Abstract
Background
Expanding the population’s access to colonoscopy screening can reduce colorectal cancer disparities. Innovative strategies are needed to address the prevailing 50% colonoscopy screening gap, partly attributable to inadequate specialist workforce. This study examined the quality of colonoscopies by primary care physicians (PCPs) with standby specialist support at a licensed ambulatory surgery center.
Methods
Retrospective data on 10,958 consecutive colonoscopies performed by 51 PCPs on 9815 patients from October 2002 to November 2007 were used to calculate the rates of cecal intubation, detection of polyps, adenomas, advanced neoplasia and cancer, adverse events, and time taken for endoscope insertion and withdrawal. The center’s protocol requires a 2-person technique (using a trained technician), polyp search and removal during both scope insertion and withdrawal, and onsite expert always available for rescue assistance (either navigational or therapeutic).
Findings
Mean patient age was 58.3 (±10.9) years, 48.0% were male, and 48.1% African-American. The cecal intubation rate was 98.1%, polyp detection rate 63.1%, hyperplastic polyp 27.5%, adenoma 29.9%, advanced neoplasia 5.7%, cancer 0.63%, major adverse events 0.06% (including 2 perforations; no death). Mean insertion and withdrawal times were 14.4 (±9.3) and 10.9 (±6.8) minutes, respectively; 13.2 (±8.6) and 8.0 (±4.5) minutes without polyps found, and 15.1 (±9.6) and 12.5 (±7.3) minutes when ≥1 polyp was found.
Conclusions
In the largest published study of PCP-performed colonoscopies with standby specialist support, we observed performance quality indicators and lesion detection rates that are comparable to documented rates for experienced gastroenterologists. Systems that use PCPs with specialist backup support enable high-quality colonoscopy performance by PCPs.
Keywords: screening colonoscopy, primary care physicians, colorectal neoplasms, colonic polyps, adenomatous polyps, patient safety, performance quality, procedure time
Screening colonoscopy of asymptomatic adults aged >50 years enables timely removal of adenomas and other precancerous lesions. Studies suggest that this may reduce incident colorectal cancers (CRC) by 60% to 90%;1–3 about 149,000 new cases and 50,000 deaths annually.4 The American Cancer Society recommends colonoscopy as 1 of 4 screening strategies.5 The American College of Gastroenterology (ACG) and the American Society for Gastrointestinal Endoscopy (ASGE) prefer colonoscopy over other screening methods for average-risk individuals after 50 years of age to facilitate the prevention of proximal colon cancers which account for about one-third of all colorectal cancers.6
Despite mounting evidence of the superiority of colonoscopy over other screening tests and of the cost-effectiveness of colonoscopy screening relative to treating cancer cases,6–9 colorectal cancer screening guidelines continue to recommend a range of screening strategies, reflecting in part the widely acknowledged shortage of endoscopy specialists.10 Innovative approaches such as utilizing primary care physicians (PCPs) could help to address the colonoscopy screening gap as well as meet the rapidly expanding demand.10
PCPs perform a small fraction of colonoscopies; 2% nationally in 2002, and 5.7% in South Carolina in 2005.11 Most cohort studies of PCP-performed colonoscopy are handicapped by poorly documented training processes, performance settings, and practice protocols. Highly variable quality of performance is documented, even among endoscopists.12 A high-quality colonoscopy requires both technical expertise and a thorough inspection of the colonic mucosa, which calls for patience and skill to navigate through the folds and twists of the colon. Because of the strong link between performance quality and the CRC protective effect of screening colonoscopies, the ASGE/ACG Taskforce on Quality in Endoscopy has established quality indicator norms, including cecal intubation rates (>95%), withdrawal time (mean ≥6 minutes), adenoma detection rates (≥25% in men and ≥ 15% in women), and perforation rates (<0.1%).13 Evidence regarding the quality of PCP-performed colonoscopies is limited, and results from the few existing studies are equivocal. 14–17 A recent meta-analysis was able to identify only 12 studies documenting PCP performance quality; none of the studies had documented the associated training process, performance setting, and access to technical support.17 Our study documents these contextual factors concurrent with the quality indicators to clarify the context in which the reported efficacy and quality indicators were achieved. We report on 10,958 consecutive colonoscopies performed by PCPs in a highly structured and technically supported endoscopy center environment.
METHODS
Setting
Since 2001, the South Carolina Medical Endoscopy Center (SCMEC), a licensed ambulatory surgery center for colonoscopy, trains and assists PCPs to conduct colonoscopies for their patients in a highly structured and technically supported practice setting. The training process, post-training clinical performance protocol, and technical support mechanisms are designed to compensate for PCPs’ lack of formal gastroenterology training. The SCMEC’s training protocol consists of didactic instruction, use of models and simulators, and hands-on assistance by an expert up to the ASGE-specified number of procedures for hospital credentialing (since August 2007, 140 procedures).18 The expert is a gastroenterologist or colorectal surgeon, or the director of the center’s colonoscopy training program. The director is a board-certified internist, trained, and credentialed for colonoscopy at the University of South Carolina Medical School teaching hospital. Over the course of training the PCP, hands-on assistance is gradually reduced to verbal assistance, sporadic manual assistance to navigate difficult turns, and therapeutic assistance to remove large, flat or cecal polyps. Regardless of PCP training status, the SCMEC protocol requires a 2-person technique for all PCPs. During the training procedures, the expert advances the endoscope, whereas the PCP visualizes and manipulates the tip deflection and hand-piece to search and remove polyps. Every PCP colonoscopy thus benefits from: (a) the dexterity of 2 “right-hands,” and, (b) the skill of a second technically trained person to provide operational and clinical support. After training, PCPs perform their cases at the SCMEC (with an experienced gastrointestinal [GI] technician assisting with scope manipulation), and an onsite expert available for backup assistance (but engaged in training other PCPs, performing their own procedures, or performing quality assurance oversight duties). In-house evaluation of the frequency of expert rescue assistance among post-training procedures yielded a rate of 14.0% (manual or therapeutic assistance) among 406 consecutive post-training procedures performed by PCPs from November 2006 to February 2007.19 Colonoscopies are conducted using the Olympus America video-colonoscope 160 series, predominantly PCF 160 AL with variable stiffness. Consistent with the state of art, the standard bowel preparation regimen is prescribed (currently, 4 Dulcolax tablets and 2 doses of 10 oz. citrate of magnesia, 4 hours apart, with additional dietary and fluid intake guidelines). Each patient receives a note itemizing all the bowel preparation instructions, along with item-wise explanation by the PCP. These instructions are reinforced by the SCMEC nurse calling the patient 3 days prior to the procedure. The sedation protocol consists of propofol slow intravenous administration titrated to patient response.
Study Sample/Data Sources
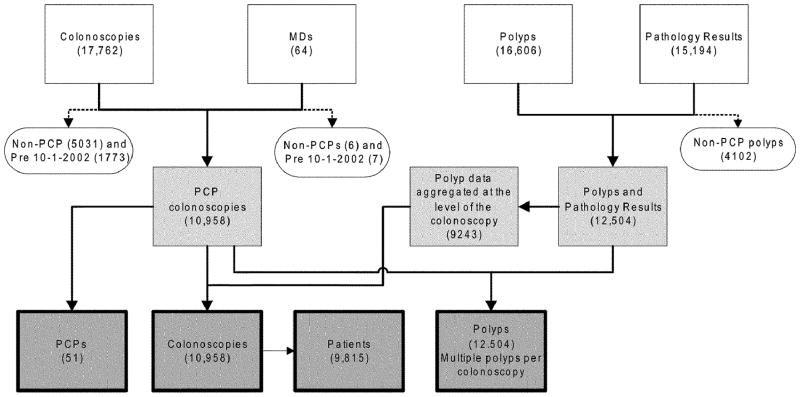
The PCPs and patient population are drawn from a 70-mile radius around Columbia, South Carolina. De-identified data on all screening colonoscopies conducted from October 1, 2002 up to November 1, 2007 were extracted from the SCMEC’s administrative and clinical databases. (Although the SCMEC’s PCP training had started earlier, our study cohort begins in October 2002 when standardized patient medical records were implemented to capture all currently recognized indicators of performance quality, including procedure times.) The SCMEC has expanded its electronic data documentation formats over time to include fields such as patient’s race, polyp histology, and reasons for incomplete exams. The study team populated these missing data on earlier cases from patient charts. Figure 1 shows the distribution of all colonoscopies conducted at the center, and the inclusion/exclusion criteria leading upto the study sample of 10,958 consecutive colonoscopies conducted by 51 PCPs during the study period. The study was approved by the University of South Carolina’s Institutional Review Board.
FIGURE 1.
Clinical database sources merged to create the study analytic databases, showing exclusion and inclusion criteria. Clear boxes represent the endoscopy center’s source databases; clear ovals show excluded cases and criteria for exclusion; lightly shaded boxes are the intermediate, merged datasets restricted to study cases; the dark-shaded boxes show the final denominators of PCPs, patients, colonoscopies and polyps used for analysis.
To assess data quality, a 2% random sample of patient charts was selected (using the RANUNI function in SAS, Version 9.0) and reviewed to verify 12 key variables (procedure date; patient age; city; zip code; performing physician; appendix seen; times procedure started, cecum was intubated, and procedure ended; polyp size, and distance from anal verge). We found an error rate of 0.3% which confirmed the accuracy of the database relative to patient charts. Standard logic and range checks were applied to identify and correct obvious typographical errors (eg, year of birth yielding patient age >100 years).
Under the SCMEC protocol, cecal intubation or satisfactory completion is defined based on either, or both, of the following criteria, as appropriate: (a) visualizing the appendiceal orifice, and when not seen, visualizing both cusps of the ileocecal valve or crow foot in the cecum (photographs documented in all cases), and (b) intubating the ileal tip and identifying lymphoid follicles to verify that complete navigation of the colon was achieved. A board-certified colorectal surgeon evaluated the photographic evidence of all 1112 PCP colonoscopies conducted from July 1, 2006 to June 30, 2007, which were recorded by the PCP as “cecal intubation completed;” 99.6% (1108 cases) were verified as accurate.19 The SCMEC training protocol emphasizes a careful and gradual polyp search and removal during both insertion and withdrawal, because polyps found during insertion are sometimes lost to visualization during scope withdrawal. Therefore, the SCMEC protocol requires documentation of both insertion and withdrawal times.
We calculated insertion time, withdrawal time, and total time (all in minutes). We present the overall cecal intubation rate, the US Multi-Society Task Force criterion-adjusted intubation rate (excluding incomplete cases because of severe colitis and poor preparation),20 and the circumstance-adjusted cecal intubation rate (excluding incomplete cases because of poor preparation, tortuous colon, obstruction or stricture, severe colitis or diverticulosis, vital sign instability, prior surgical removal of the cecum, and patient discomfort).21 We report on major adverse events (perforation, bleeding or other complication requiring a hospital/emergency room referral).22 We present percent of patients with lesions classified by a combination of polyp morphology and histology;23,24 ie, any polyp (growth with normal histology, hyperplastic, adenomatous or cancerous histology), adenomas (adenomatous histology with tubular, serrated, villous or tubulovillous features, or cancer), hyperplastic polyps, advanced neoplasms (adenomas ≥10 mm in diameter, villous histologic features, high-grade dysplasia or cancers), cancer, and carcinoids. We also present mean adenomas per case and mean advanced neoplasms per case.
Statistical Methods
Descriptive statistics are presented for the patient and PCP samples. Rates and proportions are used to present cecal intubation and lesion detection rates. Means (±SD) were used to present procedure times and mean adenomas detected per subject.
RESULTS
Table 1 shows the sample distribution by demographic characteristics of the performing physicians and patients. Among physicians, 21.6% were female, 29.4% African American, and 96.1% board certified, almost all in family medicine or internal medicine. Their mean age was 47.6 years (±8.9), and their mean colonoscopy volume was 214.9 procedures (±209.1; median = 124, range = 2–733). Of 9815 patients, 52% were female, 48.1% were African American, and their mean age was 58.3 years (±10.9).
TABLE 1.
Demographic Characteristics of Physicians and Patients
| Physicians | |
| Total | 51 |
| Gender—male | 40 (78.4%) |
| Race | |
| White | 33 (64.7%) |
| Black | 15 (29.4%) |
| Other | 3 (5.9%) |
| Specialty | |
| Internal medicine | 21 (41.2%) |
| Family medicine | 29 (56.9%) |
| Ob/gyn | 1 (1.9%) |
| Board certified | 49 (96.1%) |
| Age (yr) | 47.6 (±8.9); median = 47.0 |
| Years since MD degree | 19.8 (±9.1); median = 17.5 |
| No. colonoscopies | 214.9 (±209.1); median = 124.0 |
| Patients | |
| Total patients | 9815 |
| Gender—male | 4709 (48.0%) |
| Race* | |
| White | 4891 (51.4%) |
| Black | 4576 (48.1%) |
| Other | 55 (0.6%) |
| Age (yr) | 58.3 (±10.9) |
| No. colonoscopies | |
| 1 | 8790 (89.6%) |
| 2 | 919 (9.4%) |
| 3 + | 106(1.1%) |
| Total colonoscopies | 10,958 |
Race information was missing for 293 patients.
Table 2 shows the performance quality, case yield, and safety indicators across all colonoscopies, including the training procedures. Cecal intubation was achieved in 98.1% of total cases. The rate increased to 98.8% after applying the US Multi-Society Task Force adjustment,20 and to 99.0% for the circumstance-adjusted rate.21 Among cecum-intubated cases, 63.1% had ≥1 polyp(s) (including those with normoplastic tissue or lymphoid aggregates), 27.5% had hyperplastic polyps, 29.9% had adenomas (men = 34.6%; women = 25.4%), 5.7% had advanced neoplasms, and 0.15% had carcinoids (16 cases). The advanced neoplasm group included 69 cancers (0.63% of total colonoscopies). Mean adenomas per subject was 0.46 (±0.86 SD), and advanced neoplasms, 0.07 (±0.34 SD). Mean procedure time was 25.2 minutes (±11.7), insertion time 14.4 minutes (±9.3), and withdrawal time 10.9 minutes (±6.8).
TABLE 2.
Primary Care Physicians’ Colonoscopy Performance Quality: Cecal Intubation, Polyp, Adenoma and Cancer Detection Rates, and Procedure Times
| No. colonoscopies | 10,958 |
| No. performing physicians | 51 |
| Cecal intubation rate* | |
| Unadjusted cecal intubation rate (%) | 98.1 |
| US MSTF cecal intubation rate (%) | 98.8 |
| Circumstance-adjusted cecal intubation rate (%) | 99.0 |
| Lesion detection rates† | |
| Any polyp, incl. normoplastic polyps (% of cases) | 63.1 |
| Hyperplastic polyp (% of cases) | 27.5 |
| Adenoma (% of cases)‡ | 29.9 |
| Mean adenomas per subject (mean, SD) | 0.46 (±0.86) |
| Advanced neoplasms (%)‡ | 5.7 |
| Mean advanced neoplasms (mean, SD) | 0.07 (±0.34) |
| Cancer (% of cases) | 0.63 |
| Carcinoids (% of cases) | 0.15 |
| Procedure times: (mean, SD)§ | |
| Colonoscopies with no polyp found | 3804 |
| Insertion time | 13.2 (±8.6) |
| Withdrawal time | 8.0 (±4.5) |
| Total procedure time | 21.2(9.9) |
| Colonoscopies with polyp(s) found | 6663 |
| Insertion time | 15.1 (±9.6) |
| Withdrawal time | 12.5 (±7.3) |
| Total procedure time | 27.6 (±12.0) |
| All colonoscopies | 10,467 |
| Insertion time | 14.4 (±9.3) |
| Withdrawal time | 10.9 (±6.8) |
| Total procedure time | 25.2 (±11.7) |
Cecum intubation rate is based on 10,952 procedures for which data were available; US Multi-Society Task Force rate excludes from the denominator, 78 incomplete colonoscopy cases (cecal intubation not achieved) with severe colitis (3 cases) and poor preparation (75 cases).20 The circumstance-adjusted rate excludes from the denominator incomplete colonoscopy cases with a medical/clinical indication for stopping the colonoscopy procedure (n = 107).21
Polyp detection rate is based on 10,745 procedures where the cecum was intubated; hyperplastic, adenoma, and advanced adenoma rates were based on 8751 procedures for which histology data are available.
Adenomas include polyps with adenomatous histology and tubular, serrated, villous or rubulovillous features, or cancers. Advanced neoplasms include adenomas 10 mm or more in diameter, villous histologic features, high-grade dysplasia, or cancer.
Procedure times for 10,467 procedures where cecal intubation was achieved and time data were available.
MSTF indicates Multi-Society Task Force.
Because polyp removal consumes time, colonoscopies were categorized into those with ≥ 1 polyp(s) (regardless of histology), and those without a polyp. The corresponding procedure times when no polyp was found (n = 3804) were 21.2 (±9.9), 13.2 (±8.6), and 8.0 (±4.5) minutes, respectively, and when ≥1 polyp(s) were found, 27.6 (±12.0), 15.1 (±9.6), and 12.5 (±7.3) minutes, respectively. Differences between cases with and without polyps were statistically significant (all P < 0.0001). Among cases in which cecal intubation was either not achieved or not recorded (n = 27), the corresponding mean procedure times were 34.6 (±15.2), 23.1 (±15.5), and 11.5 (±7.8) minutes, respectively (not shown).
The major adverse event rate was 0.06% (6 cases), no death. The adverse events were 2 perforations (of diverticulae, unrelated to scope advancement or manipulation or polypectomy), 1 bleeding (5 days after a large polyp removal), 1 postpolypectomy syndrome, 1 aspiration, 1 renal failure (treated for 10 days in-hospital without dialysis and discharged without sequelae). All patients were discharged without residual major morbidity. Being a licensed ambulatory surgery center, the SCMEC is equipped to manage complications appropriately prior to hospital or emergency room (ER) referral.
DISCUSSION
Reporting on the performance of a novel PCP-delivered colonoscopy program, we found that the rates of cecal intubation and adenoma detection as well as mean endoscopy withdrawal time meet or exceed the ASGE benchmarks. Uniquely, the SCMEC’s protocol requires colonic inspection for polyps during both intubation and scope withdrawal. The ASGE recommends a mean withdrawal time for a normal examination of at least 6 minutes. Withdrawal times are directly linked to adenoma detection rates, because a more careful inspection leads to a greater yield.24–26 Gastroenterologists who adopted an 8-minute minimum withdrawal protocol, doubled their total adenoma and advanced neoplasia detection rates.25 Because specialists who complete procedures in less than the minimum recommended time do poorly, as do trainee endoscopists, Rex emphasizes that, “… colonoscopy is a highly operator-dependent procedure.”10 There also is emerging evidence that inspection during endoscope insertion may increase adenoma detection.27 Compared with the ASGE-recommended minimum withdrawal time of 6 minutes when no polyp is found, our cohort had a mean withdrawal time of 8 minutes (when no polyp was found). Polyp yield is also influenced by the endoscopist’s skill level.10 In the study setting, the performing PCP’s skill is enhanced by the 2-person technique, which provides the benefit of right-handed dexterity for both “hands”; the PCP’s right (or dominant) hand managing tip deflection, and the assistant’s dominant hand advancing the shaft guided by the PCP. The SCMEC protocol in using the GI technicians is consistent with the Society for Gastrointestinal Nurses and Associates policy guidelines.28 A recent study documented that even for attending gastroenterologists at academic medical centers, having a GI fellow serve as a second observer improved the adenoma detection rate.29 Unlike the SCMEC protocol, however, they do not document a 2-person technique in managing the endoscope, one (trainer-expert or technician) to advance the scope and the performing physician to manage tip deflection and polyp removal.
Our observed cecal intubation rate (98.1%) is higher than the ASGE standard of 95% for screening colonoscopies, which is generally met by gastroenterologists.21,24 Compared with the documented (highly variable) rates of 49% to 95% for PCP-performed colonoscopies,16,17 our study rate is higher and more uniform across physicians. Variations in cecal intubation rates are due to physician variables (skill including dexterity, training level), patient variables (age, gender, body mass index, past surgeries, tortuous colon, pain threshold, and response to anesthesia), and adequacy of bowel preparation.24,30 Adequate bowel preparation facilitates higher cecal intubation rates.21 In our cohort, 91% of patients were documented to have excellent to fair bowel preparation, which is relatively high. We suggest that these PCPs’ high performance on cecal intubation (and lesion detection) is attributable to a combination of factors: a uniformly applied protocol of bowel preparation that is regularly updated to the state-of-art, a standard protocol for ensuring that patients understand and are provided timely instruction reminders, and PCPs performing in a highly structured and supported environment designed to compensate for their lack of formal gastroenterology training. A careful and exhaustive mucosal inspection, and high lesion yield rates are also facilitated by the SCMEC’s emphasis on a team approach, where all persons present, including the PCP, the assisting technician, the nurse anesthetist, and the documenter observe the video-screen, thus increasing the polyp detection rate and ensuring cecal intubation. Finally, if the expert determined that a case is high-risk, the PCP will not perform the procedure. Conditions that trigger patient referral to the expert are frail or elderly patients aged over 75 years, and presence of acute symptoms or medically unstable conditions.
The study PCPs’ high rates of adenoma detection in men (34.6%) and women (25.4%), exceeding the ASGE benchmarks (men ≥25%; women ≥15%>),12 may be due to patient variables such as age gender, and race. African Americans (AAs) constitute about half of this study population, and it is thought that adenoma rates are often higher in AAs.31,32
The major adverse event rate of 0.06% (that included 2 perforations, both perforated diverticulae, unrelated to scope advancement or polyp removal; no death) is statistically similar to the documented 0.067% perforation rate for specialist-performed screening colonoscopies.33 Two large-scale studies on screening procedures reported death rates of 0.074/1000 and 0.34,35 The dynamics of perforation due to barotrauma are documented, as are mechanical accidents during navigation and ileal intubation, and electrocautery/thermal injury mishaps.36–38 These injuries are linked primarily to suboptimal dexterity and technical judgment. Again, the study PCPs’ low complication rate is plausibly because of the 2-person technique, specialist backup, and referral of potentially high-risk patients to the specialist for evaluation.
Our study has some limitations. As with most clinical studies, we had some missing data because of using retrospective data from a clinical practice setting and merging administrative and clinical data files, even though we were able to enter missing data from patient charts. Although this reduced some denominators (Table 2), the magnitude is negligible. Another study limitation is the lack of concurrent comparisons with expert-performed colonoscopies in the same setting (because of inadequate financial resources to populate the missing data fields from patient records for these procedures). Also, because our series had disproportionate numbers of African Americans, our findings may not be comparable to other studies with smaller proportions of African Americans. Finally, we are unable to present data on “independent PCP-performance;” that is, cecal intubation and lesion yield status prior to the specialist’s rescue assistance, because intraprocedure events without clinical significance are not documented in patient charts.
Beyond these limitations, our study makes a major contribution by documenting the efficacy and patient safety profile of PCP-performed colonoscopies with standby specialist support, including training colonoscopies, concurrent with the clinical practice protocol, and systemic safeguards that generated the performance. It documents a large sample of 10,958 consecutive PCP-performed procedures across 51 physicians, including 3827 training cases, analyzed at a high level of data quality (database accuracy verified from patient charts, and missing data populated from patient charts). The evidence shows that under conditions of technical support and specialist backup, PCPs’ quality and safety measures are comparable to those of well-performing gastroenterologists.20,23,24
A recent meta-analysis of 12 studies of PCP-performed colonoscopies spanning 1992 to 2006 reported an overall cecal intubation rate of 90.5% (when performed under conscious sedation), an adenoma detection rate of 28.9%, and an adenocarcinoma detection rate of 1.7%, with substantial variability between studies.17 One of their studies used data from the same setting as the current study, reporting somewhat different rates because of differences in time periods and completeness of the data (the current study used updated data after populating missing data fields with primary data from patient charts). The remaining studies of their meta-analysis are limited by the lack of contextual information (on training protocols, competency credentialing standards, and performance settings), which impedes valid comparisons across studies and with specialist-performed colonoscopies. Undocumented variations in these parameters together with disparate performance settings (including physician offices) may account for the wide variations in the quality of PCP colonoscopies that are documented across studies.
A few studies report a higher risk of colorectal cancer following a negative examination when conducted by a PCP or nongastroenterologist.13–15 These outcomes may be driven in part by specific circumstances that are not documented, such as the performance setting (physician office versus hospital; rural vs. urban), lack of uniform practice protocols and support systems, lack of standardized training protocols, and time-related changes in the state-of-art (sedation technology and instrumentation). In any event, it is well-documented that when quality standards are not met, there is an increased risk of missed precancerous lesions and, subsequently, frank cancer.39
Although colonoscopy screening is documented to confer a high degree of protection against CRC in clinical trials, its population-based field efficacy is contingent on a high-quality procedure39 and adequate colonoscopy capacity in the health system.40,41 Given the limited availability of GI specialists, and the negligible annual increments to their numbers,42,43 innovations are needed to expand capacity rapidly. The SCMEC model offers a mechanism to use PCPs for colonoscopy screening, without compromising quality or safety, achieved by leveraging the expertise of an onsite expert. Between rescue assistance episodes (14% of trained PCPs’ cases), the expert remains engaged in other value-added activities, including training/supervising trainee physicians, performing quality management activities, or performing endoscopies on their own patients. Economically, this model has sustained well over 9 years in a service facility setting with no external subsidies or funding sources other than reimbursements by payers. The SCMEC’s payer mix consists of 30.2% Medicare, 4.3% Medicaid, 57.1% private insurance, and 8.4% uninsured. The specialist is salaried, paid by pooling the retainer fees paid by PCPs on a per-case basis, with any deficit covered from the facility fee received by the center.
Documentation of the protocols underlying these PCPs’ high-quality performance provides an empirical foundation for expanding colonoscopy capacity. Of the estimated annual need for 23 million colonoscopies, about half (12 million) are conducted by the available 11,000 gastroenterologists, 3500 colorectal surgeons, and general surgeons, the latter performing about 7.2% of colonoscopies.5,11,40 Addressing the colonoscopy gap is particularly important because of steep declines in sigmoidoscopy screening and fecal occult blood testing in recent years.44,45 To address this gap, an additional 7340 specialists are required.40 The annual graduation of 380 gastroenterology and colorectal surgery fellows42 addresses barely 5% of the capacity gap43 (if retirement and attrition among the existing pool is disregarded). Therefore, specialists alone can neither address the current 50% screening gap nor the growing demand because of the following 2 factors: changes in AA screening guidelines (ACG recommends AA screening begin at age 45),46 and aging of the US population. PCPs perform about 2% of colonoscopies nationwide,11 and a small fraction of graduating PCPs acquire a credentialing level of colonoscopy training during their residency (eg, 0.6% of family medicine graduates).47 Our study provides a foundation to systematically use PCPs as specialist-extenders, facilitating high-quality performance through appropriate technical and specialist backup support.
A specialist-led, PCP-driven capacity expansion may be particularly relevant for reducing colorectal cancer disparities. African Americans, in particular, may benefit from such an expansion because of their unique risk profile (preponderance of, and rapid progress of proximal colon adenomas,30,48 earlier age of onset, and poorer outcomes at all cancer stages).49,50 A prospective study is needed to confirm whether trained PCPs truly add to colonoscopy capacity or whether specialists’ time is merely displaced from performing procedures to supporting PCPs.
Acknowledgments
Supported by 3 grants from the National Cancer Institute: Grant: 3U01CA114601–03S5 Pilot Project under the Community Networks Program parent grant awarded to the South Carolina Cancer Disparities Community Network (SCCDCN) (U01 CA114601–01), and K05 CA136975, Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute to JR Hébert. Additional support was received from the University of South Carolina’s Division of Health Sciences and the Center for Colon Cancer Research.
The authors thank the South Carolina Medical Endoscopy Center for sharing de-identified data for the study. We acknowledge data entry assistance by AH Mansaray and Philip Cavicchia, graduate research assistants. We are grateful to the National Cancer Institute which funded this work through 2 grants: Grant 3U01CA114601-03S5 Pilot Project under the Community Networks Program parent grant awarded to the South Carolina Cancer Disparities Community Network (SCCDCN) (1 U01 CA114601-01), and K05 CA136975, Established Investigator Award in Cancer Prevention and Control from the Cancer Training Branch of the National Cancer Institute to J. R. Hebert. We are also grateful to the University of South Carolina’s Division of Health Sciences and the Center for Colon Cancer Research for providing additional financial support.
References
- 1.Winawer SJ, Zauber AG, Ho MN, et al. The National Polyp Study Workgroup. Prevention of colorectal cancer by colonoscopic polypectomy. N Engl J Med. 1993;329:1977–1981. doi: 10.1056/NEJM199312303292701. [DOI] [PubMed] [Google Scholar]
- 2.Singh H, Turner D, Xue L, et al. Risk of developing colorectal cancer following a negative colonoscopy examination: evidence for a 10-year interval between colonoscopies. JAMA. 2006;295:2366–2373. doi: 10.1001/jama.295.20.2366. [DOI] [PubMed] [Google Scholar]
- 3.Ransohoff DF. How much does colonoscopy reduce colon cancer mortality? Ann Intern Med. 2009;150:50–52. doi: 10.7326/0003-4819-150-1-200901060-00308. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. [Accessed June 16, 2009];Guidelines for the Early Detection of Cancer. Available at: http://www.cancer.org/docroot/ped/content/ped_2_3x_acs_cancer_detection_guidelines_36.asp.
- 6.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2008. Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 7.Lieberman DA, Weiss DG, Bond JH, et al. Veterans Affairs Cooperative Study Group 380. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 8.Sonnenberg A, Delco F, Inadomi JM. Cost-effectiveness of colonoscopy in screening for colorectal cancer [comment] Ann Intern Med. 2000;133:573–584. doi: 10.7326/0003-4819-133-8-200010170-00007. [DOI] [PubMed] [Google Scholar]
- 9.Vijan S, Hwang I, Inadomi J, et al. The cost-effectiveness of CT colonography in screening for colorectal neoplasia. Am J Gastroenterol. 2007;102:380–390. doi: 10.1111/j.1572-0241.2006.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voelker R. Colonoscopy: prime time for primary care? JAMA. 2009;301:921–922. doi: 10.1001/jama.2009.238. [DOI] [PubMed] [Google Scholar]
- 11.Seeff L. Final report. Seattle, WA: Battelle Centers for Public Health Research and Evaluation; 2006. South Carolina survey of endoscopic capacity. [Google Scholar]
- 12.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 13.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 14.Bressler B, Paszat LF, Chen ZL, et al. Rates of new or missed colorectal cancers after colonoscopy and their risk factors: a population-based analysis. Gastroenterology. 2007;132:96–102. doi: 10.1053/j.gastro.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Rabeneck L, Paszat L, Saskin R. Endoscopist specialty is associated with incident colorectal cancer after a negative colonoscopy. Clin Gastroenterol Hepatol. 2010;8:275–279. doi: 10.1016/j.cgh.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 16.Kolber M, Szafran O, Suwal J, et al. Outcomes of 1949 endoscopic procedures performed by a Canadian rural family physician. Can Fam Physician. 2009;55:170–175. [PMC free article] [PubMed] [Google Scholar]
- 17.Wilkins T, LeClair B, Smolkin M, et al. Screening colonoscopies by primary care physicians: a meta-analysis. Ann Fam Med. 2009;7:56–62. doi: 10.1370/afm.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Faigel DO, Baron TH, Lewis B, et al. [Accessed August 10, 2009];Ensuring Competence in Endoscopy. 2007 Available at: http://www.asge.org/WorkArea/showcontent.aspx?id=3384.
- 19.Sweeney BW, Lloyd SC. An onsite expert is required for a quality endoscopy performed by primary care physicians. Paper presented at: The 2007 Digestive Disease Week Meeting (Sponsored by the American Gastroenterological Association, American Society of Gastrointestinal Endoscopy, American Association for the Study of Liver Disorders and The Society for Surgery of the Alimentary Tract); May 20–23, 2007; Washington, DC. [Google Scholar]
- 20.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U. S. Multi-Society Task Force on colorectal cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 21.Aslinia F, Uradomo L, Steele A, et al. Quality assessment of colonoscopic cecal intubation: an analysis of 6 years of continuous practice at a university hospital. Am J Gastroenterol. 2006;101:721–731. doi: 10.1111/j.1572-0241.2006.00494.x. [DOI] [PubMed] [Google Scholar]
- 22.Kavic SM, Basson MD. Complications of endoscopy. Am J Surg. 2001;181:319–332. doi: 10.1016/s0002-9610(01)00589-x. [DOI] [PubMed] [Google Scholar]
- 23.Kudo SI, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68(suppl 4):S3–S47. doi: 10.1016/j.gie.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 24.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 25.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–1098. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 26.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856–861. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 27.Morini S, Hassan C, Zullo A, et al. Detection of colonic polyps according to insertion/withdrawal phases of colonoscopy. Int J Colorectal Dis. 2009;24:527–530. doi: 10.1007/s00384-009-0633-2. [DOI] [PubMed] [Google Scholar]
- 28.Society of Gastroenterology Nurses and Associates. [Accessed August 10, 2009];SGNA Position Statement: Manipulation of Endoscopes During Endoscopic Procedures. 2006 Available at: http://www.sgna.org/resources/ManipulationofEndoscopesrevisedbodversion.pdf. [PubMed]
- 29.Rogart JN, Siddiqui UD, Jamidar PA, et al. Fellow involvement may increase adenoma detection rates during colonoscopy. Am J Gastroenterol. 2008;103:2841–2846. doi: 10.1111/j.1572-0241.2008.02085.x. [DOI] [PubMed] [Google Scholar]
- 30.Cotton PB, Connor P, McGee D, et al. Colonoscopy: practice variation among 69 hospital-based endoscopists. Gastrointest Endosc. 2003;57:352–357. doi: 10.1067/mge.2003.121. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman DA, Holub JL, Moravec MD, et al. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417–1422. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rex DK, Khan AM, Shah P, et al. Screening colonoscopy in asymptomatic average-risk African Americans. Gastrointest Endosc. 2000;51:524–527. doi: 10.1016/s0016-5107(00)70283-5. [DOI] [PubMed] [Google Scholar]
- 33.Arora G, Mannalithara A, Singh G, et al. Risk of perforation from a colonoscopy in adults: a large population-based study. Gastrointest Endosc. 2009;69:654–664. doi: 10.1016/j.gie.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia [comment] N Engl J Med. 2006;355:1863–1872. doi: 10.1056/NEJMoa054967. [DOI] [PubMed] [Google Scholar]
- 35.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–1906. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 36.Woltjen JA. A retrospective analysis of cecal barotrauma caused by colonoscope air flow and pressure. Gastrointest Endosc. 2005;61:37–45. doi: 10.1016/s0016-5107(04)02453-8. [DOI] [PubMed] [Google Scholar]
- 37.Tulchinsky H, Madhala-Givon O, Wasserberg N, et al. Incidence and management of colonoscopic perforations: 8 years’ experience. World J Gastroenterol. 2006;12:4211–4213. doi: 10.3748/wjg.v12.i26.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson ML, Pasha TM, Leighton JA. Endoscopic perforation of the colon: lessons from a 10-year study. Am J Gastroenterol. 2000;95:3418–3422. doi: 10.1111/j.1572-0241.2000.03356.x. [DOI] [PubMed] [Google Scholar]
- 39.Lieberman D. A call to action—measuring the quality of colonoscopy. N Engl J Med. 2006;355:2588–2589. doi: 10.1056/NEJMe068254. [DOI] [PubMed] [Google Scholar]
- 40.Vijan S, Inadomi J, Hayward RA, et al. Projections of demand and capacity for colonoscopy related to increasing rates of colorectal cancer screening in the United States. Aliment Pharmacol Ther. 2004;20:507–515. doi: 10.1111/j.1365-2036.2004.01960.x. [DOI] [PubMed] [Google Scholar]
- 41.Brown ML, Klabunde CN, Mysliwiec P. Current capacity for endoscopic colorectal cancer screening in the United States: data from the National Cancer Institute survey of colorectal cancer screening practices. Am J Med. 2003;115:129–133. doi: 10.1016/s0002-9343(03)00297-3. [DOI] [PubMed] [Google Scholar]
- 42.Graduate medical education. JAMA. 2005;294:1129–1143. [PubMed] [Google Scholar]
- 43.Seeff LC, Manninen DL, Dong FB, et al. Is there endoscopic capacity to provide colorectal cancer screening to the unscreened population in the United States? [comment] Gastroenterology. 2004;127:1661–1669. doi: 10.1053/j.gastro.2004.09.052. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro JA, Seeff LC, Thompson TD, et al. Colorectal cancer test use from the 2005 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2008;17:1623–1630. doi: 10.1158/1055-9965.EPI-07-2838. [DOI] [PubMed] [Google Scholar]
- 45.Phillips KA, Liang SY, Ladabaum U, et al. Trends in colonoscopy for colorectal cancer screening. Med Care. 2007;45:160–167. doi: 10.1097/01.mlr.0000246612.35245.21. [DOI] [PubMed] [Google Scholar]
- 46.Agrawal S, Bhupinderjit A, Bhutani MS, et al. Colorectal cancer in African Americans. Am J Gastroenterol. 2005;100:515–523. doi: 10.1111/j.1572-0241.2005.41829.x. discussion 514. [DOI] [PubMed] [Google Scholar]
- 47.Wilkins T, Jester D, Kenrick J, et al. The current state of colonoscopy training in family medicine residency programs. Fam Med. 2004;36:407–411. [PubMed] [Google Scholar]
- 48.Jarvinen HJ, Aarnio M, Mustonen H, et al. Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer [comment] Gastroenterology. 2000;118:829–834. doi: 10.1016/s0016-5085(00)70168-5. [DOI] [PubMed] [Google Scholar]
- 49.Alexander D, Chatla C, Funkhouser E, et al. Postsurgical disparity in survival between African Americans and Caucasians with colonic adenocarcinoma [comment] Cancer. 2004;101:66–76. doi: 10.1002/cncr.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rex DK, Rawl SM, Rabeneck L, et al. Colorectal cancer in African Americans. Rev Gastroenterol Disord. 2004;4:60–65. [PubMed] [Google Scholar]