Abstract

Professor Joseph Loscalzo
Dr. Joseph Loscalzo (M.D., 1978; Ph.D., 1977) is recognized here as a Redox Pioneer because he has published two articles in the field of antioxidant/redox biology that have been cited more than 1,000 times and 22 articles that have been cited more than 100 times. Dr. Loscalzo is known for his seminal contributions to our understanding of the vascular biology of nitric oxide. His initial discovery that the antiplatelet effects of organic nitrates are potentiated by thiols through a mechanism that involved metabolism to S-nitrosothiols was followed by the demonstration that S-nitrosothiols are formed endogenously through S-transnitrosation, stabilize nitric oxide, and facilitate the transport and transfer of nitric oxide between and within cells of the vessel wall. These properties led to the development of S-nitrosothiol–containing pharmacotherapies to treat disease states characterized by nitric oxide deficiency. Dr. Loscalzo's other scientific contributions include identifying the vascular functional consequences of genetic deficiencies of antioxidant enzymes that decrease nitric oxide bioavailability, collectively termed the “oxidative enzymopathies,” and demonstrating the role of mitochondria in modulating the disulfide subproteome, and in redox signaling in hypoxia. He has received numerous awards and honors for his scientific contributions, including election to the Institute of Medicine of the National Academy of Sciences. Antioxid. Redox Signal. 13, 1125–1132.
Biomedical research is an extraordinarily gratifying enterprise. Solving a perplexing problem in an innovative and rigorous way, creating new knowledge in the process, and using that knowledge to (re)define an underlying mechanism are each highly rewarding aspects of the research process. Perhaps the most rewarding feature, however, is the ability to influence the next generation of scientists through the impact of one's scientific contributions, and the guidance and advice given to one's trainees. Those of us who have succeeded in developing biomedical research careers have been given a great and special opportunity for which I, for one, am most grateful.
—Professor Joseph Loscalzo
Educational and Professional Training
Dr. Loscalzo is a graduate of the University of Pennsylvania (A.B., M.D., and Ph.D. in Biochemistry). He completed his residency in internal medicine and a cardiology fellowship at Brigham and Women's Hospital. He also completed his postdoctoral training at Harvard Medical School as a Research Fellow in Medicine.
Summary of Dr. Loscalzo's Top Contributions
Organic nitrates and nitric oxide (NO•) donors were known to limit platelet aggregation; however, the mechanism underlying this response remained unknown for more than a decade. In 1985, Dr. Loscalzo discovered that the antiplatelet effects of organic nitrates are dependent on their reaction with thiols to form S-nitrosothiols. He demonstrated that S-nitrosothiols are a stable reservoir of NO•, facilitate NO• transport within and between cells, and promote the vascular biologic effects of NO•. His research increased our understanding of NO• signaling and metabolism in the vasculature and the pathophysiologic significance of NO•-deficiency states.
Dr. Loscalzo and his granddaughter Charlotte
Background, Development, and Training
Joseph Loscalzo was born in 1951 in Camden, New Jersey, where he spent his childhood with his three siblings and a large extended family. His great-uncle, a pediatrician who served on the first board of the American Academy of Pediatrics, was a significant influence on Dr. Loscalzo's decision to become a physician. Family has always been important to Dr. Loscalzo, and he and his wife Anita are the parents of two highly accomplished grown children, Julie and Alex, and the proud grandparents of Charlotte.
Dr. Loscalzo joined the faculty of Harvard Medical School at Brigham and Women's Hospital in 1984, after completing his training, and remained on staff until 1994. During this time, he was appointed Chief of the Cardiology Section at the Brockton/West Roxbury VA Medical Center, a Harvard Medical School affiliate institution, and Director of the Center for Research in Thrombolysis and of the Center for Research in Vascular Biology.
In 1994, Dr. Loscalzo moved to Boston University School of Medicine to assume the roles of Chief of Cardiology and Director of the Whitaker Cardiovascular Institute. In 1997, he was named the Wade Professor and Chairman of the Department of Medicine. While there, he expanded the Whitaker Cardiovascular Institute by recruiting a number of talented investigators. In 2005, he returned to Harvard Medical School and Brigham and Women's Hospital as the Hersey Professor of the Theory and Practice of Medicine and Chairman of the Department of Medicine.
Area of Interest in Redox Biology
Mitochondrial ROS and the disulfide subproteome
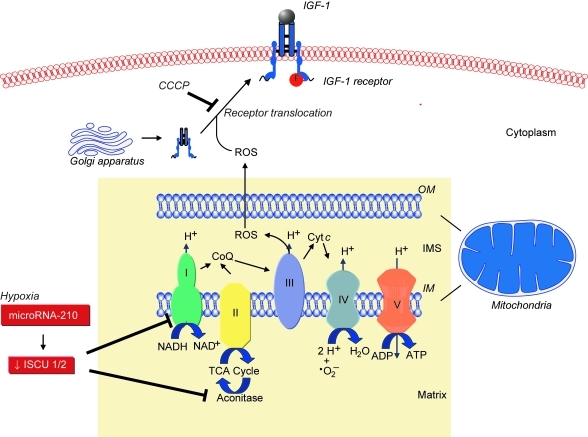
Loscalzo's group found that mitochondrial ROS were involved in de novo disulfide bond formation, a key event in protein synthesis and function, by demonstrating that global disulfide bond formation paralleled mitochondrial ROS levels. Disulfide bond formation influenced the activation and cell-surface translocation of selected disulfide-containing proteins such as insulin growth factor-1 receptor, indicating the existence of a mitochondrial ROS-dependent redox-sensitive disulfide subproteome (44). Sparsely cultured endothelial cells were found to have a lower protein disulfide content compared with confluent cells, associated with decreased mitochondrial membrane potential, superoxide production, and increased levels of reduced glutathione, indicating a more reductive state. This, in turn, was associated with diminished ligand-induced phosphorylation of insulin growth factor-1 receptor, suggesting that differences in receptor function observed in sparsely cultured cells may result from insufficient oxidative potential (44). Other studies revealed that GPx-1 regulates epidermal growth factor receptor signaling, in part, through a similar mechanism. Cells that overexpressed GPx-1 were found to have decreased epidermal growth factor receptor–mediated activation of Akt, resulting in decreased proliferation, owing to a reduction in global disulfide bond formation, mitochondrial membrane potential, and ATP production (14). These studies highlight the importance of mitochondrial ROS and intracellular reductive potential for protein disulfide formation, select disulfide-containing cell-surface receptor expression, and growth factor receptor–mediated signaling (Fig. 1).
FIG. 1.
Mitochondrial ROS, metabolism, and role in translocation and function of disulfide-containing cell-surface protein receptors. ROS generated by mitochondrial respiration play an integral role in the cell-surface translocation and activation of selected disulfide containing proteins, such as insulin growth factor-1 (IGF-1) receptor. In the presence of mitochondrial ROS inhibitors, such as the mitochondrial uncoupler carbonylcyanide m-chlorophenylhydrazone (CCCP), translocation of the IGF-1 receptor from the cytoplasm to the cell membrane is inhibited. Inhibition of mitochondrial ROS also limits phosphorylation and activation of the receptor in CCCP-treated endothelial cells. Under hypoxic conditions, mitochondrial metabolism shifts, leading to a repression of electron transport and oxidative phosphorylation in favor of glycolysis. This phenomenon is known as the “Pasteur effect.” This metabolic switch is regulated by microRNA-210, which is upregulated by hypoxia, and downregulates the expression of iron-sulfur cluster assembly proteins 1 and 2 (ISCU 1/2). These proteins are necessary for the activity of Complex I and aconitase. Thus, microRNA-210 limits mitochondrial electron transport, ROS, and the TCA cycle. CoQ, Coenzyme Q; OM, outer mitochondrial membrane; IM, inner mitochondrial membrane. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Hypoxia, endothelial cells, and microRNA-210
Hypoxia is associated with inflammation and ischemia, and, under these conditions, NO• levels may be markedly elevated, rendering vascular endothelial cells susceptible to apoptosis and cell death. Loscalzo's laboratory demonstrated that this occurred as a result of increased peroxynitrite formation that activated the mitochondria-independent pathway of apoptosis, as well as induced mitochondrial dysfunction and cytochrome c release to activate caspase-9 (39).
Under hypoxic conditions, repression of mitochondrial electron transport and oxidative phosphorylation in favor of glycolysis is known as the “Pasteur effect.” In endothelial, vascular smooth muscle, and transformed cells, as well as in vivo, this metabolic switch was shown by Dr. Loscalzo's group to be regulated by hypoxia-mediated upregulation of microRNA-210 to decrease the expression of iron-sulfur cluster assembly proteins 1 and 2. These proteins facilitate the assembly of iron-sulfur clusters, which are necessary for Complex I and aconitase activity to facilitate electron transport and mitochondrial oxidation–reduction reactions. Thus, microRNA-210 is a key regulator of the cellular adaptation to hypoxia (4). These data suggest that microRNA-210 functions as a master microRNA under hypoxic conditions and modulates disease states characterized by hypoxia, such as ischemia and tumorigenesis (3) (Fig. 1).
Description of Key Finding 1
S-nitrosothiols and the biologic effects of NO•
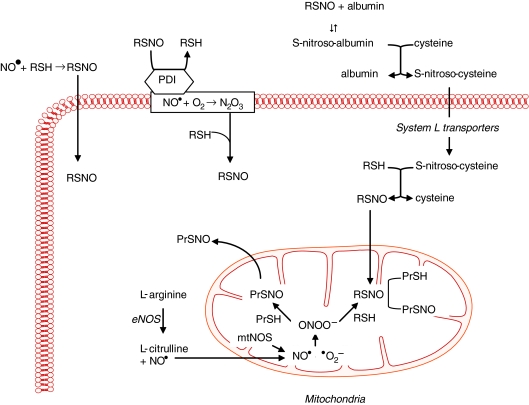
Dr. Loscalzo first demonstrated that the antiplatelet effects of organic nitrates or endothelium-derived NO• were dependent on thiols to generate S-nitrosothiols, and he confirmed this finding by showing that S-nitrosoproteins, such as S-nitroso-albumin, were potent antiplatelet agents (22, 29). This discovery by Dr. Loscalzo established the fundamental role of S-nitrosothiols in modulating the biologic effects of NO•. Subsequently, S-nitrosoglutathione was shown to enhance platelet formation from megakaryocytes by inducing apoptosis (1, 2). Loscalzo's group demonstrated that S-nitrosothiols are formed naturally in vivo and serve as a stable reservoir of NO• (28, 33). Subsequently, they found that de novo formation of S-nitrosothiols occurred through several mechanisms, including S-transnitrosation (Fig. 2) (34, 35). This process was shown to be catalyzed by the cell-surface protein disulfide isomerase, which, through S-transnitrosation, facilitated the transfer of NO• from the extracellular to the intracellular compartment (45). In vivo, S-nitrosoproteins with limited intracellular access were shown to exert their biologic actions by undergoing thiol-S-nitrosothiol exchange with low-molecular-weight thiols to form low-molecular-weight S-nitrosothiols (28). Loscalzo's group demonstrated further that endogenous NO• or exogenous S-nitrosothiols promoted the formation of S-nitrosoproteins in endothelial cells. These S-nitrosoproteins were localized principally to the mitochondria and the perimitochondrial compartment, with a half-life of ∼1 h, and their formation paralleled eNOS activity and mitochondrial function. Mass spectrometry revealed that a limited repertoire of S-nitrosoproteins exists in resting endothelial cells, with GAPDH being the most abundant, suggesting that NO• may play a role in regulating glycolysis by this mechanism (43).
FIG. 2.
S-nitrosothiol and S-nitrosoprotein formation. S-Nitrosothiols (RSNO) are formed when NO• reacts with a low- or high-molecular-weight thiol (RSH) by the exchange of −H for −NO between sulfur groups, a process known as S-transnitrosation. This reaction is catalyzed by cell-surface protein disulfide isomerase (PDI); PDI also facilitates the transport of NO• by localizing the molecule to the cell-membrane space, where it may react with molecular oxygen to generate N2O3, which is a nitrosating agent. In plasma, S-nitrosothiols may react with albumin to generate S-nitrosoalbumin, the most abundant circulating S-nitrosated protein. S-Nitrosoalbumin may also participate in thiol-nitrosothiol exchange with low-molecular-weight thiols such as cysteine. Once S-nitrosocysteine is formed, it is transported across the cell membrane by the System L transporters to reside in the cytoplasm. Here, S-nitrosocysteine may once again participate in thiol-nitrosothiol exchange reactions to generate intracellular S-nitrosothiols. S-Nitrosothiols also play an important role in the formation of S-nitrosoproteins (PrSNO). The majority of S-nitrosoproteins are localized to the mitochondria. Within the mitochondria, NO• synthesized by eNOS or mitochondrial NOS may react with superoxide (•O2−) to form peroxynitrite (ONOO−). Peroxynitrite, in turn, may react with thiol-containing proteins (PrSHs) to yield S-nitrosoproteins or may first react with RSH to generate an S-nitrosothiol, which then facilitates S-nitrosoprotein formation. Adapted from (10). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Description of Key Finding 2
Nitric oxide and platelet activation
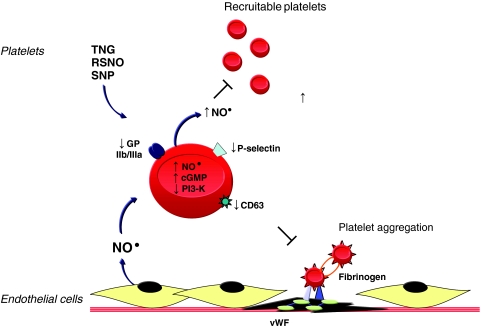
Dr. Loscalzo's laboratory has shown that endogenous or exogenous NO• modulates platelet activation, recruitment, and aggregation (Fig. 3). Endothelium-derived NO•, in the presence of N-acetylcysteine, inhibited ex vivo aggregation by increasing platelet cGMP levels (33). In vivo, in a coronary artery stenosis model, NO• inhibited periodic platelet thrombus formation through a similar mechanism (6, 27). Nitroglycerin acts synergistically with tissue plasminogen activator or the platelet inhibitory prostaglandins I2 and E1 to promote platelet disaggregation, whereas inhibiting NO• with NG-mono-methyl-l-arginine had the opposite effect and decreased bleeding time (30, 36). The mechanisms by which NO• inhibits agonist-induced platelet aggregation include decreasing the expression of platelet P-selectin, CD63, and the fibrinogen receptor GPIIb/IIIa (25); preventing the binding of fibrinogen to platelets (24); and inhibiting PI3-kinase activation in platelets, a step that renders aggregation irreversible (26). Further studies revealed that NO• production by platelets themselves did not influence platelet activation significantly but played a role in limiting platelet recruitment to the growing thrombus (10). In vivo studies in eNOS-/- mice revealed that bleeding times in these mice were decreased significantly compared with wild-type mice, and this was associated with increased platelet recruitment (12). Clinically, patients with acute coronary syndromes were also found to produce less platelet-derived NO• (13). These findings highlight the importance of endothelium- and platelet-derived NO• to limit platelet aggregation and further to identify the antiplatelet properties associated with the administration of exogenous NO• donors.
FIG. 3.
Nitric oxide and platelet aggregation. Nitric oxide donors, including nitroglycerin (TNG), S-nitrosothiols (RSNOs), and sodium nitroprusside (SNP), or endothelium-derived NO• inhibit agonist-induced platelet aggregation by increasing platelet cGMP levels; decreasing expression of the fibrinogen receptor GPIIb/IIIa, P-selectin, and CD63; and inhibiting PI3-kinase activation, to limit irreversible platelet aggregation. Platelet-derived NO•, in turn, inhibits the recruitment of circulating platelets to sites of vascular injury. vWF, von Willebrand factor. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Description of Key Finding 3
Oxidative enzymopathies
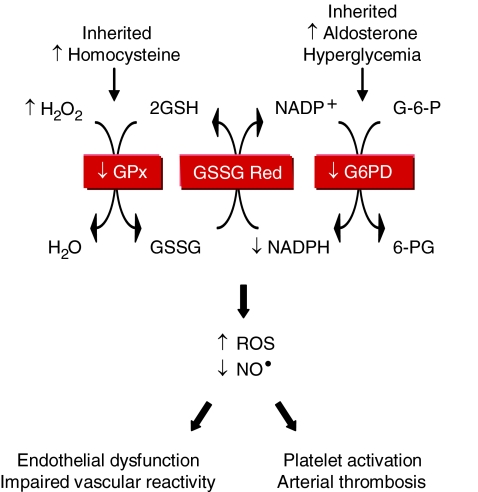
Loscalzo's laboratory found that inherited or acquired deficiency of glucose-6-phosphate dehydrogenase or the antioxidant enzyme glutathione peroxidase(s) is associated with increased oxidant stress, decreased bioavailable NO•, and vascular dysfunction (Fig. 4). Deficient glucose-6-phosphate dehydrogenase activity increased ROS levels (19); inhibited endothelial cell proliferation, migration, and tube formation in vitro, as well as angiogenesis in vivo (21); impaired vascular reactivity to endothelium-dependent and -independent vasodilators (19, 20); and mediated the adverse effects of aldosterone on vascular function by contributing to endothelial dysfunction and oxidative posttranslational modification of soluble guanylyl cyclase (20, 23).
FIG. 4.
Antioxidant enzyme deficiency and oxidative enzymopathies. The antioxidant enzymes glutathione peroxidase-1 and -3 (GPx) and glucose-6-phosphate dehydrogenase (G6PD) limit intracellular oxidant stress through a series of coupled reactions that (re)generate reduced glutathione (GSH). GPx activity may be decreased by a heritable deficiency of the enzyme or acquired, as occurs with hyperhomocysteinemia. Similarly, G6PD activity may be decreased by a heritable deficiency of the enzyme or acquired, as occurs in the hyperaldosteronism or hyperglycemia associated with diabetes mellitus. The net result of deficient activity of either of these antioxidant enzymes is an increase in ROS and a decrease in bioavailable NO•. This pathobiologic state results in endothelial dysfunction and impaired vascular reactivity, and, in the case of glutathione peroxidase-3 deficiency, platelet activation and arterial thrombosis. H2O2, hydrogen peroxide; GSSG, oxidized glutathione; GSSG reductase, glutathione reductase; G-6-P, glucose-6-phosphate; 6-PG, 6-phosphogluconate. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
They also found that GPx-1 deficiency was associated with endothelial dysfunction and vascular structural abnormalities, including increased matrix deposition and intimal thickening (7, 8). Deficiency of plasma GPx, or GPx-3, promoted platelet insensitivity to NO•, thrombosis, and was associated with stroke (9, 11). This led to the identification of a unique promoter haplotype (H2) of seven tightly linked polymorphisms in the GPx-3 gene that is overrepresented in young patients with arterial ischemic stroke and central venous thrombosis (37, 38).
In related studies, Loscalzo's laboratory demonstrated that mild-to-moderate hyperhomocysteinemia was associated with endothelial dysfunction, enhanced lipid peroxidation, and impaired vasodilation (5, 17, 41). These effects occurred, in part, as a result of homocysteine-mediated inhibition of GPx-1 translation (16), and overexpression of GPx-1 rescued the adverse vascular phenotype (42). Elevated levels of homocysteine also were shown to underlie the negative effects of l-arginine supplementation on vascular function as a result of conversion of l-arginine to creatine and, thereby, homocysteine (15).
Other Achievements
5-Lipoxygenase and pulmonary hypertension
In pulmonary artery endothelial cells and animal models of pulmonary hypertension, Dr. Loscalzo's group demonstrated the adverse biologic effects of 5-lipoxygenase, a nonheme iron-containing dioxygenase that generates the biologically active leukotrienes and 5-hydroxyeicosatetraenoic acid. Increased 5-lipoxygenase expression decreased bioavailable NO• and cGMP levels in pulmonary artery endothelial cells, whereas inhibition of 5-lipoxygenase limited cell proliferation by causing a cell-cycle block at G0/G1 (40, 46). In the monocrotaline-rat model of pulmonary hypertension, pulmonary overexpression of 5-lipoxygenase increased right ventricular systolic pressure, hypertrophy, inflammation, and muscularization of small- and medium-sized pulmonary vessels (18). Bone morphogenetic protein receptor-2 (BMPR2) mutations have also been linked to pulmonary arterial hypertension. In BMPR2 heterozygous mutant mice, pulmonary vascular overexpression of 5-lipoxygenase increased pulmonary artery pressures, vascular remodeling, and thromboxane A2 production compared with unstressed mice (32). Challenge with 5-lipoxygenase and monocrotaline resulted in early and severe increases in pulmonary pressures, vascular endothelial injury, and perivascular infiltration of inflammatory and immune cells. This work suggests that increased 5-lipoxygenase expression enhances the susceptibility to pulmonary hypertension when the BMPR2 mutation is present (31).
Current Position
Dr. Loscalzo is the Hersey Professor of the Theory and Practice of Medicine at Harvard Medical School, Vice Director of the Biomedical Research Institute, and Chairman of the Department of Medicine at Brigham and Women's Hospital, a department of approximately 900 full-time clinicians and researchers. Dr. Loscalzo's research laboratory comprises 15 members currently, and his scientific discoveries have led to 30 patents for his work. He currently holds several NIH awards, including a Method to Extend Research in Time (MERIT) Award from the NHLBI. He is a member of the Council of Councils at the NIH and an elected member of the American Society of Clinical Investigation and the Institute of Medicine of the National Academy of Sciences.
Dr. Loscalzo is the Editor-in-Chief of Circulation; an Associate Editor for the Interactive Medical Case Series at the New England Journal of Medicine; a Consulting Editor for the Journal of Clinical Investigation, Circulation Research, and Arteriosclerosis, Thrombosis and Vascular Biology; and is a member of the editorial board of 16 journals. He is also a senior editor of Harrison's Principles of Internal Medicine.
Dr. Loscalzo has a profound commitment to teaching and has trained more than 50 investigators in his laboratory, many of whom have gone on to successful careers in biomedical research and leadership positions at prominent institutions. According to Dr. Loscalzo, “Biomedical research is an extraordinarily gratifying enterprise. Solving a perplexing problem in an innovative and rigorous way, creating new knowledge in the process, and using that knowledge to (re)define an underlying mechanism are each highly rewarding aspects of the research process. Perhaps the most rewarding feature, however, is the ability to influence the next generation of scientists through the impact of one's scientific contributions, and the guidance and advice given to one's trainees. Those of us who have succeeded in developing biomedical research careers have been given a great and special opportunity for which I, for one, am most grateful.”
Supplementary Material
Abbreviations Used
- eNOS
endothelial isoform of nitric oxide synthase
- GPx-1
glutathione peroxidase-1
- GPx-3
glutathione peroxidase-3
- NO•
nitric oxide
- ROS
reactive oxygen species
Footnotes
Reviewing Editors: Dipak K. Das, Aron Fisher, Santiago Lamas, Elizabeth Murphy, and Pasquale Pagliaro
Author note: I met Dr. Loscalzo in 1994 as a Cardiology Fellow at Boston University School of Medicine and joined his research laboratory. Since then, we have been collaborating on studies that examine the pathophysiologic consequences of perturbations of redox homeostasis and nitric oxide deficiency on vascular function.
For a list of frequently cited articles published by Prof. Loscalzo, see Supplemental Tables 1 and 2, available online at www.liebertonline.com/ars.
Acknowledgments
Professor Joseph Loscalzo thanks the many extremely talented individuals with whom he has had the great good fortune to work with over the past 30 years, including his mentors and collaborators, but especially his former and current trainees. “It is their intellectual contributions, commitment, and hard work that form the basis for the recognition I have received. I wish to thank them deeply for what they have taught me, and what we have scientifically contributed together.”
This work was supported by NIH grants HL81110 and HL70819.
References
- 1.Battinelli E. Loscalzo J. Nitric oxide induces apoptosis in megakaryocytic cell lines. Blood. 2000;95:3451–3459. [PubMed] [Google Scholar]
- 2.Battinelli E. Willoughby SR. Foxall T. Valeri CR. Loscalzo J. Induction of platelet formation from megakaryocytoid cells by nitric oxide. Proc Natl Acad Sci U S A. 2001;98:14458–14463. doi: 10.1073/pnas.241427398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan SY. Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle. 2010;9:1072–1083. doi: 10.4161/cc.9.6.11006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan SY. Zhang YY. Hemann C. Mahoney CE. Zweier JL. Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–284. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eberhardt RT. Forgione MA. Cap A. Leopold JA. Rudd MA. Trolliet M. Heydrick S. Stark R. Klings ES. Moldovan NI. Yaghoubi M. Goldschmidt-Clermont PJ. Farber HW. Cohen R. Loscalzo J. Endothelial dysfunction in a murine model of mild hyperhomocyst(e)inemia. J Clin Invest. 2000;106:483–491. doi: 10.1172/JCI8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Folts JD. Stamler J. Loscalzo J. Intravenous nitroglycerin infusion inhibits cyclic blood flow responses caused by periodic platelet thrombus formation in stenosed canine coronary arteries. Circulation. 1991;83:2122–2127. doi: 10.1161/01.cir.83.6.2122. [DOI] [PubMed] [Google Scholar]
- 7.Forgione MA. Cap A. Liao R. Moldovan NI. Eberhardt RT. Lim CC. Jones J. Goldschmidt-Clermont PJ. Loscalzo J. Heterozygous cellular glutathione peroxidase deficiency in the mouse: abnormalities in vascular and cardiac function and structure. Circulation. 2002;106:1154–1158. doi: 10.1161/01.cir.0000026820.87824.6a. [DOI] [PubMed] [Google Scholar]
- 8.Forgione MA. Weiss N. Heydrick S. Cap A. Klings ES. Bierl C. Eberhardt RT. Farber HW. Loscalzo J. Cellular glutathione peroxidase deficiency and endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2002;282:H1255–H1261. doi: 10.1152/ajpheart.00598.2001. [DOI] [PubMed] [Google Scholar]
- 9.Freedman JE. Frei B. Welch GN. Loscalzo J. Glutathione peroxidase potentiates the inhibition of platelet function by S-nitrosothiols. J Clin Invest. 1995;96:394–400. doi: 10.1172/JCI118047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freedman JE. Loscalzo J. Barnard MR. Alpert C. Keaney JF. Michelson AD. Nitric oxide released from activated platelets inhibits platelet recruitment. J Clin Invest. 1997;100:350–356. doi: 10.1172/JCI119540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freedman JE. Loscalzo J. Benoit SE. Valeri CR. Barnard MR. Michelson AD. Decreased platelet inhibition by nitric oxide in two brothers with a history of arterial thrombosis. J Clin Invest. 1996;97:979–987. doi: 10.1172/JCI118522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freedman JE. Sauter R. Battinelli EM. Ault K. Knowles C. Huang PL. Loscalzo J. Deficient platelet-derived nitric oxide and enhanced hemostasis in mice lacking the NOSIII gene. Circ Res. 1999;84:1416–1421. doi: 10.1161/01.res.84.12.1416. [DOI] [PubMed] [Google Scholar]
- 13.Freedman JE. Ting B. Hankin B. Loscalzo J. Keaney JF., Jr Vita JA. Impaired platelet production of nitric oxide predicts presence of acute coronary syndromes. Circulation. 1998;98:1481–1486. doi: 10.1161/01.cir.98.15.1481. [DOI] [PubMed] [Google Scholar]
- 14.Handy DE. Lubos E. Yang Y. Galbraith JD. Kelly N. Zhang YY. Leopold JA. Loscalzo J. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J Biol Chem. 2009;284:11913–11921. doi: 10.1074/jbc.M900392200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handy DE. Scolaro J. Chen J. Huang P. Loscalzo J. l-Arginine increases plasma homocysteine in apoE-/-/iNOS-/- double knockout mice. Cell Mol Biol (Noisy-le-grand) 2004;50:903–909. [PubMed] [Google Scholar]
- 16.Handy DE. Zhang Y. Loscalzo J. Homocysteine down-regulates cellular glutathione peroxidase (GPx1) by decreasing translation. J Biol Chem. 2005;280:15518–15525. doi: 10.1074/jbc.M501452200. [DOI] [PubMed] [Google Scholar]
- 17.Heydrick SJ. Weiss N. Thomas SR. Cap AP. Pimentel DR. Loscalzo J. Keaney JF., Jr. l-Homocysteine and l-homocystine stereospecifically induce endothelial nitric oxide synthase-dependent lipid peroxidation in endothelial cells. Free Radic Biol Med. 2004;36:632–640. doi: 10.1016/j.freeradbiomed.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Jones JE. Walker JL. Song Y. Weiss N. Cardoso WV. Tuder RM. Loscalzo J. Zhang YY. Effect of 5-lipoxygenase on the development of pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol. 2004;286:H1775–H1784. doi: 10.1152/ajpheart.00281.2003. [DOI] [PubMed] [Google Scholar]
- 19.Leopold JA. Cap A. Scribner AW. Stanton RC. Loscalzo J. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J. 2001;15:1771–1773. doi: 10.1096/fj.00-0893fje. [DOI] [PubMed] [Google Scholar]
- 20.Leopold JA. Dam A. Maron BA. Scribner AW. Liao R. Handy DE. Stanton RC. Pitt B. Loscalzo J. Aldosterone impairs vascular reactivity by decreasing glucose-6-phosphate dehydrogenase activity. Nat Med. 2007;13:189–197. doi: 10.1038/nm1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leopold JA. Walker J. Scribner AW. Voetsch B. Zhang YY. Loscalzo AJ. Stanton RC. Loscalzo J. Glucose-6-phosphate dehydrogenase modulates vascular endothelial growth factor-mediated angiogenesis. J Biol Chem. 2003;278:32100–32106. doi: 10.1074/jbc.M301293200. [DOI] [PubMed] [Google Scholar]
- 22.Loscalzo J. N-Acetylcysteine potentiates inhibition of platelet aggregation by nitroglycerin. J Clin Invest. 1985;76:703–708. doi: 10.1172/JCI112024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maron BA. Zhang YY. Handy DE. Beuve A. Tang SS. Loscalzo J. Leopold JA. Aldosterone increases oxidant stress to impair guanylyl cyclase activity by cysteinyl thiol oxidation in vascular smooth muscle cells. J Biol Chem. 2009;284:7665–7672. doi: 10.1074/jbc.M809460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mendelsohn ME. O'Neill S. George D. Loscalzo J. Inhibition of fibrinogen binding to human platelets by S-nitroso-N-acetylcysteine. J Biol Chem. 1990;265:19028–19034. [PubMed] [Google Scholar]
- 25.Michelson AD. Benoit SE. Furman MI. Breckwoldt WL. Rohrer MJ. Barnard MR. Loscalzo J. Effects of nitric oxide/EDRF on platelet surface glycoproteins. Am J Physiol. 1996;270:H1640–H1648. doi: 10.1152/ajpheart.1996.270.5.H1640. [DOI] [PubMed] [Google Scholar]
- 26.Pigazzi A. Heydrick S. Folli F. Benoit S. Michelson A. Loscalzo J. Nitric oxide inhibits thrombin receptor-activating peptide-induced phosphoinositide 3-kinase activity in human platelets. J Biol Chem. 1999;274:14368–14375. doi: 10.1074/jbc.274.20.14368. [DOI] [PubMed] [Google Scholar]
- 27.Rovin JD. Stamler JS. Loscalzo J. Folts JD. Sodium nitroprusside, an endothelium-derived relaxing factor congener, increases platelet cyclic GMP levels and inhibits epinephrine-exacerbated in vivo platelet thrombus formation in stenosed canine coronary arteries. J Cardiovasc Pharmacol. 1993;22:626–631. doi: 10.1097/00005344-199310000-00017. [DOI] [PubMed] [Google Scholar]
- 28.Scharfstein JS. Keaney JF., Jr Slivka A. Welch GN. Vita JA. Stamler JS. Loscalzo J. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994;94:1432–1439. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon DI. Stamler JS. Jaraki O. Keaney JF. Osborne JA. Francis SA. Singel DJ. Loscalzo J. Antiplatelet properties of protein S-nitrosothiols derived from nitric oxide and endothelium-derived relaxing factor. Arterioscler Thromb. 1993;13:791–799. doi: 10.1161/01.atv.13.6.791. [DOI] [PubMed] [Google Scholar]
- 30.Simon DI. Stamler JS. Loh E. Loscalzo J. Francis SA. Creager MA. Effect of nitric oxide synthase inhibition on bleeding time in humans. J Cardiovasc Pharmacol. 1995;26:339–342. doi: 10.1097/00005344-199508000-00022. [DOI] [PubMed] [Google Scholar]
- 31.Song Y. Coleman L. Shi J. Beppu H. Sato K. Walsh K. Loscalzo J. Zhang YY. Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice. Am J Physiol Heart Circ Physiol. 2008;295:H677–690. doi: 10.1152/ajpheart.91519.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song Y. Jones JE. Beppu H. Keaney JF., Jr Loscalzo J. Zhang YY. Increased susceptibility to pulmonary hypertension in heterozygous BMPR2-mutant mice. Circulation. 2005;112:553–562. doi: 10.1161/CIRCULATIONAHA.104.492488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stamler J. Mendelsohn ME. Amarante P. Smick D. Andon N. Davies PF. Cooke JP. Loscalzo J. N-Acetylcysteine potentiates platelet inhibition by endothelium-derived relaxing factor. Circ Res. 1989;65:789–795. doi: 10.1161/01.res.65.3.789. [DOI] [PubMed] [Google Scholar]
- 34.Stamler JS. Jaraki O. Osborne J. Simon DI. Keaney J. Vita J. Singel D. Valeri CR. Loscalzo J. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci USA. 1992;89:7674–7677. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stamler JS. Singel DJ. Loscalzo J. Biochemistry of nitric oxide and its redox-activated forms. Science. 1992;258:1898–1902. doi: 10.1126/science.1281928. [DOI] [PubMed] [Google Scholar]
- 36.Stamler JS. Vaughan DE. Loscalzo J. Synergistic disaggregation of platelets by tissue-type plasminogen activator, prostaglandin E1, and nitroglycerin. Circ Res. 1989;65:796–804. doi: 10.1161/01.res.65.3.796. [DOI] [PubMed] [Google Scholar]
- 37.Voetsch B. Jin RC. Bierl C. Benke KS. Kenet G. Simioni P. Ottaviano F. Damasceno BP. Annichino-Bizacchi JM. Handy DE. Loscalzo J. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: a novel risk factor for arterial ischemic stroke among young adults and children. Stroke. 2007;38:41–49. doi: 10.1161/01.STR.0000252027.53766.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voetsch B. Jin RC. Bierl C. Deus-Silva L. Camargo EC. Annichino-Bizacchi JM. Handy DE. Loscalzo J. Role of promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene as a risk factor for cerebral venous thrombosis. Stroke. 2008;39:303–307. doi: 10.1161/STROKEAHA.107.490094. [DOI] [PubMed] [Google Scholar]
- 39.Walford GA. Moussignac RL. Scribner AW. Loscalzo J. Leopold JA. Hypoxia potentiates nitric oxide-mediated apoptosis in endothelial cells via peroxynitrite-induced activation of mitochondria-dependent and -independent pathways. J Biol Chem. 2004;279:4425–4432. doi: 10.1074/jbc.M310582200. [DOI] [PubMed] [Google Scholar]
- 40.Walker JL. Loscalzo J. Zhang YY. 5-Lipoxygenase and human pulmonary artery endothelial cell proliferation. Am J Physiol Heart Circ Physiol. 2002;282:H585–H593. doi: 10.1152/ajpheart.00003.2001. [DOI] [PubMed] [Google Scholar]
- 41.Weiss N. Heydrick S. Zhang YY. Bierl C. Cap A. Loscalzo J. Cellular redox state and endothelial dysfunction in mildly hyperhomocysteinemic cystathionine beta-synthase-deficient mice. Arterioscler Thromb Vasc Biol. 2002;22:34–41. doi: 10.1161/hq1201.100456. [DOI] [PubMed] [Google Scholar]
- 42.Weiss N. Zhang YY. Heydrick S. Bierl C. Loscalzo J. Overexpression of cellular glutathione peroxidase rescues homocyst(e)ine-induced endothelial dysfunction. Proc Natl Acad Sci USA. 2001;98:12503–12508. doi: 10.1073/pnas.231428998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang Y. Loscalzo J. S-Nitrosoprotein formation and localization in endothelial cells. Proc Natl Acad Sci U S A. 2005;102:117–122. doi: 10.1073/pnas.0405989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang Y. Song Y. Loscalzo J. Regulation of the protein disulfide proteome by mitochondria in mammalian cells. Proc Natl Acad Sci USA. 2007;104:10813–10817. doi: 10.1073/pnas.0702027104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zai A. Rudd MA. Scribner AW. Loscalzo J. Cell-surface protein disulfide isomerase catalyzes transnitrosation and regulates intracellular transfer of nitric oxide. J Clin Invest. 1999;103:393–399. doi: 10.1172/JCI4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang YY. Walker JL. Huang A. Keaney JF. Clish CB. Serhan CN. Loscalzo J. Expression of 5-lipoxygenase in pulmonary artery endothelial cells. Biochem J. 2002;361:267–276. doi: 10.1042/0264-6021:3610267. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.