Abstract
Brassica vegetable intake has been associated with decreased risk and well-done meat intake has been associated with increased risk of cancers at multiple organ sites in epidemiologic studies. Experimental studies suggest a role of modulation of phase I and phase II metabolizing enzymes as one mechanism for these associations. Heterocyclic aromatic amines (HAAs) are carcinogens formed in meat that has been cooked to well-done and at high temperatures. Phase I metabolizing enzymes catalyze the activation of HAAs, and phase II metabolizing enzymes serve to detoxify the active carcinogens. The glutathione S-transferases (GSTs) are a family of phase II metabolizing enzymes that are induced by, and act to conjugate, isothiocyanates (ITCs), phytochemicals found in Brassica vegetables. This review summarizes the results of feeding studies in humans that examine effects of polymorphisms in GSTs on ITC metabolite excretion, reviews the evidence for modulation of HAA mutagenicity by ITCs, and discusses the need for feeding studies examining potential interactions among polymorphic genes encoding phase I and phase II metabolizing enzymes, meat intake, and Brassica intake to elucidate their role in cancer etiology.
Keywords: Brassica, heterocyclic aromatic amines, glutathione S-transferase, isothiocyanate
INTRODUCTION
Brassica vegetables are members of the plant family Brassicaceae. Vegetables of the genus Brassica represent virtually all members of this family that are consumed by humans. The genus includes broccoli, cauliflower, cabbage, kale, collard greens, mustard, watercress, and Brussels sprouts, among others. Isothiocyanates (ITCs) are phytochemicals in Brassica vegetables that have anticarcinogenic properties in cell culture and animal models, and intake of Brassica vegetables is inversely associated with cancer in many epidemiologic studies [Verhoeven et al., 1997; Talalay and Fahey, 2001]. The mechanism of action of these phytochemicals is believed to involve their ability to inhibit phase I and induce phase II metabolizing enzymes, the effect of which may protect cells from the damaging effects of carcinogens, such as heterocyclic aromatic amines (HAAs) found in meat cooked to well-done at high temperatures [Verhoeven et al., 1997]. One class of phase II metabolizing enzymes that has been extensively studied with regard to Brassica vegetables and cancer are the glutathione S-transferases (GSTs). We review here the feeding studies that have examined interactions among the GSTs, ITC metabolite excretion, and/or HAA metabolite excretion in humans, providing context for a large and growing cancer epidemiologic literature on the subject. We refer to feeding studies as studies in humans that provide a known dose of a food (in this case, Brassica vegetable) to individuals in a controlled setting over a defined course of time to investigate biologic mechanisms. Outcomes, such as metabolite excretion, gene expression or enzyme activity, are measured to examine effects of the feeding regimen on biologic processes. These studies are typically small in size (less than 100 subjects) and short in duration (e.g., may measure the effects of one meal to up to daily meals for several weeks).
BRASSICA VEGETABLES MAY REDUCE CANCER RISK
Brassica vegetables have been implicated in cancer prevention for a large number of organ sites [van Poppel et al., 1999; Talalay and Fahey, 2001; Lampe and Peterson, 2002; Seow et al., 2005; Higdon et al., 2007; Jeffery and Keck, 2008]. In addition, numerous animal studies have shown that administration of Brassica vegetables or phytochemicals from Brassica vegetables reduces tumor incidence and size [Verhoeven et al., 1997] and has beneficial effects on cell cycle progression and apoptosis [Gamet-Payrastre et al., 2000; Keum et al., 2004; Chiao et al., 2004; Zhang, 2004; Zhang et al., 2006].
The epidemiologic evidence for the association between Brassica intake and cancer risk has been reviewed previously [Higdon et al., 2007], and remains mixed, with most studies showing either an inverse or null association. In our ecological study of breast cancer and nutritional, socioeconomic and reproductive factors using data from 59 countries, we found that on a per-calorie exposure basis, the strongest protective effect was due to Brassica vegetable consumption [Hebert and Rosen, 1996]. In another paper, utilizing data from the Long Island breast cancer study project, we found a borderline significant reduced risk of breast cancer among postmenopausal women, but an increased risk among premenopausal women who consumed ≥6 servings of Brassica vegetables per week as compared with those women consuming 0 or 1 servings per week [Gaudet et al., 2004a]. The increased risk observed in premenopausal women is perplexing as it is inconsistent with other studies (see for example, [Fowke et al., 2003a; Ambrosone et al., 2004]). Inconsistencies in study results may reflect the different dietary assessment tools used or the limited range in intake of some populations studied [Hebert and Miller, 1988; Hebert, 2005]. Some dietary assessment methods may not have included all, or even the most common, Brassica vegetables consumed in the population under study (e.g., kale), whereas others may have include non-Brassica vegetables (e.g., spinach) resulting in inaccurate exposure data or narrow ranges of intake that limit the ability to observe an association if one exists. Intakes of Brassica vegetables are highest in East Asian countries, where individuals eat foods rarely eaten in the West, but only a few studies have tested for associations in populations from this geographic region [London et al., 2000; Seow et al., 2002; Zhao et al., 2001; Fowke et al., 2003b]. Perhaps more importantly, it may be necessary to categorize individuals by genetic susceptibility, such as by polymorphisms for genes encoding phase I or phase II metabolizing enzymes known to be affected by phytochemicals in Brassica vegetables, to clearly observe an effect of Brassica vegetable intake on cancer risk. The interaction between ITCs and polymorphisms in one family of phase II metabolizing enzymes, the GSTs, has been examined in numerous epidemiologic studies in relation to lung [London et al., 2000; Spitz et al., 2000; Zhao et al., 2001; Lewis et al., 2002; Wang et al., 2004; Brennan et al., 2005], colon and colorectal [Lin et al., 1998; Slattery et al., 2000; Seow et al., 2002], prostate [Joseph et al., 2004], head and neck [Gaudet et al., 2004b], renal cell [Moore et al., 2007], and breast [Fowke et al., 2003a; Ambrosone et al., 2004; Steck et al., 2007b; Lee et al., 2008] cancers. However, the mechanisms underlying these associations have been explored in only a few feeding studies, as discussed later.
ITCs ARE PHYTOCHEMICALS RELEASED UPON BRASSICA VEGETABLE CONSUMPTION
Brassica vegetables contain glucosinolates, which upon chewing or chopping, are hydrolyzed into ITCs, indoles, and other substances by an enzyme called myrosinase that is present in a separate cellular compartment in the vegetable. The ITCs are responsible for the pungent flavor or biting taste that is experienced, acutely by many individuals, upon consumption of Brassica vegetables [Zhang and Talalay, 1994]. It is estimated that significant portions of ITCs are released upon intake of average servings of Brassica vegetables. In one study, consumption of 2 oz of watercress resulted in the release of ~12 mg of a specific ITC, phenethyl ITC (PEITC), representing a 30 to 67% conversion rate [Chung et al., 1992]. Brassica vegetables contain varying amounts of different glucosinolates, giving rise to different ITCs [Kushad et al., 1999; Vermeulen et al., 2006; Velasco et al., 2007; Cartea et al., 2008]. See review by Zhang [2004] and study by Shapiro et al. [1998] for chemical structures and metabolic pathways of ITCs. The most commonly studied of the ITCs include sulforaphane (primarily from broccoli), PEITC (from Chinese cabbage, radishes and watercress), and allyl ITC (AITC; from mustard, collard greens and kale). The amount of different glucosinolates, and hence, ITC byproducts, can vary at least 10-fold within and between Brassica vegetables, and even within varieties of a single vegetable type [Finley, 2005]. Cooking reduces ITC availability by inactivating myrosinase [Conaway et al., 2000]. However, even in the absence of myrosinase activity, small amounts of ITCs are released by the gut microflora [Krul et al., 2002]. ITCs are metabolized by the mercapturic acid pathway, which involves initial conjugation with glutathione (GSH) and further enzymic modification to N-acetylcysteine (NAC) conjugates (mercapturic acids) that are rapidly excreted in the urine [Mennicke et al., 1983; Ye et al., 2002]. The rapid accumulation of ITC in cells is due primarily to this conjugation with GSH [Zhang et al., 2006].
HAAs ARE CARCINOGENS FORMED IN WELL-DONE MEAT COOKED AT HIGH TEMPERATURES
HAAs are formed when amino acids pyrolyze in meat juice. Their concentrations are particularly high in panfried, grilled and, to a lesser extent, broiled meat [Knize et al., 1999]. The amount of HAAs formed is dependent upon the method, temperature, and duration of cooking, with greater doneness and higher temperatures associated with higher concentrations of HAAs [Sinha et al., 1998a, b]. Concentrations of three HAAs, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), and 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQX), are detectable in meat cooked by various methods [Sinha, 2002]. Foods high in MeIQx include panfried hamburger, sausage, and steak cooked to well-done or very well-done. By contrast, DiMeIQx has been detected in small quantities in pan-fried very well-done steak and in pan-fried and grilled/barbecued chicken [Sinha et al., 1995, 1998b]. Steak cooked to very well-done by frying or grilling/barbecuing and chicken are major contributors to PhIP intake [Sinha et al., 1998b; Skog and Solyakov, 2002].
HAAs are known carcinogens in animals, and are involved in the development of tumors through direct damage to DNA (formation of DNA adducts) [el-Bayoumy et al., 1995; Snyderwine et al., 2002]. MeIQx and PhIP are activated via hydroxylation with CYP1A2 in the liver [Boobis et al., 1994], as well as with other CYPs including CYP1A1 and CYP1B1 in extrahepatic tissue [Crofts et al., 1998]. Additional reactions such as conjugation with sulfotransferases, N-acetyltransferases, UDP-glucuronosyltransferases, and perhaps GSTs then occur to detoxify the active carcinogen [Pool-Zobel et al., 2005; Turesky, 2005]. [For chemical structures and metabolic pathways, see Felton et al., 2004; Walters et al., 2004; Turesky, 2005].
Dietary intake of these meat and meat-derived HAAs has been linked consistently to colorectal cancer [Cross and Sinha, 2004], and has been linked to breast cancer in some [De Stefani et al., 1997], but not all [Delfino et al., 2000; Sinha et al., 2000] epidemiologic studies. In a previous study, we found that high lifetime intake of grilled/barbecued and smoked meats was associated with increased risk of breast cancer among women consuming few fruits and vegetables [Steck et al., 2007c]. This is consistent with experimental studies that suggest that chemopreventive constituents of fruits and vegetables, such as ITCs, may protect against HAA-induced genotoxicity [Dingley et al., 2003; Conaway et al., 2005].
A few feeding studies in humans have examined the effect of Brassica vegetable intake on HAA metabolism and DNA adduct formation after meat and Brassica consumption [Murray et al., 2001; Steinkellner et al., 2001; Hoelzl et al., 2008]. In one study, excretion of MeIQx and PhIP in urine following a fried meat meal was reduced after 14 days of intake of 250 g each of Brussels sprouts and broccoli compared with a control period in which no Brassica was consumed [Murray et al., 2001]. Hoelzl et al. [2008] found that after consumption of 300 g Brussels sprouts per day for 6 days, PhIP-induced DNA damage was reduced in lymphocytes collected from participants before and after the intervention. Similarly, Steinkellner et al. [2001] report results from feeding studies showing that urinary mutagenicity following meat consumption was decreased with the consumption of red cabbage prior to the meat meals, while a larger portion of the excreted mutagenic substances were conjugated following cabbage consumption. Finally, in one small pilot study, eight individuals were fed fried meat daily for 6 weeks, with half of the subjects consuming Brassica vegetables daily, and the other half consuming no Brassica vegetables [DeMarini et al., 1997]. In that study, conjugated mutagens in urine doubled among the subjects consuming Brassica vegetables but decreased among those not consuming Brassica vegetables. This finding is consistent with the hypothesis that Brassica vegetables are working through a phase II enzyme-inducing mechanism. This study also genotyped participants for GSTM1 and other genes encoding phase II metabolizing enzymes, but because of the small sample size (n = 8), did not allow for characterizing the effect of GST genotype on response to the combination of Brassica vegetable and meat intake. Thus, more work in this area is needed to examine whether these in vivo effects are mediated by genotypes for genes encoding both phase I and phase II metabolizing enzymes.
ITCs FROM BRASSICA VEGETABLES MODULATE PHASE I AND PHASE II METABOLIZING ENZYMES AND HAVE ANTICARCINOGENIC PROPERTIES
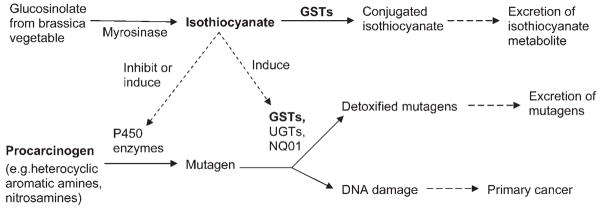
The biologic rationale supporting the role of ITCs in cancer prevention is related, in part, to their ability to modulate phase I and phase II enzymes [Hecht, 1999; Talalay and Fahey, 2001; Lampe and Chang, 2007; Clarke et al., 2008]; thereby detoxifying carcinogens such as the HAAs (see Fig. 1). The cytochrome P450 enzymes are phase I enzymes that are responsible for transforming precarcinogens to their carcinogenic form(s) and for metabolizing carcinogens to more carcinogenic form(s). In the process, electrophilic intermediates may be formed that can react with macromolecules to form DNA adducts. If not repaired, these DNA adducts can cause mutations in critical genes involved in cell cycle control or tumor suppression. In contrast, phase II enzymes, such as the family of GSTs and NAD(P)H:quinone oxidoreductase (NQO1), are capable of detoxifying these P450-activated carcinogens through the addition of polar moieties that make the carcinogen readily excretable (GSTs) and suppress the creation of reactive oxygen species (NQO1). Thus, inhibiting certain phase I enzymes or inducing the phase II enzymes (to a greater extent than any phase I induction) are two potential ways to reduce carcinogenicity. ITCs have been shown to do both in animal models and in vitro [Hecht, 1999; Steinkellner et al., 2001; Hwang and Jeffery, 2005; Zhou et al., 2007; Clarke et al., 2008].
Fig. 1.
Biologic pathways relating ITCs, HAAs, GSTs, and primary cancer. Isothiocyanates are substrates for GSTs. Isothiocyanates may inhibit or induce phase I metabolizing enzymes (CYPs such as CYP1A1, CYP1A2, CYP2A1, CYP2A6, CYP2B1, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4) that activate heterocyclic aromatic amines into active mutagens, and induce phase II metabolizing enzymes, such as the GSTs, that detoxify the active mutagens. Adapted with permission from Lin HJ, et al. 1998. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev 7:647–652, American Association for Cancer Research.
The effects of specific ITCs on CYP enzymes in cell culture and animal studies have been reported, albeit with mixed results. Sulforaphane has been shown to inhibit CYP1A1 and CYP2B1/2 in rat hepatocytes, and to decrease mRNA levels of CYP3A4 in human hepatocytes [Maheo et al., 1997], as well as inhibit steroid and xenobiotic receptor (SXR)-mediated induction of CYP3A4 in human hepatocytes and intestinal cells [Zhou et al., 2007]. PEITC also has been shown to inhibit CYP1A1, CYP1A2, and CYP2B1 in rat liver microsomes [Conaway et al., 1996], inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C9, CYP2D6, CYP2E1, and CYP3A4 human isoforms in bactulovirus-infected insect cells, and function as a mechanism-based inactivator of CYP2E1 [Nakajima et al., 2001]. Similarly, another ITC, BITC, was shown to be a mechanism-based inactivator of CYP2E1 [Moreno et al., 1999]. However, sulforaphane had no effect on CYP activity in human or rat liver in another study [Hanlon et al., 2008]. In contrast to previous studies, sulforaphane induced hepatic CYP1A2 in rats fed sulforaphane for 10 days [Yoxall et al., 2005], and PEITC was shown to upregulate CYP1A1 and activate CYP1A2 transcription in human hepatocytes [Gross-Steinmeyer et al., 2004]. Thus, the variation in Phase I enzyme response to ITCs appears to depend on experimental conditions and the different assays used to measure enzyme activity.
In human feeding studies, Brassica vegetables have the effect of upregulating CYP1A2 activity. This has been reported for intake of broccoli [Vistisen et al., 1992; Kall et al., 1996; Lampe et al., 2000b], cabbage and Brussels sprouts combined [Pantuck et al., 1979], and broccoli and Brussels sprouts combined [Murray et al., 2001]. Given the variety of phytochemicals present in Brassica vegetables, this effect may not entirely be contradictory to the cell culture studies showing inhibition of CYP1A2 by ITCs, if, in fact, other compounds in Brassica vegetables (such as indole-3-carbinol) have the effect of upregulating CYP1A2 beyond the inhibition exhibited by the ITCs.
In humans, a few feeding studies have reported changes in GST activity upon Brassica consumption [Bogaards et al., 1994; Sreerama et al., 1995; Lampe et al., 2000a; Lampe et al., 2000b]. In one study of males only, GST-α was increased in plasma following consumption of 300 g/d cooked Brussels sprouts for 2 weeks [Bogaards et al., 1994]. Similarly, Nijhoff et al. [1995b], found that plasma GST-α was increased in males, but not in females, after 6 days of consuming 300 g/d cooked Brussels sprouts, while urinary GST-alpha and both plasma and urinary GST-pi levels were not significantly changed by the intervention. A study by Lampe et al. [2000a], found that GST-α activity was increased in fasting blood samples from GSTM1-null women (but not in GSTM-positive women or in men of either genotype) after consuming Brassica vegetables for 6 days. One small crossover feeding study also found increased GST-α and -π levels in rectal biopsies from humans after intake of 300 g/d Brussels sprouts for 7 days [Nijhoff et al., 1995a]. However, a recent feeding study showed no change in expression of GSTs in human gastric mucosa after one dose of standard broccoli or high-glucosinolate broccoli [Gasper et al., 2007]. Possible reasons for this discrepant result may be the fact that only a single dose of broccoli was administered in that study precluding the examination of effects of more chronic intake.
ITCs ARE SUBSTRATES FOR GSTs WHICH ARE POLYMORPHIC IN HUMAN POPULATIONS
In addition to inducing GSTs, ITCs are known substrates for the GSTs (see Fig. 1). Functional polymorphisms in the GST family of genes have been identified and the frequencies of some of these differ by race, as found in the Carolina Breast Cancer Study [Millikan et al., 2000]. The most studied of the GST genes in relation to Brassica vegetables and cancer are GSTM1, GSTT1, and GSTP1. A deletion in both alleles of GSTM1 results in no enzyme activity in an estimated 52% of European Americans but only 28% of African Americans. A deletion in both alleles of GSTT1 exists equally among African Americans and European Americans at ~20%. For GSTP1, an amino acid substitution (Ile105Val) is associated with alterations in enzyme activity; approximately 49% of European Americans and 55% of African Americans are heterozygous, whereas 11% of European Americans and 23% of African Americans carry the homozygous variant. The functional effect of this polymorphism is not entirely clear, as there is evidence that the Val allele has reduced specific activity to sulforaphane as compared with the Ile allele in one study [Lin et al., 2003], whereas the Ile allele has lower activity toward detoxifying benzo(a)pyrene (BaP) compared with the Val allele in another study [Sundberg et al., 1998],
In vitro studies reveal that different classes of GSTs are more efficient catalysts for specific ITCs than others, with GSTM1 and GSTP1 being the most efficient, GSTA1 less efficient, and GSTM2 and GSTM4 least efficient for each of four ITCs studied (AITC, benzyl-ITC, PEITC, and sulforaphane) [Kolm et al., 1995; Zhang et al., 1995]. There is evidence from controlled feeding studies that individuals can be separated into high and low excreters of ITC, even at constant dosing [Shapiro et al., 1998]. One hypothesis to explain is that having the inactive form of GSTs may result in reduced excretion of ITC metabolites due to reduced metabolism of these phytochemicals. This hypothesis was supported in one observational study, where the null genotype for GSTT1, as compared with non-null genotype, was associated with lower ITC excretion levels in Singapore Chinese, eating mostly green leafy crucifers such as Chinese cabbage [Seow et al., 1998]. However, more recently, a broccoli feeding study in the UK found that individuals with the GSTM1-null genotype had significantly higher excretion of sulforaphane metabolites following broccoli intake than GSTM1-positive subjects [Gasper et al., 2005]. We conducted a one-meal feeding study examining the relationship between polymorphisms in GSTM1, GSTT1, GSTP1 and GSTA1, and ITC metabolite excretion after broccoli consumption in humans [Steck et al., 2007a]. Similar to the Gasper et al. study, we did not observe an increase in ITC metabolite excretion in individuals with the non-null or more active genotypes.
Even within a single family of vegetables, such as the crucifers and a specific genus, Brassica, effects may differ between different vegetables, and there may be alternative routes of metabolism for the different ITC (namely sulforaphane in the broccoli studies). There is speculation that there may be differential effects of different Brassica vegetables on cancer risk because of differences in their phytochemical constitution [Gasper et al., 2005]. Other factors that may explain the differential excretion of ITCs between individuals include the amount of chewing, which affects the release of myrosinase from the vegetable. In the case of cooked vegetables, where myrosinase has been inactivated, conversion of glucosinolates to ITCs still occurs via the gastrointestinal microflora, so differences in ITC excretion between individuals also may be related to differences in the gastrointestinal microflora. Finally, there is speculation that the GSTs affect the rate of ITC excretion, rather than the absolute amount excreted; in which case, a single urinary measurement of ITC excretion would have little value within either a feeding study or epidemiologic study designed to account for this difference in rate.
The relationship between GST genotypes and excretion of specific ITC from other Brassica vegetables besides broccoli has not been tested in human feeding studies. These types of feeding studies are needed to identify whether certain GSTs are more important in specific ITC metabolism and excretion than others in vivo, and whether one class of GSTs may compensate when another class is missing or less active. Interestingly, sulforaphane was the poorest substrate for GSTM1, GSTP1, GSTA1, and GSTM2 enzymes, yet was the most potent inducer as compared with three other ITC in vitro [Zhang et al., 1995]. This suggests that it may be sulforaphane’s ability to induce the GSTs rather than its role as a substrate for the GSTs that is most crucial in cancer prevention. This is consistent with findings from some studies in the United States showing that intake of Brassica vegetables (the majority being broccoli) is associated with greater cancer risk reduction in individuals with the active forms of the GST genes as compared with individuals with the inactive forms [Spitz et al., 2000; Joseph et al., 2004; Wang et al., 2004].
CONCLUSION
Intake of Brassica vegetables in relation to GST polymorphisms represents a well-studied gene-diet interaction in cancer epidemiology. As described earlier, interaction between GSTs, HAAs, and ITCs may exist and two possible mechanisms include: (1) polymorphisms in GSTs that reduce enzyme activity may predispose meat-eaters to cancer by reducing HAA detoxification; in turn, intake of Brassica vegetables may counteract this effect because of the ability of ITCs to induce GSTs and other phase II metabolizing enzymes and enhance HAA detoxification; (2) polymorphisms in GSTs that reduce enzyme activity may enable individuals consuming high amounts of Brassica vegetables to sequester ITCs in the body to act via chemopreventive mechanisms; these individuals may then be more protected against meat-derived HAA intake than individuals with the fully active enzymes and low Brassica vegetable intake. Of course, these mechanisms are not mutually exclusive. One small pilot study in humans provides suggestive evidence that both GST polymorphism and ITC intake may modulate effects of HAA on urinary mutagenicity [DeMarini et al., 1997]. More work is needed to elucidate the full capabilities of these interactions to beneficially affect health and disease risk. Feeding studies that enroll adequate numbers of individuals of varying genotypes; and then examine the effects of cooked vs. raw Brassica vegetables, of different types of ITCs from various Brassica vegetables, and both ITC and HAA metabolite excretion patterns by genotype are warranted as these factors cannot be controlled in observational epidemiologic studies.
Intake of Brassica vegetables is estimated to be low in the American population. Based on data from the Continuing Survey of Food Intakes by Individuals on over 4,800 Americans, fewer than 20% of the study sample consumed a Brassica vegetable (broccoli, cauliflower, kale or Brussels sprouts) in the two-day reporting period [Johnston et al., 2000]. Given the substantial scope for increasing intake of Brassica vegetables in the American population, efforts to increase consumption may be more effective and economical if tailored to individuals who will receive the most benefit, i.e., those who are genetically susceptible to the benefits of Brassica vegetable intake. Thus, it is extremely important to study the biologic mechanisms linking ITCs to inhibition of carcinogenicity by HAAs and to study the effect of GST genotype on response to ITC and HAA intake in humans to determine whether the observational data on the association between ITC, GST genotype and cancer are merely chance findings or whether they have etiological implications.
Abbreviations
- AITC
allyl isothiocyanate
- BaP
benzo(a)pyrene
- DiMeIQX
2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline
- GSH
glutathione
- GST
glutathione S-transferase
- HAA
heterocylic aromatic amine
- ITC
isothiocyanate
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- NAC
N-acetylcysteine
- NQO1, NAD(P)H
quinone oxidoreductase
- PEITC
phenethyl isothiocyanate
- PhIP
2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridine
- SXR
steroid and xenobiotic receptor
References
- Ambrosone CB, McCann SE, Freudenheim JL, Marshall JR, Zhang Y, Shields PG. Breast cancer risk in premenopausal women is inversely associated with consumption of broccoli, a source of isothiocyanates, but is not modified by GST genotype. J Nutr. 2004;134:1134–1138. doi: 10.1093/jn/134.5.1134. [DOI] [PubMed] [Google Scholar]
- Bogaards JJ, Verhagen H, Willems MI, van Poppel G, van Bladeren PJ. Consumption of Brussels sprouts results in elevated alpha-class glutathione S-transferase levels in human blood plasma. Carcinogenesis. 1994;15:1073–1075. doi: 10.1093/carcin/15.5.1073. [DOI] [PubMed] [Google Scholar]
- Boobis AR, Lynch AM, Murray S, de la Torre R, Solans A, Farre M, Segura J, Gooderham NJ, Davies DS. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994;54:89–94. [PubMed] [Google Scholar]
- Brennan P, Hsu CC, Moullan N, Szeszenia-Dabrowska N, Lissowska J, Zaridze D, Rudnai P, Fabianova E, Mates D, Bencko V, Foretova L, Janout V, Gemignani F, Chabrier A, Hall J, Hung RJ, Boffetta P, Canzian F. Effect of cruciferous vegetables on lung cancer in patients stratified by genetic status: A mendelian randomisation approach. Lancet. 2005;366:1558–1560. doi: 10.1016/S0140-6736(05)67628-3. [DOI] [PubMed] [Google Scholar]
- Cartea ME, Velasco P, Obregon S, Padilla G, de Haro A. Seasonal variation in glucosinolate content in Brassica oleracea crops grown in northwestern Spain. Phytochemistry. 2008;69:403–410. doi: 10.1016/j.phytochem.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Chiao JW, Wu H, Ramaswamy G, Conaway CC, Chung FL, Wang L, Liu D. Ingestion of an isothiocyanate metabolite from cruciferous vegetables inhibits growth of human prostate cancer cell xenografts by apoptosis and cell cycle arrest. Carcinogenesis. 2004;25:1403–1408. doi: 10.1093/carcin/bgh136. [DOI] [PubMed] [Google Scholar]
- Chung FL, Morse MA, Eklind KI, Lewis J. Quantitation of human uptake of the anticarcinogen phenethyl isothiocyanate after a watercress meal. Cancer Epidemiol Biomarkers Prev. 1992;1:383–388. [PubMed] [Google Scholar]
- Clarke JD, Dashwood RH, Ho E. Multi-targeted prevention of cancer by sulforaphane. Cancer Lett. 2008;269:291–304. doi: 10.1016/j.canlet.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway CC, Getahun SM, Liebes LL, Pusateri DJ, Topham DK, Botero-Omary M, Chung FL. Disposition of glucosinolates and sulforaphane in humans after ingestion of steamed and fresh broccoli. Nutr Cancer. 2000;38:168–178. doi: 10.1207/S15327914NC382_5. [DOI] [PubMed] [Google Scholar]
- Conaway CC, Jiao D, Chung FL. Inhibition of rat liver cytochrome P450 isozymes by isothiocyanates and their conjugates: A structure-activity relationship study. Carcinogenesis. 1996;17:2423–2427. doi: 10.1093/carcin/17.11.2423. [DOI] [PubMed] [Google Scholar]
- Conaway CC, Wang CX, Pittman B, Yang YM, Schwartz JE, Tian D, McIntee EJ, Hecht SS, Chung FL. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005;65:8548–8557. doi: 10.1158/0008-5472.CAN-05-0237. [DOI] [PubMed] [Google Scholar]
- Crofts FG, Sutter TR, Strickland PT. Metabolism of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine by human cytochrome P4501A1, P4501A2 and P4501B1. Carcinogenesis. 1998;19:1969–1973. doi: 10.1093/carcin/19.11.1969. [DOI] [PubMed] [Google Scholar]
- Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- De Stefani E, Ronco A, Mendilaharsu M, Guidobono M, Deneo-Pellegrini H. Meat intake, heterocyclic amines, and risk of breast cancer: A case-control study in Uruguay. Cancer Epidemiol Biomarkers Prev. 1997;6:573–581. [PubMed] [Google Scholar]
- Delfino RJ, Sinha R, Smith C, West J, White E, Lin HJ, Liao SY, Gim JS, Ma HL, Butler J, Anton-Culver H. Breast cancer, heterocyclic aromatic amines from meat and N-acetyltransferase 2 genotype. Carcinogenesis. 2000;21:607–615. doi: 10.1093/carcin/21.4.607. [DOI] [PubMed] [Google Scholar]
- DeMarini DM, Hastings SB, Brooks LR, Eischen BT, Bell DA, Watson MA, Felton JS, Sandler R, Kohlmeier L. Pilot study of free and conjugated urinary mutagenicity during consumption of pan-fried meats: Possible modulation by cruciferous vegetables, glutathione S-transferase-M1, and N-acetyltransferase-2. Mutat Res. 1997;381:83–96. doi: 10.1016/s0027-5107(97)00152-8. [DOI] [PubMed] [Google Scholar]
- Dingley KH, Ubick EA, Chiarappa-Zucca ML, Nowell S, Abel S, Ebeler SE, Mitchell AE, Burns SA, Steinberg FM, Clifford AJ. Effect of dietary constituents with chemopreventive potential on adduct formation of a low dose of the heterocyclic amines PhIP and IQ, phase II hepatic enzymes. Nutr Cancer. 2003;46:212–221. doi: 10.1207/S15327914NC4602_15. [DOI] [PubMed] [Google Scholar]
- el-Bayoumy K, Chae YH, Upadhyaya P, Rivenson A, Kurtzke C, Reddy B, Hecht SS. Comparative tumorigenicity of benzo[a] pyrene, 1-nitropyrene and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine administered by gavage to female CD rats. Carcinogenesis. 1995;16:431–434. doi: 10.1093/carcin/16.2.431. [DOI] [PubMed] [Google Scholar]
- Felton JS, Knize MG, Bennett LM, Malfatti MA, Colvin ME, Kulp KS. Impact of environmental exposures on the mutagenicity/carcinogenicity of heterocyclic amines. Toxicology. 2004;198:135–145. doi: 10.1016/j.tox.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Finley JW. Proposed criteria for assessing the efficacy of cancer reduction by plant foods enriched in carotenoids, glucosinolates, polyphenols and selenocompounds. Ann Bot (Lond) 2005;95:1075–1096. doi: 10.1093/aob/mci123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowke JH, Chung FL, Jin F, Qi D, Cai Q, Conaway C, Cheng JR, Shu XO, Gao YT, Zheng W. Urinary isothiocyanate levels, brassica, and human breast cancer. Cancer Res. 2003a;63:3980–3986. [PubMed] [Google Scholar]
- Fowke JH, Shu XO, Dai Q, Shintani A, Conaway CC, Chung FL, Cai Q, Gao YT, Zheng W. Urinary isothiocyanate excretion, brassica consumption, and gene polymorphisms among women living in Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2003b;12:1536–1539. [PubMed] [Google Scholar]
- Gamet-Payrastre L, Li P, Lumeau S, Cassar G, Dupont MA, Chevolleau S, Gasc N, Tulliez J, Terce F. Sulforaphane, a naturally occurring isothiocyanate, induces cell cycle arrest and apoptosis in HT29 human colon cancer cells. Cancer Res. 2000;60:1426–1433. [PubMed] [Google Scholar]
- Gasper AV, Al-Janobi A, Smith JA, Bacon JR, Fortun P, Atherton C, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- Gasper AV, Traka M, Bacon JR, Smith JA, Taylor MA, Hawkey CJ, Barrett DA, Mithen RF. Consuming broccoli does not induce genes associated with xenobiotic metabolism and cell cycle control in human gastric mucosa. J Nutr. 2007;137:1718–1724. doi: 10.1093/jn/137.7.1718. [DOI] [PubMed] [Google Scholar]
- Gaudet MM, Britton JA, Kabat GC, Steck-Scott S, Eng SM, Teitelbaum SL, Terry MB, Neugut AI, Gammon MD. Fruits, vegetables, and micronutrients in relation to breast cancer modified by menopause and hormone receptor status. Cancer Epidemiol Biomarkers Prev. 2004a;13:1485–1494. [PubMed] [Google Scholar]
- Gaudet MM, Olshan AF, Poole C, Weissler MC, Watson M, Bell DA. Diet, GSTM1 and GSTT1 and head and neck cancer. Carcinogenesis. 2004b;25:735–740. doi: 10.1093/carcin/bgh054. [DOI] [PubMed] [Google Scholar]
- Gross-Steinmeyer K, Stapleton PL, Liu F, Tracy JH, Bammler TK, Quigley SD, Farin FM, Buhler DR, Safe SH, Strom SC, Eaton DL. Phytochemical-induced changes in gene expression of carcinogen-metabolizing enzymes in cultured human primary hepatocytes. Xenobiotica. 2004;34:619–632. doi: 10.1080/00498250412331285481. [DOI] [PubMed] [Google Scholar]
- Hanlon N, Coldham N, Sauer MJ, Ioannides C. Upregulation of the CYP1 family in rat and human liver by the aliphatic isothiocyanates erucin and sulforaphane. Toxicology. 2008;252:92–98. doi: 10.1016/j.tox.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Hebert JR. Epidemiologic studies of diet and cancer: The case for international collaboration. Austro-Asian J Cancer. 2005;4:125–134. [Google Scholar]
- Hebert JR, Miller DR. Methodologic considerations for investigating the diet-cancer link. Am J Clin Nutr. 1988;47:1068–1077. doi: 10.1093/ajcn/47.6.1068. [DOI] [PubMed] [Google Scholar]
- Hebert JR, Rosen A. Nutritional, socioeconomic, and reproductive factors in relation to female breast cancer mortality: Findings from a cross-national study. Cancer Detect Prev. 1996;20:234–244. [PubMed] [Google Scholar]
- Hecht SS. Chemoprevention of cancer by isothiocyanates, modifiers of carcinogen metabolism. J Nutr. 1999;129:768S–774S. doi: 10.1093/jn/129.3.768S. [DOI] [PubMed] [Google Scholar]
- Higdon JV, Delage B, Williams DE, Dashwood RH. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol Res. 2007;55:224–236. doi: 10.1016/j.phrs.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoelzl C, Glatt H, Meinl W, Sontag G, Haidinger G, Kundi M, Simic T, Chakraborty A, Bichler J, Ferk F, Angelis K, Nersesyan A, Knasmuller S. Consumption of Brussels sprouts protects peripheral human lymphocytes against 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and oxidative DNA-damage: Results of a controlled human intervention trial. Mol Nutr Food Res. 2008;52:330–341. doi: 10.1002/mnfr.200700406. [DOI] [PubMed] [Google Scholar]
- Hwang ES, Jeffery EH. Induction of quinone reductase by sulforaphane and sulforaphane N-acetylcysteine conjugate in murine hepatoma cells. J Med Food. 2005;8:198–203. doi: 10.1089/jmf.2005.8.198. [DOI] [PubMed] [Google Scholar]
- Jeffery EH, Keck AS. Translating knowledge generated by epidemiological and in vitro studies into dietary cancer prevention. Mol Nutr Food Res. 2008;52(Suppl 1):S7–S17. doi: 10.1002/mnfr.200700226. [DOI] [PubMed] [Google Scholar]
- Johnston CS, Taylor CA, Hampl JS. More Americans are eating “5 a day” but intakes of dark green and cruciferous vegetables remain low. J Nutr. 2000;130:3063–3067. doi: 10.1093/jn/130.12.3063. [DOI] [PubMed] [Google Scholar]
- Joseph MA, Moysich KB, Freudenheim JL, Shields PG, Bowman ED, Zhang Y, Marshall JR, Ambrosone CB. Cruciferous vegetables, genetic polymorphisms in glutathione S-transferases M1 and T1, and prostate cancer risk. Nutr Cancer. 2004;50:206–213. doi: 10.1207/s15327914nc5002_11. [DOI] [PubMed] [Google Scholar]
- Kall MA, Vang O, Clausen J. Effects of dietary broccoli on human in vivo drug metabolizing enzymes: Evaluation of caffeine, oestrone and chlorzoxazone metabolism. Carcinogenesis. 1996;17:793–799. doi: 10.1093/carcin/17.4.793. [DOI] [PubMed] [Google Scholar]
- Keum YS, Jeong WS, Kong AN. Chemoprevention by isothiocyanates and their underlying molecular signaling mechanisms. Mutat Res. 2004;555:191–202. doi: 10.1016/j.mrfmmm.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Knize MG, Salmon CP, Pais P, Felton JS. Food heating and the formation of heterocyclic aromatic amine and polycyclic aromatic hydrocarbon mutagens/carcinogens. Adv Exp Med Biol. 1999;459:179–193. doi: 10.1007/978-1-4615-4853-9_12. [DOI] [PubMed] [Google Scholar]
- Kolm RH, Danielson UH, Zhang Y, Talalay P, Mannervik B. Isothiocyanates as substrates for human glutathione transferases: Structure-activity studies. Biochem J. 1995;311(Part 2):453–459. doi: 10.1042/bj3110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krul C, Humblot C, Philippe C, Vermeulen M, van Nuenen M, Havenaar R, Rabot S. Metabolism of sinigrin (2-propenyl glucosinolate) by the human colonic microflora in a dynamic in vitro large-intestinal model. Carcinogenesis. 2002;23:1009–1016. doi: 10.1093/carcin/23.6.1009. [DOI] [PubMed] [Google Scholar]
- Kushad MM, Brown AF, Kurilich AC, Juvik JA, Klein BP, Wallig MA, Jeffery EH. Variation of glucosinolates in vegetable crops of Brassica oleracea. J Agric Food Chem. 1999;47:1541–1548. doi: 10.1021/jf980985s. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Chang JL. Interindividual differences in phytochemical metabolism and disposition. Semin Cancer Biol. 2007;17:347–353. doi: 10.1016/j.semcancer.2007.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampe JW, Peterson S. Brassica, biotransformation and cancer risk: Genetic polymorphisms alter the preventive effects of cruciferous vegetables. J Nutr. 2002;132:2991–2994. doi: 10.1093/jn/131.10.2991. [DOI] [PubMed] [Google Scholar]
- Lampe JW, Chen C, Li S, Prunty J, Grate MT, Meehan DE, Barale KV, Dightman DA, Feng Z, Potter JD. Modulation of human glutathione S-transferases by botanically defined vegetable diets. Cancer Epidemiol Biomarkers Prev. 2000a;9:787–793. [PubMed] [Google Scholar]
- Lampe JW, King IB, Li S, Grate MT, Barale KV, Chen C, Feng Z, Potter JD. Brassica vegetables increase and apiaceous vegetables decrease cytochrome P450 1A2 activity in humans: Changes in caffeine metabolite ratios in response to controlled vegetable diets. Carcinogenesis. 2000b;21:1157–1162. [PubMed] [Google Scholar]
- Lee SA, Fowke JH, Lu W, Ye C, Zheng Y, Cai Q, Gu K, Gao YT, Shu XO, Zheng W. Cruciferous vegetables, the GSTP1 Ile105Val genetic polymorphism, and breast cancer risk. Am J Clin Nutr. 2008;87:753–760. doi: 10.1093/ajcn/87.3.753. [DOI] [PubMed] [Google Scholar]
- Lewis S, Brennan P, Nyberg F, Ahrens W, Constantinescu V, Mukeria A, Benhamou S, Batura-Gabryel H, Bruske-Hohlfeld I, Simonato L, Menezes A, Boffetta P. Cruciferous vegetable intake, GSTM1 genotype and lung cancer risk in a non-smoking population. IARC Sci Publ. 2002;156:507–508. [PubMed] [Google Scholar]
- Lin HJ, Probst-Hensch NM, Louie AD, Kau IH, Witte JS, Ingles SA, Frankl HD, Lee ER, Haile RW. Glutathione transferase null genotype, broccoli, and lower prevalence of colorectal adenomas. Cancer Epidemiol Biomarkers Prev. 1998;7:647–652. [PubMed] [Google Scholar]
- Lin HJ, Johansson AS, Stenberg G, Materi AM, Park JM, Dai A, Zhou H, Gim JS, Kau IH, Hardy SI, Parker MW, Mannervik B. Naturally occurring Phe151Leu substitution near a conserved folding module lowers stability of glutathione transferase P1-1. Biochim Biophys Acta. 2003;1649:16–23. doi: 10.1016/s1570-9639(03)00149-3. [DOI] [PubMed] [Google Scholar]
- London SJ, Yuan JM, Chung FL, Gao YT, Coetzee GA, Ross RK, Yu MC. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: A prospective study of men in Shanghai, China. Lancet. 2000;356:724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- Maheo K, Morel F, Langouet S, Kramer H, Le Ferrec E, Ketterer B, Guillouzo A. Inhibition of cytochromes P-450 and induction of glutathione S-transferases by sulforaphane in primary human and rat hepatocytes. Cancer Res. 1997;57:3649–3652. [PubMed] [Google Scholar]
- Mennicke WH, Gorler K, Krumbiegel G. Metabolism of some naturally occurring isothiocyanates in the rat. Xenobiotica. 1983;13:203–207. doi: 10.3109/00498258309052256. [DOI] [PubMed] [Google Scholar]
- Millikan R, Pittman G, Tse CK, Savitz DA, Newman B, Bell D. Glutathione S-transferases M1, T1, and P1 and breast cancer. Cancer Epidemiol Biomarkers Prev. 2000;9:567–573. [PubMed] [Google Scholar]
- Moore LE, Brennan P, Karami S, Hung RJ, Hsu C, Boffetta P, Toro J, Zaridze D, Janout V, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Mukeria A, Holcatova I, Welch R, Chanock S, Rothman N, Chow WH. Glutathione S-transferase polymorphisms, cruciferous vegetable intake and cancer risk in the Central and Eastern European kidney cancer study. Carcinogenesis. 2007;28:1960–1964. doi: 10.1093/carcin/bgm151. [DOI] [PubMed] [Google Scholar]
- Moreno RL, Kent UM, Hodge K, Hollenberg PF. Inactivation of cytochrome P450 2E1 by benzyl isothiocyanate. Chem Res Toxicol. 1999;12:582–587. doi: 10.1021/tx9900019. [DOI] [PubMed] [Google Scholar]
- Murray S, Lake BG, Gray S, Edwards AJ, Springall C, Bowey EA, Williamson G, Boobis AR, Gooderham NJ. Effect of cruciferous vegetable consumption on heterocyclic aromatic amine metabolism in man. Carcinogenesis. 2001;22:1413–1420. doi: 10.1093/carcin/22.9.1413. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Yoshida R, Shimada N, Yamazaki H, Yokoi T. Inhibition and inactivation of human cytochrome p450 isoforms by phenethyl isothiocyanate. Drug Metab Dispos. 2001;29:1110–1113. [PubMed] [Google Scholar]
- Nijhoff WA, Grubben MJ, Nagengast FM, Jansen JB, Verhagen H, van Poppel G, Peters WH. Effects of consumption of Brussels sprouts on intestinal and lymphocytic glutathione S-transferases in humans. Carcinogenesis. 1995a;16:2125–2128. doi: 10.1093/carcin/16.9.2125. [DOI] [PubMed] [Google Scholar]
- Nijhoff WA, Mulder TP, Verhagen H, van Poppel G, Peters WH. Effects of consumption of brussels sprouts on plasma and urinary glutathione S-transferase class-alpha and -pi in humans. Carcinogenesis. 1995b;16:955–957. doi: 10.1093/carcin/16.4.955. [DOI] [PubMed] [Google Scholar]
- Pantuck EJ, Pantuck CB, Garland WA, Min BH, Wattenberg LW, Anderson KE, Kappas A, Conney AH. Stimulatory effect of brussels sprouts and cabbage on human drug metabolism. Clin Pharmacol Ther. 1979;25:88–95. doi: 10.1002/cpt197925188. [DOI] [PubMed] [Google Scholar]
- Pool-Zobel B, Veeriah S, Bohmer FD. Modulation of xenobiotic metabolising enzymes by anticarcinogens—focus on glutathione S-transferases and their role as targets of dietary chemoprevention in colorectal carcinogenesis. Mutat Res. 2005;591:74–92. doi: 10.1016/j.mrfmmm.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Seow A, Shi CY, Chung FL, Jiao D, Hankin JH, Lee HP, Coetzee GA, Yu MC. Urinary total isothiocyanate (ITC) in a population-based sample of middle-aged and older Chinese in Singapore: Relationship with dietary total ITC, glutathione S-transferase M1/T1/P1 genotypes. Cancer Epidemiol Biomarkers Prev. 1998;7:775–781. [PubMed] [Google Scholar]
- Seow A, Yuan JM, Sun CL, Van Den Berg D, Lee HP, Yu MC. Dietary isothiocyanates, glutathione S-transferase polymorphisms and colorectal cancer risk in the Singapore Chinese health study. Carcinogenesis. 2002;23:2055–2061. doi: 10.1093/carcin/23.12.2055. [DOI] [PubMed] [Google Scholar]
- Seow A, Vainio H, Yu MC. Effect of glutathione-S-transferase polymorphisms on the cancer preventive potential of isothiocyanates: An epidemiological perspective. Mutat Res. 2005;592:58–67. doi: 10.1016/j.mrfmmm.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Shapiro TA, Fahey JW, Wade KL, Stephenson KK, Talalay P. Human metabolism and excretion of cancer chemoprotective glucosinolates and isothiocyanates of cruciferous vegetables. Cancer Epidemiol Biomarkers Prev. 1998;7:1091–1100. [PubMed] [Google Scholar]
- Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat Res. 2002;506(507):197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Sinha R, Rothman N, Brown ED, Salmon CP, Knize MG, Swanson CA, Rossi SC, Mark SD, Levander OA, Felton JS. High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo-4,5-b pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res. 1995;55:4516–4519. [PubMed] [Google Scholar]
- Sinha R, Knize MG, Salmon CP, Brown ED, Rhodes D, Felton JS, Levander OA, Rothman N. Heterocyclic amine content of pork products cooked by different methods and to varying degrees of doneness. Food Chem Toxicol. 1998a;36:289–297. doi: 10.1016/s0278-6915(97)00159-2. [DOI] [PubMed] [Google Scholar]
- Sinha R, Rothman N, Salmon CP, Knize MG, Brown ED, Swanson CA, Rhodes D, Rossi S, Felton JS, Levander OA. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol. 1998b;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- Sinha R, Gustafson DR, Kulldorff M, Wen WQ, Cerhan JR, Zheng W. 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine, a carcinogen in high-temperature-cooked meat, and breast cancer risk. J Natl Cancer Inst. 2000;92:1352–1354. doi: 10.1093/jnci/92.16.1352. [DOI] [PubMed] [Google Scholar]
- Skog K, Solyakov A. Heterocyclic amines in poultry products: A literature review. Food Chem Toxicol. 2002;40:1213–1221. doi: 10.1016/s0278-6915(02)00062-5. [DOI] [PubMed] [Google Scholar]
- Slattery ML, Kampman E, Samowitz W, Caan BJ, Potter JD. Interplay between dietary inducers of GST, the GSTM-1 genotype in colon cancer. Int J Cancer. 2000;87:728–733. [PubMed] [Google Scholar]
- Snyderwine EG, Venugopal M, Yu M. Mammary gland carcinogenesis by food-derived heterocyclic amines and studies on the mechanisms of carcinogenesis of 2-amino-1-methyl-6-phenylimi-dazo[4,5-b]pyridine (PhIP) Mutat Res. 2002;506–507:145–152. doi: 10.1016/s0027-5107(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Spitz MR, Duphorne CM, Detry MA, Pillow PC, Amos CI, Lei L, de Andrade M, Gu X, Hong WK, Wu X. Dietary intake of isothiocyanates: Evidence of a joint effect with glutathione S-transferase polymorphisms in lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2000;9:1017–1020. [PubMed] [Google Scholar]
- Sreerama L, Hedge MW, Sladek NE. Identification of a class 3 aldehyde dehydrogenase in human saliva and increased levels of this enzyme, glutathione S-transferases, and DT-diaphorase in the saliva of subjects who continually ingest large quantities of coffee or broccoli. Clin Cancer Res. 1995;1:1153–1163. [PubMed] [Google Scholar]
- Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J Nutr. 2007a;137:904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steck SE, Gaudet MM, Britton JA, Teitelbaum SL, Terry MB, Neugut AI, Santella RM, Gammon MD. Interactions among GSTM1, GSTT1 and GSTP1 polymorphisms, cruciferous vegetable intake and breast cancer risk. Carcinogenesis. 2007b;28:1954–1959. doi: 10.1093/carcin/bgm141. [DOI] [PubMed] [Google Scholar]
- Steck SE, Gaudet MM, Eng SM, Britton JA, Teitelbaum SL, Neugut AI, Santella RM, Gammon MD. Cooked meat and risk of breast cancer-lifetime versus recent dietary intake. Epidemiology. 2007c;18:373–382. doi: 10.1097/01.ede.0000259968.11151.06. [DOI] [PubMed] [Google Scholar]
- Steinkellner H, Rabot S, Freywald C, Nobis E, Scharf G, Chabicovsky M, Knasmuller S, Kassie F. Effects of cruciferous vegetables and their constituents on drug metabolizing enzymes involved in the bioactivation of DNA-reactive dietary carcinogens. Mutat Res. 2001;480(481):285–297. doi: 10.1016/s0027-5107(01)00188-9. [DOI] [PubMed] [Google Scholar]
- Sundberg K, Johansson AS, Stenberg G, Widersten M, Seidel A, Mannervik B, Jernstrom B. Differences in the catalytic efficiencies of allelic variants of glutathione transferase P1-1 towards carcinogenic diol epoxides of polycyclic aromatic hydrocarbons. Carcinogenesis. 1998;19:433–436. doi: 10.1093/carcin/19.3.433. [DOI] [PubMed] [Google Scholar]
- Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131(11 Suppl):3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- Turesky RJ. Interspecies metabolism of heterocyclic aromatic amines and the uncertainties in extrapolation of animal toxicity data for human risk assessment. Mol Nutr Food Res. 2005;49:101–117. doi: 10.1002/mnfr.200400076. [DOI] [PubMed] [Google Scholar]
- van Poppel G, Verhoeven DT, Verhagen H, Goldbohm RA. Brassica vegetables and cancer prevention. Epidemiology and mechanisms. Adv Exp Med Biol. 1999;472:159–168. doi: 10.1007/978-1-4757-3230-6_14. [DOI] [PubMed] [Google Scholar]
- Velasco P, Cartea ME, Gonzalez C, Vilar M, Ordas A. Factors affecting the glucosinolate content of kale (Brassica oleracea acephala group) J Agric Food Chem. 2007;55:955–962. doi: 10.1021/jf0624897. [DOI] [PubMed] [Google Scholar]
- Verhoeven DT, Verhagen H, Goldbohm RA, van den Brandt PA, van Poppel G. A review of mechanisms underlying anticarcinogenicity by brassica vegetables. Chem Biol Interact. 1997;103:79–129. doi: 10.1016/s0009-2797(96)03745-3. [DOI] [PubMed] [Google Scholar]
- Vermeulen M, van den Berg R, Freidig AP, van Bladeren PJ, Vaes WH. Association between consumption of cruciferous vegetables and condiments and excretion in urine of isothiocyanate mercapturic acids. J Agric Food Chem. 2006;54:5350–5358. doi: 10.1021/jf060723n. [DOI] [PubMed] [Google Scholar]
- Vistisen K, Poulsen HE, Loft S. Foreign compound metabolism capacity in man measured from metabolites of dietary caffeine. Carcinogenesis. 1992;13:1561–1568. doi: 10.1093/carcin/13.9.1561. [DOI] [PubMed] [Google Scholar]
- Walters DG, Young PJ, Agus C, Knize MG, Boobis AR, Gooderham NJ, Lake BG. Cruciferous vegetable consumption alters the metabolism of the dietary carcinogen 2-amino-1-methyl-6-phenyl-imidazo[4,5-b]pyridine (PhIP) in humans. Carcinogenesis. 2004;25:1659–1669. doi: 10.1093/carcin/bgh164. [DOI] [PubMed] [Google Scholar]
- Wang LI, Giovannucci EL, Hunter D, Neuberg D, Su L, Christiani DC. Dietary intake of cruciferous vegetables, glutathione S-transferase (GST) polymorphisms and lung cancer risk in a Caucasian population. Cancer Causes Control. 2004;15:977–985. doi: 10.1007/s10552-004-1093-1. [DOI] [PubMed] [Google Scholar]
- Ye L, Dinkova-Kostova AT, Wade KL, Zhang Y, Shapiro TA, Talalay P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin Chim Acta. 2002;316:43–53. doi: 10.1016/s0009-8981(01)00727-6. [DOI] [PubMed] [Google Scholar]
- Yoxall V, Kentish P, Coldham N, Kuhnert N, Sauer MJ, Ioannides C. Modulation of hepatic cytochromes P450 and phase II enzymes by dietary doses of sulforaphane in rats: Implications for its chemopreventive activity. Int J Cancer. 2005;117:356–362. doi: 10.1002/ijc.21191. [DOI] [PubMed] [Google Scholar]
- Zhang Y. Cancer-preventive isothiocyanates: Measurement of human exposure and mechanism of action. Mutat Res. 2004;555:173–190. doi: 10.1016/j.mrfmmm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Talalay P. Anticarcinogenic activities of organic isothiocyanates: Chemistry and mechanisms. Cancer Res. 1994;54(7 Suppl):1976s–1981s. [PubMed] [Google Scholar]
- Zhang Y, Kolm RH, Mannervik B, Talalay P. Reversible conjugation of isothiocyanates with glutathione catalyzed by human glutathione transferases. Biochem Biophys Res Commun. 1995;206:748–755. doi: 10.1006/bbrc.1995.1106. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yao S, Li J. Vegetable-derived isothiocyanates: Anti-proliferative activity and mechanism of action. Proc Nutr Soc. 2006;65:68–75. doi: 10.1079/pns2005475. [DOI] [PubMed] [Google Scholar]
- Zhao B, Seow A, Lee EJ, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Dietary isothiocyanates, glutathione S-transferase -M1, -T1 polymorphisms and lung cancer risk among Chinese women in Singapore. Cancer Epidemiol Biomarkers Prev. 2001;10:1063–1067. [PubMed] [Google Scholar]
- Zhou C, Poulton EJ, Grun F, Bammler TK, Blumberg B, Thummel KE, Eaton DL. The dietary isothiocyanate sulforaphane is an antagonist of the human steroid and xenobiotic nuclear receptor. Mol Pharmacol. 2007;71:220–229. doi: 10.1124/mol.106.029264. [DOI] [PubMed] [Google Scholar]