Abstract
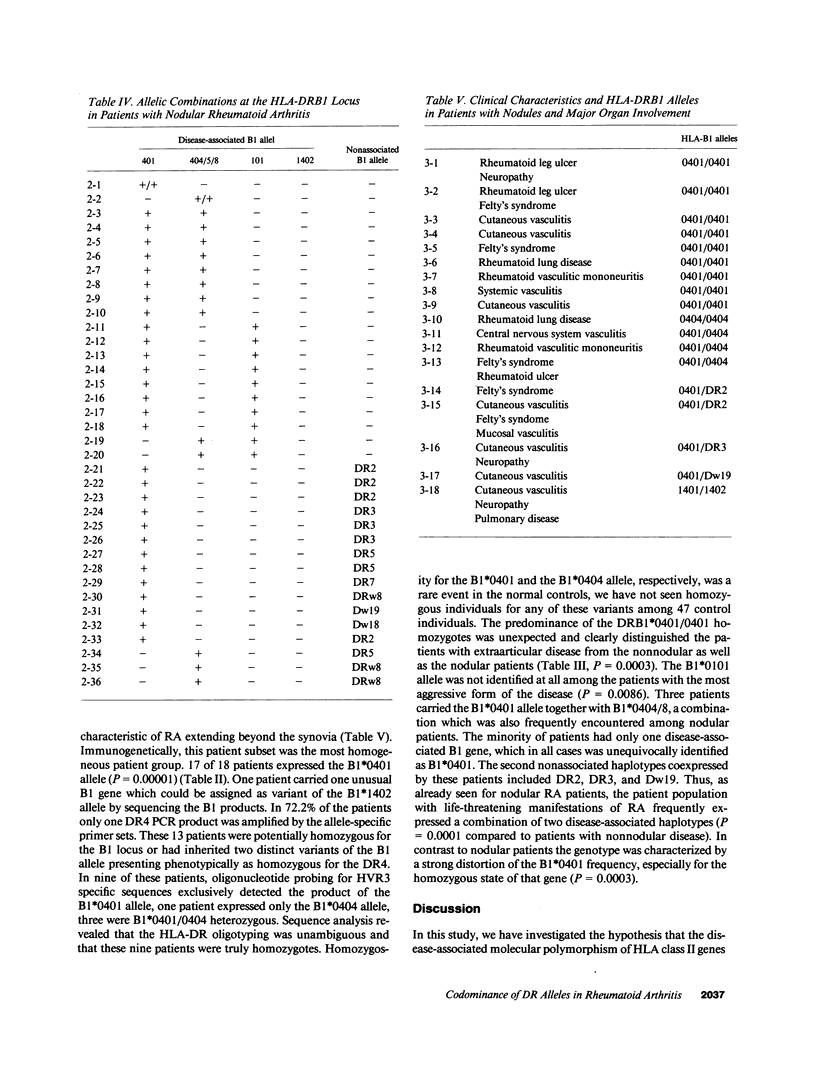
Seropositive rheumatoid arthritis is genetically linked to a group of HLA-DRB1 alleles sharing a sequence motif within the third hypervariable region. Controversy exists over the role of the distinct allelic variants in affecting not only the risk to develop disease, but also in modifying the expression of the disease. We have stratified 81 patients according to their patterns of disease manifestations and identified the HLA-DRB1 alleles by polymerase chain reaction amplification and subsequent oligonucleotide hybridization. To identify precisely the allelic combinations at the HLA-DRB1 locus, homozygosity was confirmed by locus-specific cDNA amplification and subsequent sequencing. Our study demonstrated a high correlation of allelic combinations of disease-associated HLA-DRB1 alleles with the clinical manifestations. Characteristic genotypes were identified for patients who had progressed toward nodular disease and patients who had developed major organ involvement. Rheumatoid nodules were highly associated with a heterozygosity for two disease associated HLA-DRB1 alleles. Homozygosity for the HLA-DRB1*0401 allele was a characteristic finding for RA patients with major organ involvement. Our data suggest a role of the disease-associated sequence motif in determining severity of the disease. The finding of a codominant function of HLA-DRB1 alleles suggests that the biological function of HLA-DR molecules in thymic selection might be important in the pathogenesis of RA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnett F. C., Edworthy S. M., Bloch D. A., McShane D. J., Fries J. F., Cooper N. S., Healey L. A., Kaplan S. R., Liang M. H., Luthra H. S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988 Mar;31(3):315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Gao X. J., Olsen N. J., Pincus T., Stastny P. HLA-DR alleles with naturally occurring amino acid substitutions and risk for development of rheumatoid arthritis. Arthritis Rheum. 1990 Jul;33(7):939–946. doi: 10.1002/art.1780330704. [DOI] [PubMed] [Google Scholar]
- Goronzy J., Weyand C. M., Fathman C. G. Shared T cell recognition sites on human histocompatibility leukocyte antigen class II molecules of patients with seropositive rheumatoid arthritis. J Clin Invest. 1986 Mar;77(3):1042–1049. doi: 10.1172/JCI112358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Shen M., Song Q. L., Merryman P., Degar S., Seki T., Maccari J., Goldberg D., Murphy H., Schwenzer J. Molecular diversity of HLA-DR4 haplotypes. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2642–2646. doi: 10.1073/pnas.83.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregersen P. K., Silver J., Winchester R. J. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987 Nov;30(11):1205–1213. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- Harley J. B., Reichlin M., Arnett F. C., Alexander E. L., Bias W. B., Provost T. T. Gene interaction at HLA-DQ enhances autoantibody production in primary Sjögren's syndrome. Science. 1986 May 30;232(4754):1145–1147. doi: 10.1126/science.3458307. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Nepom B. S., Nepom G. T., Mickelson E., Schaller J. G., Antonelli P., Hansen J. A. Specific HLA-DR4-associated histocompatibility molecules characterize patients with seropositive juvenile rheumatoid arthritis. J Clin Invest. 1984 Jul;74(1):287–291. doi: 10.1172/JCI111413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepom G. T., Hansen J. A., Nepom B. S. The molecular basis for HLA class II associations with rheumatoid arthritis. J Clin Immunol. 1987 Jan;7(1):1–7. doi: 10.1007/BF00915418. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Seyfried C. E., Holbeck S. L., Wilske K. R., Nepom B. S. Identification of HLA-Dw14 genes in DR4+ rheumatoid arthritis. Lancet. 1986 Nov 1;2(8514):1002–1005. doi: 10.1016/s0140-6736(86)92614-0. [DOI] [PubMed] [Google Scholar]
- Petersdorf E. W., Smith A. G., Mickelson E. M., Martin P. J., Hansen J. A. Ten HLA-DR4 alleles defined by sequence polymorphisms within the DRB1 first domain. Immunogenetics. 1991;33(4):267–275. doi: 10.1007/BF00230505. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Access to a messenger RNA sequence or its protein product is not limited by tissue or species specificity. Science. 1989 Apr 21;244(4902):331–334. doi: 10.1126/science.2565599. [DOI] [PubMed] [Google Scholar]
- Schiff B., Mizrachi Y., Orgad S., Yaron M., Gazit E. Association of HLA-Aw31 and HLA-DR1 with adult rheumatoid arthritis. Ann Rheum Dis. 1982 Aug;41(4):403–404. doi: 10.1136/ard.41.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehy M. J., Scharf S. J., Rowe J. R., Neme de Gimenez M. H., Meske L. M., Erlich H. A., Nepom B. S. A diabetes-susceptible HLA haplotype is best defined by a combination of HLA-DR and -DQ alleles. J Clin Invest. 1989 Mar;83(3):830–835. doi: 10.1172/JCI113965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Markussen G., Ek J., Gjerde H., Vartdal F., Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989 Jan 1;169(1):345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N Engl J Med. 1978 Apr 20;298(16):869–871. doi: 10.1056/NEJM197804202981602. [DOI] [PubMed] [Google Scholar]
- Stoflet E. S., Koeberl D. D., Sarkar G., Sommer S. S. Genomic amplification with transcript sequencing. Science. 1988 Jan 29;239(4839):491–494. doi: 10.1126/science.3340835. [DOI] [PubMed] [Google Scholar]
- Svejgaard A., Ryder L. P. HLA genotype distribution and genetic models of insulin-dependent diabetes mellitus. Ann Hum Genet. 1981 Jul;45(Pt 3):293–298. doi: 10.1111/j.1469-1809.1981.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Vollertsen R. S., Conn D. L. Vasculitis associated with rheumatoid arthritis. Rheum Dis Clin North Am. 1990 May;16(2):445–461. [PubMed] [Google Scholar]
- Westedt M. L., Breedveld F. C., Schreuder G. M., D'Amaro J., Cats A., de Vries R. R. Immunogenetic heterogeneity of rheumatoid arthritis. Ann Rheum Dis. 1986 Jul;45(7):534–538. doi: 10.1136/ard.45.7.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Disease-associated human histocompatibility leukocyte antigen determinants in patients with seropositive rheumatoid arthritis. Functional role in antigen-specific and allogeneic T cell recognition. J Clin Invest. 1990 Apr;85(4):1051–1057. doi: 10.1172/JCI114535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand C. M., Goronzy J. J. Mapping of allospecific T-cell recognition sites encoded by the HLA-DR4 beta 1-chain. Hum Immunol. 1989 Feb;24(2):133–143. doi: 10.1016/0198-8859(89)90053-0. [DOI] [PubMed] [Google Scholar]
- Weyand C. M., Hicok K. C., Goronzy J. J. Nonrandom selection of T cell specificities in anti-HLA-DR responses. Sequence motifs of the responder HLA-DR allele influence T cell recruitment. J Immunol. 1991 Jul 1;147(1):70–78. [PubMed] [Google Scholar]
- Willkens R. F., Nepom G. T., Marks C. R., Nettles J. W., Nepom B. S. Association of HLA-Dw16 with rheumatoid arthritis in Yakima Indians. Further evidence for the "shared epitope" hypothesis. Arthritis Rheum. 1991 Jan;34(1):43–47. doi: 10.1002/art.1780340107. [DOI] [PubMed] [Google Scholar]
- Young A., Jaraquemada D., Awad J., Festenstein H., Corbett M., Hay F. C., Roitt I. M. Association of HLA-DR4/Dw4 and DR2/Dw2 with radiologic changes in a prospective study of patients with rheumatoid arthritis. Preferential relationship with HLA-Dw rather than HLA-DR specificities. Arthritis Rheum. 1984 Jan;27(1):20–25. doi: 10.1002/art.1780270104. [DOI] [PubMed] [Google Scholar]