Abstract
Background
In the United States and particularly South Carolina, African-American women suffer disproportionately higher mortality rates than do European-American women. The timeliness of patient adherence to the follow-up of mammographic abnormalities may influence prognosis and survival. Consequently, the purpose of the present investigation was to examine racial differences in the completion and completion time of a diagnostic work-up following a finding of a suspicious breast abnormality.
Methods
Study participants of the Best Chance Network, a state-wide service program that provides free mammography screenings to economically disadvantaged and medically underserved women, were included in the study. Racial differences in tumor characteristics and adherence to recommended work-up were tested using Chi-square and t-tests. Logistic and Cox regression modeling was used to assess the relationship between work-up completion and other factors among African-American and European-American women.
Results
Completion of the work-up was associated with the number of previous procedures and income, with no significant differences noted by race. The amount of time to completion of the work-up was influenced by previous procedures, income, and race. After accounting for completion time, African-American women were 12% less likely than European-American women to complete the recommended work-up (HR=0.88, p-value=0.01).
Conclusion
This study established a racial disparity in the time to completion of a diagnostic work-up among Best Chance Network participants. These findings highlight the importance of understanding factors associated with delays and adherence in completion of recommended work-up when breast abnormalities are detected in mammograms.
Keywords: Mammography, health status disparities, African Americans, medically uninsured, breast neoplasms
Introduction
It is well established that African-American women suffer disproportionately higher mortality rates from breast cancer than do their European-American counterparts (1–3). Previous research has done little to explain why African-American women present at much younger ages, with more aggressive disease (4–7), and experience much higher breast cancer mortality rates than their European-American counterparts(4–9). Factors related to access to screening and treatment explain only part of the excess variability (2;10–12). Many important gaps in our knowledge must be filled before we can devise the best strategies to reduce these racial disparities in breast cancer.
The disparities observed on the national level are even more striking when we focus on outcomes in South Carolina. In 2000, breast cancer incidence rates were lower than the national rates for South Carolinian women; 11% lower for European-American women and 8% lower for African-American women (1;13). However, while mortality rates for European-American women in South Carolina were 7% lower than the national US mortality rate, they were 29% higher for African-American women in South Carolina (1;13). Preliminary work in this South Carolina population have found that even at the same stage and tumor size, breast cancers in African-American women tend to be more aggressive than those in European-American women (14).
Numerous studies have shown that early detection (from methods including mammography, self-breast exams, and clinical breast exams) is one of the best ways to improve a woman’s prognosis of breast cancer (9;15–19). Not only may a woman’s prognosis of breast cancer be improved by early detection, but prognosis also may be influenced by the timeliness of a patient adhering to recommended follow-up when a breast abnormality is found (20). Patient adherence to follow-up recommendations is most likely multi-faceted. Previous research has found that socioeconomic and demographic characteristics, and certain attitudes or misconceptions about cancer are associated with delayed or incomplete follow-up (21–25). Such attitudes are common among low-income, minority, and under- or uninsured populations (17;26). Furthermore, research has shown that, compared to European-American women, African-American women experience significantly longer time intervals from an abnormal mammogram to diagnostic testing or are less likely to comply with recommended diagnostic follow-up exams within six months of an abnormal mammogram (27–29).
South Carolina’s National Breast and Cervical Cancer Early Detection Program (NBCCEDP) offers an ideal opportunity to study the relationship between race and mammography follow-up in a population that is economically disadvantaged. The NBCCEDP is a nationwide program to help uninsured or underinsured women gain access to screening services for the early detection of breast cancer. South Carolina’s NBCCEDP, commonly called the Best Chance Network, provides free mammography and cervical screenings to women aged 47 to 64 years who do not have health insurance or for whom insurance pays for hospital care only, and who are at or below 200% of federal poverty guidelines. Reflecting South Carolina’s demographics, the Best Chance Network enrolls a population which is approximately 60% African American, and the majority of whom reside in a county classified as rural by the United States Census Bureau. The purpose of this investigation was to examine the relationship between race, compliance, and total time of follow-up of suspicious breast abnormalities among women participating in the Best Chance Network.
Methods
Since the research investigation utilized de-identified data collected for non-research purposes, an exemption of approval from an Institutional Review Board was granted by the University of South Carolina Office of Research.
Study Participants
Subjects for this investigation were the Best Chance Network (BCN) participants. From its inception in 1992 until June 30, 2005, the Best Chance Network performed more than 50,000 mammography screenings. The BCN is implemented through the South Carolina Department of Health and Environmental Control and funded by the Centers for Disease Control and Prevention. The program provides services for underserved women aged 47–64 years, who are at or below 200 percent of the Federal poverty level, and do not have insurance or have insurance that covers only hospital care. The BCN provides screening services, such as mammograms and clinical breast exams, diagnostic procedures, case management, and community education on breast cancer and early detection. Eligible women are recruited into the BCN through active in-reach efforts through primary care providers from federally qualified health centers; outreach through the American Cancer Society, South-Atlantic Division; and various media mechanisms. The BCN serves South Carolinian women through outreach workers throughout the entire state. The BCN is a network of public and private partnerships and comprises more than 250 health care providers: including federally-funded primary care centers in the SC Primary Health Care Association; private physicians, including surgeons and gynecologists; laboratories, university-sponsored clinics, free clinics, regional medical centers and radiology facilities providing screening and follow-up services. Community partners include over 800 volunteers, many of whom are members of local task forces who assist in referring women to screening sites. Per BCN protocol, all participants with a breast abnormality are provided case management services which work with the participant to help her receive all diagnostic services within 60 days. Breast diagnostic work-up is covered by the BCN program.
As per the standard of care, films from all women undergoing screening mammography were classified according to the Breast Imaging Reporting and Data System (BI-RADS™)(30). Coding definitions are: 1-“negative”; 2-“benign”; 3-“probably benign”; 4-“suspicious for malignancy”; and 5-“highly suggestive of malignancy”. Subjects were included in analyses if they were African American or European American and their BI-RADS™ rating was either a 4 or 5 (n=1630), indicating a need for further diagnostic procedures. The BCN protocol does not require diagnostic work-up for a BI-RADS rating of 3. The recommended follow-up time for this category is 3 – 6 months. As such, women with this BI-RAD rating were excluded from the analysis. The study sample consisted of 729 African-American women and 901 European-American women. A retrospective cohort study design was utilized to ascertain if additional diagnostic procedures including mammographic views, ultrasound, or biopsy were undertaken. For women with a pathologically diagnosed breast carcinoma, we retrieved additional information such as tumor histology, behavior, stage, and size (n=407). Among women who underwent a breast biopsy, 187 were European American and 220 were African American.
Covariates
Women were considered to have complete follow-up if their recommended work-up was recorded as complete by the Best Chance Network. As per Best Chance Network guidelines, a complete work-up indicates that the diagnostic testing is complete, and that final diagnosis (whether benign or malignant) and date of final diagnosis are known. For those diagnosed with breast cancer, stage at diagnosis and tumor size also were recorded at the time of final diagnosis. An incomplete work-up was defined as a curtailed planned work-up, pending work-up, refused work-up, or if the woman was lost to follow-up. If a woman severed her relationship with the Best Chance Network program and had her diagnostic work-up performed by another provider, her work-up was defined as refused. A woman was considered lost to follow-up if she died, moved before her work-up started, or when tracking efforts were attempted but failed. The date, at which this determination was made, was used for our analyses.
Total yearly family income, insurance status, previous mammogram and breast symptoms were self-reported at the time of the woman’s visit with the Best Chance Network. Breast symptoms included a lump, bloody nipple discharge, dimpling, ulceration, or inflammation of the skin. Diagnostic procedures included additional mammographic views, repeated clinical breast exams or surgical consultations, breast ultrasounds, breast biopsy or lumpectomies, fine needle aspirations, and other procedures such as stereotactic localization, magnetic resonance imaging, and metastatic work-up such as a bone survey.
Tumor size, stage, and behavior were reported for women with a diagnosis of breast cancer. Tumor size was categorized into the following groups: 0 to 1 centimeter (cm), 1 to 2 cm, 2 to 5 cm, greater than 5 cm, and unknown. Tumor stage was reported according to the American Joint Committee on Cancer (AJCC)(31). Tumor behavior was defined as either in-situ or invasive.
Statistical Methods
Descriptive statistics, stratified by race, were calculated for all demographic and breast screening variables. Differences between African-American and European-American women were analyzed using the χ2 test for categorical measures and a t-test for continuous measures. All p-values were two-tailed and significance was assessed at α= 0.05 Type I error rate.
Logistic regression was conducted to model the relationship between race and whether recommended workup was completed. Other covariates included in these models were age, healthcare provider, number of procedures, income, and insurance type. Because there were a finite number of mammography providers, we had to consider the possibility of correlation among observations within the provider variable. After investigating several parametric structures for within-provider correlation, all testing concluded that the pooled model was the most appropriate approach where inference adjusted for possible within-provider correlation using the empirical (modified sandwich) variance estimator (32).
Kaplan-Meier survival curves were compared using the log-rank test for equality. Cox proportional hazard modeling was the primary statistical method used for analysis of follow-up time. Those women with incomplete work-ups were categorized as censored observations. Two measures of time, the number of days between the first mammogram and the date that the status of the work-up was finalized and the number of days between the first clinical breast exam and the date that the status was finalized, were analyzed in this investigation. For women who completed their work-up, the date that the status was finalized corresponds to the date of a final diagnosis (either benign or malignant). A final diagnosis was documented only after the individual had completed all recommended diagnostic procedures including additional mammographic views, ultrasound, and biopsy (if recommended). For those women with incomplete work-up, the date corresponds to the date when their status (incomplete, refused, lost-to-follow up, or pending) was assessed.
As in our logistic models, we evaluated the possible correlation of repeated observations within each mammography provider for our estimated Cox models. Further testing indicated that the pooled (independence) Cox regression model was the most appropriate method (33). The proportional hazards assumption was tested and the assumption was not violated for the main predictor variable of race (p-value = 0.11).
Results
Study population characteristics are displayed in Table 1. The mean age of the women was 52 years old and was not significantly different between African-American and European-American women. However, income levels varied by race, with African-American women having a mean income of $1,294 less than European-American women (p=0.0003). African-American women were also less likely to have insurance as compared to their European-American counterparts. The majority of women did not experience breast symptoms at the time of their appointment. African-American women were more likely to have an abnormal clinical breast exam than European-American women. For both groups of women, 91% had completed follow-up of abnormal breast findings. Additionally, there was not a significant difference in the number of diagnostic procedures performed or in the number of previous mammograms performed between African-American and European-American women.
Table 1.
Demographic Characteristics of the Study Population According to Race: Best Chance Network
| African American (n = 729) |
European American (n = 901) |
||
|---|---|---|---|
| % (Number) Mean (SD) |
% (Number) Mean (SD) |
p-value* | |
| Demographic Characteristics | |||
| Income* | $5,887.60 ($6,838.50) | $7,181.50 ($7,706.00) | 0.0003 |
| Age at diagnosis | 52 (9.3) | 52 (10.18) | 0.46 |
| Breast Symptoms | |||
| Yes | 20 (184) | 24 (174) | 0.089 |
| No | 77 (695) | 75 (545) | |
| Unknown | 3 (10) | 1 (10) | |
| Clinical Breast Exam Result | |||
| Normal | 51 (463) | 45 (329) | 0.04 |
| Abnormal | 44 (396) | 50 (363) | |
| Not needed | 5 (42) | 5 (37) | |
| Previous Mammogram | |||
| Yes | 52 (471) | 48 (352) | 0.27 |
| No | 46 (413) | 50 (362) | |
| Unknown | 2 (17) | 2 (15) | |
| Status of Mammography Final Diagnosis | |||
| Work-up complete | 91 (817) | 91 (662) | 0.93 |
| Refused, pending, incomplete | 9 (84) | 9 (67) | |
| Insurance Status** | |||
| None | 78 (701) | 73 (527) | 0.008 |
| Hospitalization only | 6 (51) | 5 (37) | |
| Unreported | 16 (149) | 22 (165) | |
| Number of procedures | |||
| 0 | 7 (48) | 7 (64) | 0.28 |
| 1 | 48 (354) | 44 (396) | |
| 2 | 29 (215) | 33 (300) | |
| 3 | 12 (86) | 13 (119) | |
| >4 | 4 (26) | 3 (22) | |
| Final Diagnosis | |||
| In Situ | 4 (40) | 5 (38) | 0.73 |
| Invasive | 20 (180) | 20 (149) | |
| Breast cancer not diagnosed/unknown | 76 (681) | 75 (542) |
p < 0.05 based on t-test test or chi-square test as appropriate between African American and European American
The clinical characteristics of those women who were diagnosed with breast cancer within the Best Chance Network are shown in Table 2. Among women diagnosed with breast cancer, there was not a difference between African-American and European-American women with regard to tumor size, tumor stage, or tumor behavior. For both groups, there was a larger percentage of tumors greater than 2 centimeters and less than 5 centimeters and the tumors were more likely to be invasive than in-situ.
Table 2.
Clinical Characteristics of Women Diagnosed with Breast Cancer According to Race: Best Chance Network
| African American (n = 220) |
European American (n = 187) |
||
|---|---|---|---|
| % (Number) | % (Number) | p-value* | |
| Clinical Characteristics | |||
| Tumor Size | |||
| 0 to 1 cm | 8 (18) | 8 (15) | 0.54 |
| >1 to 2 cm | 20 (43) | 22 (42) | |
| >2 to 5 cm | 30 (65) | 29 (54) | |
| >5 cm | 15 (33) | 10 (18) | |
| Unknown | 27 (61) | 31 (58) | |
| Tumor Behavior | |||
| In-situ | 18 (40) | 20 (38) | 0.58 |
| Invasive | 82 (180) | 80 (149) | |
| Tumor Stage | |||
| Stage I/Local | 23 (50) | 21 (39) | 0.90 |
| Stage II, III/Regional | 44 (98) | 48 (90) | |
| Stage IV/Distant | 4 (9) | 4 (8) | |
| Unknown | 29 (63) | 27 (50) |
p < 0.05 based on chi-square test between African American and European American
The relationship between the various covariates available and overall completion of recommended work-up is displayed in Table 3. Regression analyses showed that a complete work-up was associated with the number of previous procedures and income, but was not significantly associated with age, type of insurance, or race. Women with one previous procedure were over three times more likely to complete their recommended follow-up as compared to women not having previous procedures (95% CI: 1.71, 6,27). For each doubling of income, the odds of completing the work-up increased by 10% (95% CI: 1.03, 1.19).
Table 3.
Multivariate Logistic Analyses of Factors Influencing Complete Recommended Workup*
| Variable | Incomplete Work-up | Complete Work-up |
Relative Risk (CI) † |
|---|---|---|---|
| Age | 151 | 1479 | 0.98 (0.95, 1.01) |
| Income | 151 | 1479 | 1.10 (1.03, 1.19) |
| Number of procedures | |||
| 1 | 141 | 721 | 1.00 |
| 2 or more | 10 | 758 | 3.28 (1.71, 6.27) |
| Insurance | |||
| No insurance | 92 | 1136 | 1.00 |
| Hospital only Insurance | 59 | 343 | 0.51 (0.20, 1.30) |
| Race‡ | |||
| EA | 84 | 817 | 1.00 |
| AA | 67 | 662 | 1.29 (0.65, 2.55) |
Time was measured as the number of days between the first mammogram and the date of final diagnosis.
CI: Confidence Interval
Race described as EA = European American; AA = African American
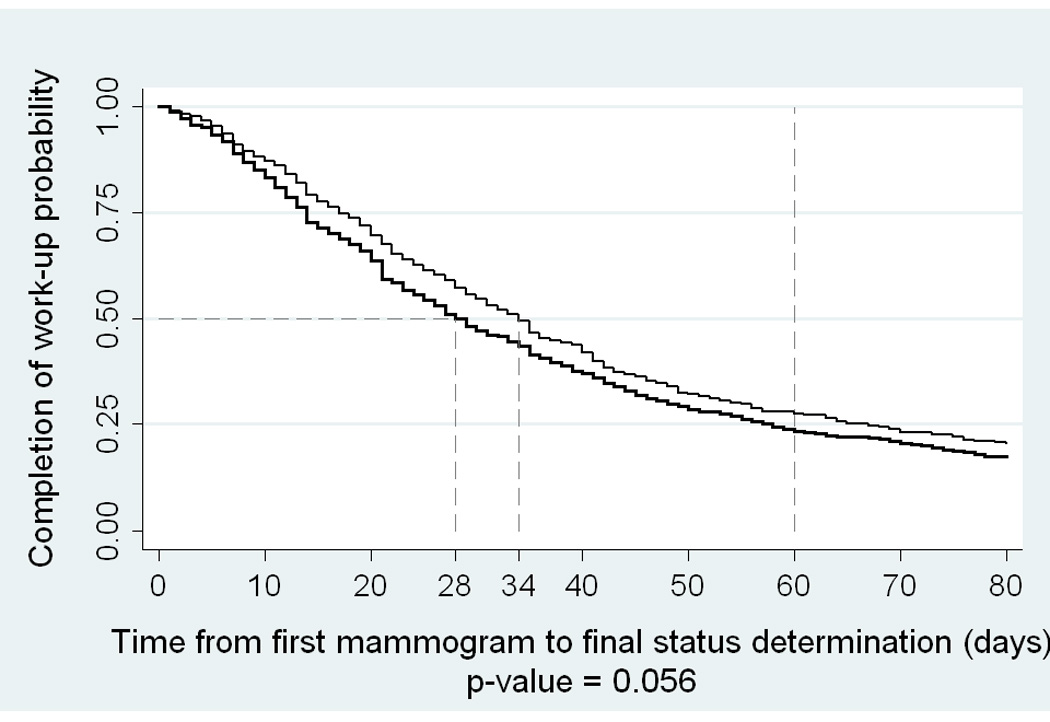
The Kaplan Meier curves comparing the number of days between the first mammogram and the date of final status by race are depicted in Figure 1. The graph of the time estimates for race showed little difference between African-American and European-American women, although the log-rank test for equality of survival functions was marginally significant (p-value=0.056). The median work-up time from the first mammogram to the final status was 34 days for African-American women and 28 days for European-American women.
Figure 1.
The Kaplan Meier curves of the time (in days) between the first clinical breast exam and the date of final status are depicted in Figure 2. Interestingly, we noted a significant difference in the time to work-up completion between African-American and European-American women (median time = 44 days for African Americans and 40 days for European Americans, p-value=0.02).
Figure 2.
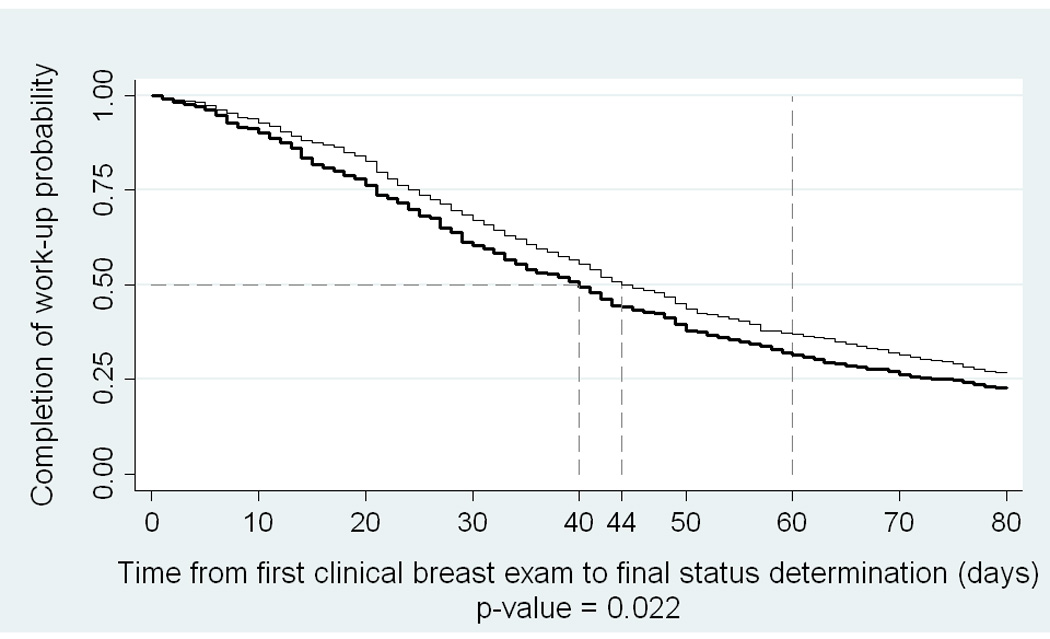
Table 4 contains the Cox Hazard analysis of factors influencing time to completion of the recommended work-up. In this analysis, time was measured as the number of days between the first mammogram and the date that final status was determined. Cox analyses suggested that after adjusting for age, income, number of previous procedures, and insurance, African-American women were 11% less likely to complete their work-up than European-American women (p-value=0.09). In addition, results indicated that for each previous procedure women were 30% more likely to complete the work-up (p-value<0.0001). With each doubling of income, the likelihood of completing the recommended work-up was 1.01 times higher (p-value=0.017). Women with insurance were 1.11 times more likely to complete the recommended work-up as compared to women without insurance or hospitalization insurance only (p-value=0.05). Age was not significantly associated with completion of the work-up.
Table 4.
Survival Analysis of Factors Influencing Completion of Recommended Workup From First Mammogram
| Variable | Incomplete Work-up | Complete Work-up | Hazard Ratio (CI)* |
|---|---|---|---|
| Age | 151 | 1479 | 1.00 (0.99, 1.01) |
| Income | 151 | 1479 | 1.01 (1.00, 1.02) |
| Number of procedures | |||
| 1 | 141 | 721 | 1.00 |
| 2 or more | 10 | 758 | 1.29 (1.21, 1.39) |
| Insurance | |||
| No insurance | 92 | 1136 | 1.00 |
| Hospital only Insurance | 59 | 343 | 1.11 (0.99, 1.24) |
| Race† | |||
| EA | 84 | 817 | 1.00 |
| AA | 67 | 662 | 0.89 (0.77, 1.02) |
CI: Confidence Interval
Race described as EA = European American; AA = African American
Table 5 contains results from the Cox analysis of factors influencing time to completion of recommended work-up when time was assessed from clinical breast exam to the date that status of the work-up was finalized. Analyses indicated that, after adjusting for age, income, number of previous procedures, and insurance, African-American women were 12% less likely to complete their work-up than European-American women (p-value=0.01). In addition, these results indicate that women having more than one previous procedure were 32% more likely to complete the work-up (p-value<0.0001) than women who had no previous procedures. Age, income, and insurance status were not significantly associated with completion of the work-up.
Table 5.
Survival Analysis of Factors Influencing Completion of Recommended Workup from First Clinical Breast Examination
| Variable | Incomplete Work-up | Complete Work-up | Hazard Ratio (CI)* |
|---|---|---|---|
| Age | 151 | 1479 | 0.99 (0.95, 1.00) |
| Income | 151 | 1479 | 1.01 (1.99, 1.02) |
| Number of procedures | |||
| 1 | 141 | 721 | 1.00 |
| 2 or more | 10 | 758 | 1.32 (1.25, 1.39) |
| Insurance | |||
| No insurance | 92 | 1136 | 1.00 |
| Hospital only Insurance | 59 | 343 | 1.07 (0.94, 1.22) |
| Race† | |||
| EA | 84 | 817 | 1.00 |
| AA | 67 | 662 | 0.88 (0.79, 0.97) |
CI: Confidence Interval
Race described as EA = European American; AA = African American
Discussion
Among economically-disadvantaged women, race was not significantly associated with overall completion of mammographic work-up. However, we did find evidence for racial disparities in the time between the first abnormal clinical breast examination and determination of final status. After accounting for time, African-American women were significantly less likely to complete their mammographic work-up than European-American women.
Interestingly, when time was measured in the number of days between the mammogram and the date of final status, a significant effect of race was no longer evident. Because the clinical breast examination typically precedes the diagnostic mammogram, these findings suggest that the racial differences may occur early in the process. Although the exact protocol will vary according to the clinical site where the patient receives care, the clinical breast examination usually will be completed by a different provider than the provider of the mammographic services. Consequently, structural and environmental factors that could affect the time between the clinical breast exam and the mammogram are communication to the patient by the provider, lengthy scheduling delays, proximity of the mammography clinic to the patient (which could be especially pertinent in a rural setting), and availability of transportation (23;34–38). Conceptualizing these factors in light of the racial differences that we found suggest several interesting areas for further research (24;24;38–40). Diagnostic delays also may be a result, in part, of deficits in the patient-provider relationship. Patient trust in the provider has been shown to be positively correlated with willingness to seek care and adhere to treatment recommendations (41). Furthermore, a lack of a usual provider is associated with inadequate follow-up after an abnormal mammogram (42). It has been noted that physician perceptions tended to be more negative regarding lower-income and minority women compared to higher-income and non-minority women (37;41;43). Therefore, African-American women may not adhere to recommendations concerning breast abnormalities because of a lack of trust with their health care provider or a lack of a consistent provider due to lower socioeconomic status. It is puzzling to find that even in the population where all services are provided free of charge, income influences a woman’s adherence to recommended follow-up. While the BCN does provide all diagnostic services, up until 2000 there were no provisions for no-cost services once a woman was diagnosed with a breast malignancy. Consequently, this may have influenced a woman’s decision to follow up after a doctor’s recommendation because of her inability to pay or her fear about paying for additional medical services should she be diagnosed with breast cancer. This is consistent with literature showing that numerous socioeconomic factors are associated with delayed follow-up of an abnormal mammogram or clinical breast exam including: low household income, other cost issues, and transportation problems (21–24;34;40;44–51).
Delayed follow-up of breast abnormalities could result in detecting the breast cancer at a later stage, thus influencing a woman’s prognosis and mortality of the disease. Richards and colleagues found in a meta-analysis that a delayed diagnosis of breast cancer of as little as 3 months was associated with lower survival than those with prompt follow-up (52). Likewise, they found that three- to six-month delays were clearly associated with increased tumor size, advanced disease stage, and poorer long-term prognosis.
In a previous investigation, our research team examined the effect of delayed diagnosis on mortality in the Best Chance Network(53). With this investigation, one of the intervals examined was the time between the suspicious mammogram or CBE and breast cancer diagnosis. Interestingly, we found no significant association between the diagnosis interval and mortality nor any significant interaction between race and the diagnosis interval. Combined with our findings, this suggests that future studies should focus on the time period between clinical breast examination and mammography.
As with any epidemiological investigation, our study had some limitations worth noting. Data elements from the NBCCEDP are dictated by the Centers for Disease Control and Prevention. While we had a wealth of information with which to work, we did not have information about beliefs about screening, health literacy, patient-provider communication and relationship, or system failures. This information would be useful to collect in future studies in order to provide a more comprehensive analysis of our findings. In addition, due to small cell sizes, we were forced to collapse the outcome variable ‘status of the work-up’ from 4 to 2 levels (refused, lost-to-follow-up, or pending were condensed to incomplete). This may have led to possible misclassification bias as the factors associated with a refusal, loss to follow-up, or pending work-up may be different. Nevertheless, one would expect such misclassification of the outcome to bias the findings toward the null value thereby strengthening the claim of a true association based on the observed relationship between race and time between the first CBE and date of final status.
This research study has many strengths. The Best Chance Network targets rural, medically underserved women of South Carolina, serving a population that is approximately 70% African-American. Hence, we were able to study a population that is chronically under-represented in the scientific literature. In addition, because the Best Chance Network is a state-wide program, we were able to follow women over time regardless of where they may have received treatment, thus minimizing losses to follow-up (e.g., a woman would not be lost upon moving to another city). Due to the cohort design of the program and our study, we were able to account for past screening history in the investigation, which does appear to affect the time to completion of the diagnostic work-up.
In conclusion, we found evidence for a racial disparity in the time to completion of a diagnostic work-up among low-income women enrolled in South Carolina’s NBCCEDP, the Best Chance Network. Given the target population of the BCN, we believe that these results are particularly applicable to economically disadvantaged AA women living in rural areas. The finding that no disparities existed in the overall completion of the work-up are also an encouraging evaluation of the NBCCEDP, because it suggests that the program is making progress toward eliminating racial disparities in breast cancer and offer areas for strengthening (i.e., in decreasing the total time interval). Overall, these findings highlight the importance of understanding factors associated with these delays. In addition, they suggest several areas for potential policy changes such as additional support to the BCN program to allow the expansion of services. Improving patient adherence to follow-up recommendations and decreasing the time lag between the detection of breast abnormalities and the date of completion may decrease breast cancer mortality rates.
Acknowledgments
Funding:
This work was supported by the University of South Carolina, Office of Research and Health Sciences. We also would like to acknowledge funding of the South Carolina Cancer Disparities Community Network (SCCDCN) through grant number 1 U01 CA114601-01 from the National Cancer Institute (Community Networks Program).
Footnotes
There are no financial disclosures to be reported for any authors.
Reference List
- 1.Adams SA, Hebert JR, Bolick-Aldrich S, Daguise VG, Mosely CM, Modayil MV, et al. Breast cancer disparities in South Carolina: Early detection, special programs, and descriptive epidemiology. J S C Med Assoc. 2006;102:231–239. [PMC free article] [PubMed] [Google Scholar]
- 2.Li CI. Racial and ethnic disparities in breast cancer stage, treatment, and survival in the United States. Ethn Dis. 2005;15:S5–S9. [PubMed] [Google Scholar]
- 3.Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 4.Freeman HP. The meaning of Race in Cancer of the Breast. Cancer J Sci Am. 1997;3:76–77. [PubMed] [Google Scholar]
- 5.Krieger N, Van Den Eden SK, Zava D, Okamoto A. Race/Ethnicity, social class, and prevalence of Breast Cancer Prognostic Biomarkers: a Study of White, Black, and Asian Women in the San Francisco bay area. Ethn.Dis. 1997;7:137–149. [PubMed] [Google Scholar]
- 6.Moorman PG, Jones BA, Millikan RC, Hall IJ, Newman B. Race, anthropometric factors, and stage at diagnosis of breast cancer. Am.J.Epidemiol. 2001;153:284–291. doi: 10.1093/aje/153.3.284. [DOI] [PubMed] [Google Scholar]
- 7.Rose DP, Royak-Schaler R. Tumor biology and prognosis in black breast cancer patients. Cancer Detect Prev. 2001;25:16–31. [PubMed] [Google Scholar]
- 8.El-Tamer MB, Homel P, Wait R. Is race a poor prognostic factor in breast cancer? J Am Coll Surg. 1999;189:41–45. doi: 10.1016/s1072-7515(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 9.Marbella AM, Layde PM. Racial trends in age-specific Breast Cancer Mortality Rates in US Women. Am.J.Public Health. 2001;91:118–121. doi: 10.2105/ajph.91.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldridge ML, Daniels JL, Jukic AM. Mammograms and healthcare access among US Hispanic and non-Hispanic women 40 years and older. Family & Community Health. 2006;29:80–88. doi: 10.1097/00003727-200604000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi M, Thacker HL, Litaker DG, Kippes C. Differences in breast cancer screening rates: an issue of ethnicity or socioeconomics? J Womens Health Gend Based Med. 2000;9:1025–1031. doi: 10.1089/15246090050200060. [DOI] [PubMed] [Google Scholar]
- 12.Wojcik B, Spinks M. Can Racial Differences in Breast Carcinoma Survival Be Explained by Access to Medical Care? CANCER(Supplement) 2000;88:1268–1269. [Google Scholar]
- 13.Sanders LC, Hardy WR, Ashford-Carroll TS, Bolick-Aldrich SW. South Carolina Cancer Facts and Figures: 2001–2002. South Carolina Central Cancer Registry, Office of Public Health Statistics and Information Services, South Carolina Department of Health and Environmental Control. American Cancer Society, Southeast Division; 2001. Ref Type: Report. [Google Scholar]
- 14.Cunningham JE, Butler WM. Racial Disparities in Female Breast Cancer in South Carolina: Clinical Evidence for a Biological Basis. Breast Cancer Res Treat. 2004;88:161–176. doi: 10.1007/s10549-004-0592-9. [DOI] [PubMed] [Google Scholar]
- 15.Wojcik BE, Spinks MK, Optenberg SA. Breast Carcinoma Survival Analysis for African American and White Women in an Equal- Access Health Care System. Cancer. 1998;82:1310–1318. doi: 10.1002/(sici)1097-0142(19980401)82:7<1310::aid-cncr14>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Chen V, Correa P, Kurman R, Wu X, Eley J, Austin D, et al. Histological characteristics of breast carcinoma in blacks and whites. Cancer Epidemiol Biomarkers Prev. 1994;3:127–135. [PubMed] [Google Scholar]
- 17.McCarthy EP, Burns RB, Coughlin SS, Freund KM, Rice J, Marwill SL, et al. Mammography use helps to explain differences in breast cancer stage at diagnosis between older black and white women. Ann Intern Med. 1998;128:729–736. doi: 10.7326/0003-4819-128-9-199805010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Tabar L, Fagerberg G, Chen HH, Duffy SW, Gad A. Screening for breast cancer in women aged under 50: mode of detection, incidence, fatality, and histology. J Med Screen. 1995;2:94–98. doi: 10.1177/096914139500200208. [DOI] [PubMed] [Google Scholar]
- 19.Kerlikowske K, Barclay J. Outcomes of modern screening mammography. J Natl Cancer Inst Monogr. 1997;22:105–111. doi: 10.1093/jncimono/1997.22.105. [DOI] [PubMed] [Google Scholar]
- 20.Jones BA, Patterson EA, Calvocoressi L. Mammography screening in African American women: evaluating the research. Cancer. 2003;97:258–272. doi: 10.1002/cncr.11022. [DOI] [PubMed] [Google Scholar]
- 21.Caplan LS, Helzlsouer KJ, Shapiro S, Wesley MN, Edwards BK. Reasons for delay in breast cancer diagnosis. Prev Med. 1996;25:218–224. doi: 10.1006/pmed.1996.0049. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy BD, Yood MU, Boohaker EA, Ward RE, Rebner M, Johnson CC. Inadequate follow-up of abnormal mammograms. Am J Prev Med. 1996;12:282–288. [PubMed] [Google Scholar]
- 23.McCarthy BD, Yood MU, Janz NK, Boohaker EA, Ward RE, Johnson CC. Evaluation of factors potentially associated with inadequate follow-up of mammographic abnormalities. Cancer. 1996;77(10):2070–2076. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2070::AID-CNCR16>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 24.Weinmann S, Taplin SH, Gilbert J, Beverly RK, Geiger AM, Yood MU, et al. Characteristics of women refusing follow-up for tests or symptoms suggestive of breast cancer. J Natl Cancer Inst Monogr. 2005;35:33–38. doi: 10.1093/jncimonographs/lgi035. [DOI] [PubMed] [Google Scholar]
- 25.Kerlikowske K. Timeliness of follow-up after abnormal screening mammography. Breast Cancer Res Treat. 1996;40:53–64. doi: 10.1007/BF01806002. [DOI] [PubMed] [Google Scholar]
- 26.Caplan LS, Schoenfeld ER, O'Leary ES, Leske MC. Breast cancer and electromagnetic fields. Ann.Epidemiol. 2000;10:31–44. doi: 10.1016/s1047-2797(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 27.Elmore JG, Wells CK, Howard DH, Feinstein AR. The impact of clinical history on mammographic interpretations. JAMA. 1997;277:49–52. [PubMed] [Google Scholar]
- 28.Chang S, Hulka BS, Baird DD, Ingle JN, Newman B, Graham ML, II, et al. Breast cancer survival and the timing of tumor removal during the menstrual cycle. Cancer Epidemiol.Biomarkers Prev. 1997;6:881–886. [PubMed] [Google Scholar]
- 29.Kerner JF, Guirguis-Blake J, Hennessy KD, Brounstein PJ, Vinson C, Schwartz RH, et al. Translating research into improved outcomes in comprehensive cancer control. Cancer Causes Control. 2005;16:27–40. doi: 10.1007/s10552-005-0488-y. [DOI] [PubMed] [Google Scholar]
- 30.American College of Radiology. Breast Imaging Reporting and Data System® (BI-RADS®) Atlas. Reston, VA: American College of Radiology; 2003. [Google Scholar]
- 31.AJCC Cancer Staging Manual. 5th Edition ed. USA: Lippincott Raven Publishers; 1997. [Google Scholar]
- 32.Williams R. A note on robust variance estimation for cluster-correlated data. Biometrics. 2000;56:645–646. doi: 10.1111/j.0006-341x.2000.00645.x. [DOI] [PubMed] [Google Scholar]
- 33.Lin DY, Wei LJ. The robust inference for the Cox proportional hazards model. J Am Stat Assoc. 1989;84:1074–1078. [Google Scholar]
- 34.Caplan LS, Helzlsouer KJ, Shapiro S, Freedman LS, Coates RJ, Edwards BK. System delay in breast cancer in whites and blacks. Am J Epidemiol. 1995;142:804–812. doi: 10.1093/oxfordjournals.aje.a117719. [DOI] [PubMed] [Google Scholar]
- 35.Garbers S, Jessop DJ, Foti H, Uribelarrea M, Chiasson MA. Barriers to breast cancer screening for low-income Mexican and Dominican women in New York City. Journal of Urban Health. 2003;80:81–91. doi: 10.1007/PL00022327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyndman JC, Holman CD, Dawes VP. Effect of distance and social disadvantage on the response to invitations to attend mammography screening. Journal of Medical Screening. 2000;7:141–145. doi: 10.1136/jms.7.3.141. [DOI] [PubMed] [Google Scholar]
- 37.van Ryn M, Burke J. The effect of patient race and socio-economic status on physicians' perceptions of patients. Soc Sci Med. 2000;50:813–828. doi: 10.1016/s0277-9536(99)00338-x. [DOI] [PubMed] [Google Scholar]
- 38.Zapka JG, Puleo E, Taplin SH, Goins KV, Ulcickas Yood M, Mouchawar J, et al. Processes of care in cervical and breast cancer screening and follow-up--the importance of communication. Prev Med. 2004;39:81–90. doi: 10.1016/j.ypmed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Cardenosa G, Eklund GW. Rate of compliance with recommendations for additional mammographic views and biopsies. Radiology. 1991;181:359–361. doi: 10.1148/radiology.181.2.1924772. [DOI] [PubMed] [Google Scholar]
- 40.Mandelblatt J, Traxler M, Lakin P, Kanetsky P, Thomas L, Chauhan P, et al. Breast and cervical cancer screening of poor, elderly, black women: clinical results and implications. Harlem Study Team. Am J Prev Med. 1993;9:133–138. [PubMed] [Google Scholar]
- 41.O'Malley AS, Sheppard VB, Schwartz M, Mandelblatt J. The role of trust in use of preventive services among low-income African-American women. Prev Med. 2004;38:777–785. doi: 10.1016/j.ypmed.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Jones BA, Dailey A, Calvocoressi L, Reams K, Kasl SV, Lee C, et al. Inadequate follow-up of abnormal screening mammograms: findings from the race differences in screening mammography process study (United States) Cancer Causes Control. 2005;16:809–821. doi: 10.1007/s10552-005-2905-7. [DOI] [PubMed] [Google Scholar]
- 43.Schneider AE, Davis RB, Phillips RS. Discussion of hormone replacement therapy between physicians and their patients. Am J Med Qual. 2000;15:143–147. doi: 10.1177/106286060001500404. [DOI] [PubMed] [Google Scholar]
- 44.Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factors are associated with diagnostic follow-up after abnormal mammograms? Findings from a U.S. National Survey. Cancer Epidemiol Biomarkers Prev. 2004;13:723–732. [PubMed] [Google Scholar]
- 45.Caplan LS, May DS, Richardson LC. Time to diagnosis and treatment of breast cancer: results from the National Breast and Cervical Cancer Early Detection Program, 1991–1995. Am J Public Health. 2000;90:130–134. doi: 10.2105/ajph.90.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gotay CC, Wilson ME. Social support and cancer screening in African American, Hispanic, and Native American women. Cancer Pract. 1998;6:31–37. doi: 10.1046/j.1523-5394.1998.1998006031.x. [DOI] [PubMed] [Google Scholar]
- 47.Katz SJ, Hofer TP. Socioeconomic disparities in preventive care persist despite universal coverage. Breast and cervical cancer screening in Ontario and the United States. JAMA. 1994;272:530–534. [PubMed] [Google Scholar]
- 48.Harris DM, Miller JE, Davis DM. Racial differences in breast cancer screening, knowledge and compliance. J Natl Med Assoc. 2003;95:693–701. [PMC free article] [PubMed] [Google Scholar]
- 49.Howard DL, Penchansky R, Brown MB. Disaggregating the effects of race on breast cancer survival. Fam Med. 1998;30:228–235. [PubMed] [Google Scholar]
- 50.Reisch LM, Barton MB, Fletcher SW, Kreuter W, Elmore JG. Breast cancer screening use by African Americans and Whites in an HMO. J Gen Intern Med. 2000;15:229–234. doi: 10.1111/j.1525-1497.2000.01339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zackrisson S, Andersson I, Manjer J, Janzon L. Non-attendance in breast cancer screening is associated with unfavourable socio-economic circumstances and advanced carcinoma. Int J Cancer. 2004;108:754–760. doi: 10.1002/ijc.11622. [DOI] [PubMed] [Google Scholar]
- 52.Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 53.Smith E, Adams S, Prabhu-Das I, Bottai M, Fulton J, Hebert JR. Breast Cancer Survival Among Economically Disadvantaged Women: The Influences of Delayed Diagnosis and Treatment on Mortality. Cancer Epidemiology Biomarkers and Prevention. 2008;17:2882–2890. doi: 10.1158/1055-9965.EPI-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]


