROA progression to facilitate our explanations wherever applicable.
Randomized Controlled Trials to Assess the Relation of a Risk Factor to ROA Progression
We begin with the premise that an ideal design to evaluate the effect of a risk factor on progression of knee ROA is a randomized clinical trial. Specifically, to test whether an intervention (e.g., a drug or weight loss) may decrease the risk of ROA progression, subjects who have mild or moderate knee ROA are randomly allocated into appropriate intervention groups. At the end of follow-up, subjects’ knee radiographs would be scored blinded to the intervention assigned. The effect of the intervention on the risk of ROA progression would be estimated by comparing the treatment groups.
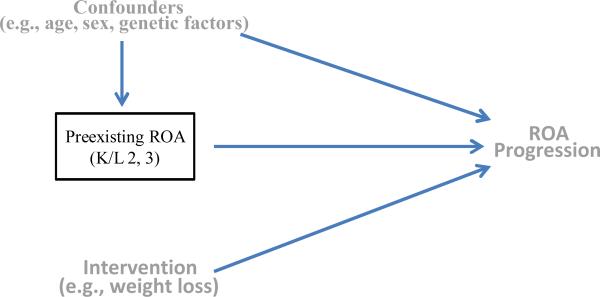
Such a trial can be illustrated using a causal diagram that consists of knees that have preexisting ROA at baseline (i.e., study sample), the intervention (i.e., exposure), a set of covariates (i.e., potential confounders), and ROA progression (i.e., outcome) (Figure 1). A causal diagram consists of a set of relevant variables (exposure, potential confounders, and outcomes) and arrows to indicate the flow of causation. An arrow placed with its base at a variable, say exposure (e.g., intervention), and its head at another variable, say outcome (e.g., ROA progression) indicates that the exposure causes the outcome. In this causal diagram, the fact that the study sample is restricted to (i.e., conditioned on) knees that have mild or moderate ROA at baseline is denoted by a box around “preexisting ROA”. Because the intervention in the trial is randomly assigned to the study participants, the exposure is associated with neither potential confounders (either known or unknown) nor preexisting ROA as indicated by the absence of arrows directed towards the exposure. Under such a scenario one can obtain an unbiased effect estimate of the intervention on ROA progression using appropriate statistical methods.
Figure 1.
A causal diagram of a randomized trial showing assessment of the effect of an intervention regimen (e.g., weight loss) on the risk of ROA progression. Because the intervention regimen in this trial is randomly assigned to the study participants, the intervention regimen is not influenced by either potential confounders (e.g., age, sex, genetic factors, etc.) or previous ROA status, as indicated by the absence of arrows or causal paths directed towards the intervention regimen.
Bias Introduced by Conditioning on Preexisting Knee ROA in Observational Studies
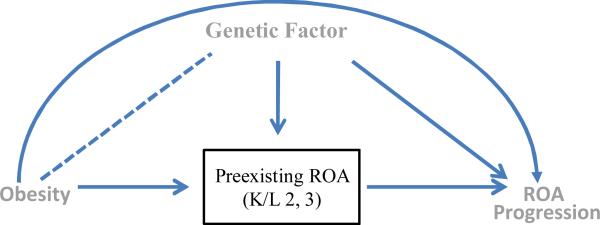
In contrast, observational studies of ROA progression can produce biased estimates of the effect of an exposure on the outcome given the very fact that the study sample is restricted to the knees that have preexisting mild or moderate knee ROA. To illustrate this, we go back to a causal diagram, this time to depict the relationship of a risk factor (e.g., obesity) to knee ROA progression in a hypothetical observational study (Figure 2). To simplify our discussion throughout this paper we consider only one potential confounder (although this can be readily extended into more complex scenarios with more potential confounders), in this case a genetic factor that increases the risk of both incident and progressive ROA. Note that the genetic factor is not associated with obesity before knees develop ROA. Thus it would not be a confounder in a study of obesity and incident ROA.
Figure 2.
A causal diagram of an observational study showing the assessment of the effect of obesity on ROA progression among knees with preexisting ROA at baseline. Conditioning on baseline ROA results in its causes (i.e., obesity and the genetic factor) becoming directly associated, as indicated by a dotted line between obesity and genetic factors, even though these two factors are not associated before the knees developed ROA. Such conditioning opens an alternative path from obesity to ROA progression (i.e., obesity --- genetic factor → ROA progression), thus biasing the effect of obesity on ROA progression.
As shown in Figure 2, knees that have preexisting ROA at baseline developed ROA because of either obesity or the genetic factor; thus preexisting ROA at baseline is a “common effect” of obesity and the genetic factor (i.e., obesity → preexisting ROA ← genetic factor). The fact that there is no association between the obesity and genetic factor before knees develop mild or moderate ROA is indicated by the lack of an arrow between obesity and the genetic factor. The only paths between these two variables are blocked by colliding arrow heads at their common effects nodes, i.e., preexisting ROA (i.e., obesity → preexisting ROA ← genetic factor) or ROA progression (i.e., obesity → ROA progression ← genetic factor).
However, as a result of conditioning on a common effect, in this case preexisting ROA at baseline, obesity and the genetic factor are no longer independent. Such conditioning opens an alternative non-causal path between obesity and the genetic factor (i.e., obesity --- genetic factor → ROA progression). This alternative path biases the effect estimate of obesity on ROA progression, unless the effect of the genetic factor is appropriately adjusted for. However, in many instances, not all confounders are measured or are known, therefore leading to confounded effect estimates.
Here we provide an intuitive example to illustrate the logic behind potential spurious associations created by conditioning on a common effect16. Suppose one has two fair coins and one bell. The bell rings whenever either coin comes up heads on a toss of the two coins. Thus, the bell ringing is a “common effect“ of heads appearing on the toss of either coin A or coin B (i.e., coin A → Bell ringing ← coin B). Obviously, heads appearing as a result of one coin toss is independent of heads appearing as a result of the other coin toss; thus the correlation coefficient between heads appearing from coin A and from coin B equals 0. However, suppose that we only examined the relationship between heads appearing with the two coin tosses in those instances where the bell did ring (analogous to only examining the effect of obesity on ROA progression in people with preexisting ROA). By conditioning on the status of the bell ringing (i.e., conditioning on a common effect), heads appearing from coin A and heads appearing from coin B are no longer independent. For example, if the bell rings and coin A came up tails, then that must mean that coin B came up heads (and vice versa if coin B came up tails). As a consequence, conditioning on the status of the bell ringing induces a negative correlation between heads appearing from coin A and from coin B.
As we assumed that there are only two causal factors (e.g., obesity and the genetic factor) for development of mild or moderate ROA in our scenario, some preexisting ROA at baseline was caused by obesity, and others, by the genetic factor. While the genetic factor is not associated with obesity before knees developed ROA, among knees that have ROA at baseline, obesity and the genetic factor are no longer independent. This is because if the cause of preexisting ROA is not obesity, it must then be the genetic factor, or vice versa. Subsequently, when evaluating the relation of obesity to the risk of ROA progression, such a negative correlation between obesity and the genetic factor would bias the effect of obesity on ROA progression downward unless the genetic factor is appropriately controlled for.
Ideally, to assess the effect of obesity on the risk of ROA progression one should compare the risk of ROA progression among persons with obesity with those without obesity, with all else being equal. However, in an observational study persons with baseline knee ROA but are not obese must have been exposed to other risk factors for ROA. Thus, the two groups (i.e., obese vs. non-obese) are not comparable in terms of distribution of potential confounders. Such a study can almost be considered as a study that compares the risk of ROA progression among persons who are exposed to obesity with those who are exposed to the genetic as well as other risk factors for ROA. Of course, one should acknowledge that some of the knees with preexisting ROA at baseline may have been exposed to obesity, the genetic factor, and other risk factors. Nonetheless, conditioning on preexisting ROA at baseline would tend to bias the effect of obesity on progression towards the null unless the analysis adjusts for genetic and other risk factors. Unfortunately, not all factors are always known or measured, and therefore cannot be adjusted for, leading to bias.
Using data from the Multicenter Osteoarthritis (MOST) Study, we explored this possibility when evaluating the effect of obesity as an example of a chronic risk factor, on progression of knee ROA to illustrate this issue. Among knees eligible for progressive knee ROA all known risk factors for ROA (e.g., female gender, knee injury, high BMD, and knee malalignment) were more prevalent among persons who were obese than those not obese. Since obesity is a strong risk factor for knee ROA at baseline, as are many of the other factors examined, one would speculate that there must be other risk factors, not yet identified, that contribute to ROA development in non-obese people at baseline. If these unknown risk factors are also associated with ROA progression, it would bias the effect of obesity towards the null.
Bias Introduced by Loss to Follow-up in Observational Studies
For logistical reasons, few large-scale observational studies of ROA progression have been able to obtain knee radiographs at multiple time points from each participant within a relatively short period of time. For example, the average follow-up time between repeated knee radiographs was 9 years in the Framingham Osteoarthritis Study, 6 years in the Rotterdam Study, 4 years in the Chingford Study, and 2.5 years in the MOST Study. When the follow-up time is long, a substantial proportion of subjects may be lost to follow-up, leading to potential selection bias. Indeed, the rate of loss to follow-up is high in studies of ROA. Of 1,473 subjects in the Framingham OA Study who have knee radiographs taken at baseline, 40% of them did not have knee radiographs at the follow-up visit17. A similar proportion of loss to follow-up (40%) was also reported in a study in England18.
Loss to follow-up in observational studies could bias the effect estimate of the risk factor of interest. As shown in Figure 3, the status of complete follow-up is a common effect of ROA progression and obesity (i.e., obesity → complete follow-up ← ROA progression). For example, more obese subjects are less likely to complete follow-up due to poor health and subjects whose ROA gets worse are less likely to return for the last study visit due to loss of mobility. Because investigators are only able to examine the relation of obesity to the risk of ROA progression among those who have follow-up knee radiographs, conditioning on a common effect (i.e., complete follow-up), as denoted by a box around “complete follow-up” in Figure 3, creates a spurious association between its causes, denoted by a dotted line between obesity and ROA progression, leading to a biased effect estimate. In this example, as in many instances in real-life, ROA progression status is unknown among subjects who are lost to follow-up; thus, the magnitude and direction of bias due to loss to follow-up is difficult to predict. One approach often used when loss to follow-up does occur is to compare the baseline characteristics between subjects who complete the follow-up with those who are lost to follow-up. However, such similarities do not guard against selection bias since reasons for loss to follow-up may not be the same between the exposed and unexposed groups and therefore could still lead to selection bias.
Figure 3.
A causal diagram of an observational study showing the assessment of the effect of obesity on ROA progression among knees that have preexisting ROA at baseline and also have follow-up knee radiographs. Conditioning on a common effect, i.e., complete follow-up status (obesity → complete follow-up ← ROA progression) results in its causes (i.e., obesity and ROA progression) becoming directly associated, and opens an alternative path from obesity to ROA progression (i.e., obesity --- ROA progression). Thus, even if there is no association between obesity and ROA progression, conditioning on complete follow-up status tends to introduce a negative association between obesity and ROA progression.
In the MOST study, we found that the proportion of loss to follow-up was higher among subjects whose BMI was ≥ 30 kg/m2 (15.6%) than those whose BMI was < 25 kg/m2 (11.3%), suggesting a positive association between obesity and proportion of loss to follow-up (as denoted by the path between obesity and complete follow-up in Figure 3). It is reasonable to assume that subjects who have ROA progression, i.e., developing more severe or end-stage ROA, have a higher proportion of loss to follow-up than those who did not have ROA progression (as shown by the path between ROA progression and complete follow-up in Figure 3). Thus, selection bias due to conditioning on those who have complete follow-up data on the outcome will introduce a spurious association between its causes (i.e., obesity --- ROA progression), which can dilute the effect of obesity.
Bias due to Measurement of Outcome and Ceiling Effect
One of the main motivations for studying risk factors for ROA progression is that the effect of a specific risk factor(s) on incident ROA may differ from that on progressive disease. If that is the case, it is also reasonable to speculate that the effect of a specific risk factor(s) on the risk of progression from mild ROA to either moderate or severe ROA may differ from that on progression from moderate to severe disease. However, the current definition of ROA progression assumes that the effect of a specific risk factor is the same regardless of the severity of ROA at the baseline which runs counter to the initial intention of evaluating whether risk factors have different effects depending on stage of disease. If the effects of risk factors on ROA progression vary with stage of ROA severity, mixing data from various transitions of ROA severity may mask the true causal association. In reality, there must be a continuum of radiographic changes in ROA, and any threshold, including the current widely used one, is inherently arbitrary18.
Radiographs for such studies are typically acquired at fixed time intervals. In most cases, the time interval between two consecutive radiographs is relatively long. Knees that have the same severity of ROA, indicated by either K/L grade or joint space narrowing (JSN) score, on the follow-up radiographs are treated the same regardless of how rapidly they actually reached that stage. While it is difficult to measure severity of ROA with frequent short time intervals to capture the exact date of change in severity of ROA, long intervals between two consecutive assessments of severity of ROA have substantial drawbacks.
Assume that knee radiographs in a study are acquired every 24 months. ROA in one knee progressed from mild to moderate disease at 16 months and in another at 22 months. Neither of them progressed further by 24 months. At the 24-month follow-up radiograph both knees would be considered as having experienced ROA progression. While one knee progressed more rapidly than the other, this information is not captured owing to the relatively long interval between two consecutive assessments. Furthermore, some measures used to assess ROA progression, such as semi-quantitative joint-space narrowing (JSN) score or K/L grade, cannot accurately estimate the rate of progression when the disease has reached its end stage according to that scale (i.e., JSN=3 or K/L grade=4), reflecting a ceiling effect in these scoring systems. Under such circumstances, even if we can obtain the risk of ROA progression and estimate the risk ratio, that effect estimate will be smaller than the rate ratio and will be closer to the null. The degree of underestimation depends on the level of the risk, being slight for small risks and greater for large risks19. This issue can be illustrated with the following example. Assume we recruit 10,000 male smokers and 10,000 male non-smokers at age 50 and follow them for a certain time period. We would expect that smokers experience a higher mortality than non-smokers over a 5-year period. However, if we follow those subjects for 50 years, almost all participants will die by the end of the study; thus the mortality ratio from smoking will be close to 1.
Alternative Approaches to Studying Risk Factors for ROA Worsening
In an ideal epidemiologic study of the association between risk factors and risk of ROA progression, it is preferable to study a risk factor that either occurs at the time of diagnosis of mild or moderate ROA or changes over time. Such a study could be considered as a natural experiment, allowing one to assess whether such a risk factor or its change are associated with the risk of ROA progression. However, most risk factors under study are typically chronic ones (e.g., BMI, nutritional factors) that either are likely to exist long before the diagnosis of mild or moderate ROA or do not change substantially over a short period of time unless under special circumstances, for example, studying the effect of weight loss on risk of knee OA progression among persons who underwent a surgical operation to induce weight loss.
Alternatively, observational studies should ideally be conducted using the measures for ROA progression that are continuous or interval-scaled, collecting and controlling for as many potential confounders as possible; assembling a large sample of knees with mild ROA (when cartilage loss in most knees has not occurred yet) and having knee imaging obtained more frequently within a short period of time; and minimizing loss to follow-up. However, these alternatives will not allow investigators to eliminate the bias induced by conditioning on preexisting ROA in observational studies when the risk factor under study is a cause of ROA at the baseline. While a randomized clinical trial is an ideal study design that would avoid the bias due to conditioning on preexisting ROA, logistical challenges, ethical issues, and potential of loss to follow-up limit its utility for studies of risk factors for ROA progression. Furthermore, the findings from a randomized clinical trial may not be generalizable to the general population if some characteristic(s) differ between participants in the trial and target population and such characteristic(s) would potentially modify the effect of the factor of interest.
Given the limitations of not having the ideal study parameters and capabilities available at present, an alternative approach is needed to assess the effect of a specific risk factor on the risk of severity of ROA, in order to minimize the potential biases that we have discussed above. We explored using such an approach to assess the effect of BMI on the risk of OA worsening. Specifically, among persons without ROA (K/L=0 or 1) at baseline, we evaluated the relation of BMI to incident mild (K/L=2) and incident moderate/severe ROA (K/L=3 or 4), respectively. Of 3,313 knees without OA at baseline in the MOST Study, 116 (3.5%) knees developed mild ROA, and 96 (2.9%) knees developed moderate/severe disease over the 30-month follow-up period. As shown in Table 1, higher BMI increased the risk of both mild and moderate to severe ROA. Compared with those with BMI < 25kg/m2, subjects with BMI ≥ 35kg/m2 had 3-fold increased risk for mild ROA and 4-fold increased risk for moderate/severe ROA. These findings clearly suggest that obesity not only increases the risk of developing knee ROA, but is also associated with more rapid worsening of ROA once it has developed. However, using the same data, BMI was not associated with an increased risk of ROA progression when the study sample was restricted to those with ROA at baseline20.
Table 1.
BMI and Risk of Mild and Moderate/Severe ROA
| BMI (kg/m2) | # Knees | Mild ROA (K/L=2) | Moderate/severe ROA (K/L=3, 4) | ||
|---|---|---|---|---|---|
| Risk (%) | RR (95% CI) | Risk (%) | RR (95% CI) | ||
| <25 | 624 | 1.8 | 1.0 | 1.1 | 1.0 |
| 25-29 | 1340 | 2.9 | 1.7 (0.9-3.3) | 2.5 | 2.3 (0.9-5.8) |
| 30-34 | 912 | 4.2 | 2.4 (1.2-4.7) | 3.0 | 3.0 (1.0-6.9) |
| 35+ | 429 | 6.3 | 3.1 (1.5-6.4) | 5.6 | 4.1 (1.5-11.1) |
| P for trend | < 0.004 | <0.001 | |||
This approach has some advantages. The risk factor is not a consequence of the disease process; thus, reversal causality is unlikely. While unmeasured confounding is still an issue, it should have a much weaker effect compared with the usual approach of studying risk of ROA progression. However, there are drawbacks as well. First, while the study would answer the question whether obesity increases the risk of mild, moderate and severe ROA among knees without preexisting disease, it does not directly test the hypothesis of whether obesity accelerates ROA progression among the knees with pre-existing disease. Second, the risk of moderate/ severe ROA among those without disease at baseline, especially severe disease, may not be high. Thus, unless the sample size is large enough or the follow-up time is long, the study may not have enough power to examine the relation of a risk factor to the risk of moderate/ severe disease.
Conclusions
While risk factors for progressive ROA may be different from that for incident ROA because of different underlying pathophysiology for various stages of ROA, there are other compelling explanations that may underlie the discrepancy between study findings for ROA progression and those for ROA incidence. We contend that using an observational study design to assess risk factors for ROA progression in joints with existing disease is subject to various biases that may account for such null findings. These biases are likely to be important threats to the validity of any observational study of risk factors for ROA progression and will make it formidably challenging to study these risk factors outside of a randomized trial. We hypothesize that risk factors may actually exist for progressive ROA, but that flaws in study design and our current measure for ROA progression may have prevented us from identifying them.
Acknowledgments
Supported by NIH AR47785
References
- 1.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. Br Med J. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DeFrances CJ, Podgornik MN. 2004 National Hospital Discharge Survey. Adv Data. 2006:1–19. [PubMed] [Google Scholar]
- 4.Felson DT. Chondroitin for pain in osteoarthritis. Ann Intern Med. 2007;146(8):611–2. doi: 10.7326/0003-4819-146-8-200704170-00014. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y, Jordan JM. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2008;34:515–29. doi: 10.1016/j.rdc.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med. 2000;133:635–46. doi: 10.7326/0003-4819-133-8-200010170-00016. [DOI] [PubMed] [Google Scholar]
- 7.Belo JN, Berger MY, Reijman M, Koes BW, Bierma-Zeinstra SM. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 2007;57:13–26. doi: 10.1002/art.22475. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Hannan MT, Chaisson CE, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27:1032–7. [PubMed] [Google Scholar]
- 9.Hart DJ, Cronin C, Daniels M, Worthy T, Doyle DV, Spector TD. The relationship of bone density and fracture to incident and progressive radiographic osteoarthritis of the knee: the Chingford Study. Arthritis Rheum. 2002;46:92–9. doi: 10.1002/1529-0131(200201)46:1<92::AID-ART10057>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 10.McAlindon TE, Jacques P, Zhang Y, et al. Do antioxidant micronutrients protect against the development and progression of knee osteoarthritis? Arthritis Rheum. 1996;39:648–56. doi: 10.1002/art.1780390417. [DOI] [PubMed] [Google Scholar]
- 11.Nevitt MC, Zhang Y, Javaid MK, et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: The MOST study. Ann Rheum Dis. 2010;69:163–8. doi: 10.1136/ard.2008.099531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet. 2006;368:666–78. doi: 10.1016/S0140-6736(06)69251-9. [DOI] [PubMed] [Google Scholar]
- 13.Hallin R, Koivisto-Hursti UK, Lindberg E, Janson C. Nutritional status, dietary energy intake and the risk of exacerbations in patients with chronic obstructive pulmonary disease (COPD). Respiratory medicine. 2006;100:561–7. doi: 10.1016/j.rmed.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 14.Tsai CL, Clark S, Cydulka RK, Rowe BH, Camargo CA., Jr Factors associated with hospital admission among emergency department patients with chronic obstructive pulmonary disease exacerbation. Acad Emerg Med. 2007;14:6–14. doi: 10.1197/j.aem.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 15.Glymour MM, Greenland S. Causal Diagrams. In: Rothman KJ, Greenland S, Lash TL, editors. Modern Epidemiology. 3rd ed. Lippicott William & Wilkins; Philadelphia, Baltimore, New York, Buenos Aires, Hong Kong, Sydney, Tokyo: 2008. pp. 183–209. [Google Scholar]
- 16.Pearl J. Causality: Models, Reasoning, and Inference. 2nd ed. Cambridge University Press; Cambridge: 2009. [Google Scholar]
- 17.Felson DT, Zhang Y, Hannan MT, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–33. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 18.Cooper C, Snow S, McAlindon TE, et al. Risk factors for the incidence and progression of radiographic knee osteoarthritis. Arthritis Rheum. 2000;43:995–1000. doi: 10.1002/1529-0131(200005)43:5<995::AID-ANR6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 19.Rothman KJ. Modern Epidemiology. 1st ed. Little, Brown and Company; Boston/Toronto: 1986. Analysis of Crude Data. pp. 154–76. [Google Scholar]
- 20.Niu J, Zhang YQ, Torner J, et al. Is obesity a risk factor for progressive radiographic knee osteoarthritis? Arthritis Rheum. 2009;61:329–35. doi: 10.1002/art.24337. [DOI] [PMC free article] [PubMed] [Google Scholar]