Abstract
Mitochondria are a principal site for generation of reactive oxygen species (ROS) in the heart. Peroxisome proliferator activated receptor γ coactivator 1α (PGC-1α) plays an important role in regulating mitochondrial biogenesis and myocardial metabolism, but whether PGC-1α can simultaneously upregulate myocardial mitochondrial antioxidants has not been studied. In the present study, we examined the effect of PGC-1α deficiency (PGC-1α−/−) on oxidative stress and expression of a group of mitochondrial antioxidants in normal hearts and in hearts exposed to chronic systolic pressure overload produced by transverse aortic constriction (TAC). We found that PGC-1α−/− caused moderate but significant decreases of myocardial mitochondrial antioxidant enzymes such as SOD2, and thioredoxin (Trx2), but had no effect on expression of myocardial oxidative stress markers and left ventricular (LV) function under basal conditions. However, in response to TAC for 6 weeks, PGC-1α−/− mice showed greater increases of myocardial oxidative stress markers 3’-nitrotyrosine and 4-hydroxynonenal, more severe LV hypertrophy and dilatation, pulmonary congestion, and a greater reduction of LV fractional shortening and dP/dtmax than did wild-type hearts. SOD mimetic MnTMPyP treatment (6 mg/kg/day) significantly attenuated TAC-induced LV hypertrophy and dysfunction in PGC-1α−/− mice. These data indicate that PGC-1α plays an important role in regulating expression of myocardial mitochondrial antioxidants SOD2 and Trx2 and in protecting hearts against TAC-induced myocardial oxidative stress, hypertrophy, and dysfunction. Antioxid. Redox Signal. 13, 1011–1022.
Introduction
In the heart, myocardial ATP is supplied almost exclusively by oxidative metabolism in the mitochondria. During respiration, a small fraction of the unpaired elections escape from the mitochondrial electron transport chain and react with molecular oxygen to produce superoxide and other reactive oxygen species (ROS) (3). As a result, mitochondria are responsible for a major fraction of the ROS generated in cardiac myocytes (3). The mitochondrial antioxidant enzymes form the first line of defense against mitochondrial ROS, including manganese superoxide dismutase (SOD2, the enzyme that scavenges superoxide anion to produce H2O2), and peroxiredoxin III (Prx3), V (Prx5), mitochondrial thioredoxin (Trx2), and mitochondrial thioredoxin reductase (TrxR2). By using global and/or cardiac specific gene deletion or overexpression of SOD2 (22), Prx3 (21), Trx2 (23), or TrxR2 (4), previous studies have demonstrated that SOD2, Prx3, Trx2, and TrxR2 can protect the heart against oxidative injury and ventricular dysfunction resulting from oxidative stress.
Peroxisome proliferator activated receptor γ coactivator 1α (PGC-1α) is a potent regulator of myocardial energy metabolism and mitochondrial biogenesis(1, 6, 13, 33). PGC-1α can bind to the transcriptional nuclear respiratory factors (NRF-1), mitochondrial DNA transcription factor A (mtTFA), and other metabolic transcriptional nuclear factors to regulate mitochondrial biogenesis and function (33). PGC-1α can also regulate mitochondrial antioxidant enzymes' expression and activity in cultured vascular endothelial cells (32) and brain tissues (29). Overexpression of PGC-1α in vascular endothelial cells increased mitochondrial antioxidant enzyme expression, and decreased oxidative stress and cell death (32). Furthermore, PGC-1α null mice had reduced expression of mitochondrial antioxidants, including SOD2 and UCP2, in brain tissue (29). In a neurodegenerative model, these PGC-1α-/- mice had greatly increased vulnerability to oxidative injury of the dopaminergic neurons in the substantia nigra and hippocampus (29). However, the effect of PGC-1α on oxidative stress and the expression of mitochondrial antioxidants in the heart has not been reported.
Congestive heart failure (CHF) is associated with depressed antioxidant reserves and increased products of ROS (12), suggesting that oxidative stress may contribute to the progression of heart failure (7). To investigate the role of PGC-1α in regulating myocardial antioxidants and the metabolism of ROS, we examined the effect of PGC-1α−/− on myocardial oxidative stress, the expression of a group of antioxidants in the heart, and the development of LV hypertrophy and CHF in mice exposed to chronic systolic pressure overload produced by transverse aortic constriction (TAC). In response to mechanical constriction of the aorta that increased systolic aortic pressure, the LV adapts to increased workload through hypertrophy of individual muscle cells (26). Although this adaptive response may provide initial salutary compensation to the stress, sustained hypertrophic stimulation becomes maladaptive, worsening morbidity and mortality risks (31). Growing evidence indicates oxidative stress plays a pivotal role for this maladaptation. Here we report that PGC-1α−/− resulted in decreased mitochondrial antioxidant enzymes (SOD2, Prx3, Prx5, Trx2, and TrxR2) and increased oxidative stress when the hearts were exposed to TAC, and this was associated with more severe LV dilation and contractile dysfunction, as well as greater hypertrophy and fibrosis. In contrast, PGC-1α−/− had no significant effect on the expression of several antioxidants that are predominantly expressed in the cytosol or extracellular space. In addition, PGC-1α−/− also significantly attenuated expressions of myocardial cytochrome C (Cyt-c), cytochrome C oxidase subunit-III (COX-III), and metabolic transcriptional factors ERRα and mtTFA under control conditions or after TAC. As PGC-1α−/− has no significant effect on LV structure, oxidative stress, and function under unstressed conditions, these findings imply that regulation of the mitochondrial ROS defense system by PGC-1α is not critical in protecting the unstressed hearts, but it is important in protecting the overloaded heart.
Methods
Mice and TAC-induced systolic overload
Male C57BL/6 (Taconic, Germantown, NY) and PGC-1α−/− mice (16) (congenic with the Taconic C57BL/6 strain) 8–10 weeks of age were used. This study was approved by the Animal Care and Use Committee of the University of Minnesota. A TAC procedure (using a 26G needle to calibrate the degree of aortic constriction) was performed on wild-type (n = 14) and PGC-1α–/– mice (n = 13) using the minimally invasive suprasternal approach as previously described (9). To ensure that similar pressure overload was produced in the PGC-1α−/− and WT mice, the TAC procedure was performed on PGC-1α−/− and corresponding Wt mice on the same day by the same surgeon who was blinded as to the genotype of the mice. Body weight and age matched WT mice (n = 7) and PGC-1α–/– mice (n = 8) with sham operated were used as controls. For SOD mimetic manganese(III) tetrakis(1-methyl-4-pyridyl) porphyrin (MnTMPyP, Calbiochem, Gibbstown, NJ) study, PGC-1α–/– mice were randomly assigned to two groups after TAC, one group treated with MnTMPyP (6 mg/kg/day i.p.) and the other treated with vehicle for 6 weeks.
Echocardiography and evaluation of LV hemodynamics
Mice were anesthetized with 1.5% isoflurane. Echocardiographic images were obtained with a Visualsonics high resolution Veve 770 system as we previously described (18, 20, 34). For pressure measurements, a 1.2 Fr. pressure catheter (Scisense Inc., Ontario, Canada) was introduced through the right common carotid artery into the ascending aorta and then advanced into the LV for measurement of systolic and end-diastolic pressures, and positive and negative LV dP/dtmax, as we previous described (18, 20, 34).
Western blot analysis
Protein content was analyzed using Western blots as previously described (n = 6 samples each group) (20) with primary antibodies against ANP (Peninsula Biolabs, Belmont, MA), nitrotyrosine, 4-HNE, ERRα, PPARα (Millipore, Temecula, CA), Cytochrome C (Cell Signaling, Danvers, MA), MCAD (Cayman Chemical, Ann Arbor, MI), COX-III, CD36, peroxiredoxin III (Prx3), V (Prx5), mitochondrial thioredoxin (Trx2), mitochondrial thioredoxin reductase (TrxR2), SOD1, SOD2, GAPDH (Santa Cruz, Santa Cruz, CA), SOD3, Catalase (Sigma, St. Louis, MO). HRP conjugated secondary antibodies were from BioRad Laboratories (Hercules, CA) and Sigma.
Quantitative real-time PCR
Total RNA was reverse-transcribed using random hexamers and Moloney murine leukemia virus (MMLV) reverse transcriptase (Clontech, Mountain View, CA). The real-time PCR reaction was carried out using the Light Cycler Thermocycler (Roche Diagnostics Corp, Basel, Switzerland). Primers are listed in Table 1. Results were normalized to 18S rRNA.
Table 1.
Primers Used in Real-time PCR
| Sense | Antisense | |
|---|---|---|
| PGC-1β | 5′-CACGGTTTTATCACCTTCCG-3′ | 5′-GCTCATTGCGCTTTCTCA GG-3′ |
| ERRα | 5′-TGGAGCGGGAGGAGTACGTC-3′ | 5′-CAGCCTCAGCATCTTCAATGTG-3′ |
| ERRγ | 5′-CTCTGTGACTTGGCTGAC CG-3′ | 5′-CCAGGGACAGTGTGGAGAAGC-3′ |
| PPARα | 5′-TTGTGGCCAAGATGGTGGCCAA-3′ | 5′-CAGTTCTAAGGCATTGAACTTC-3′ |
| NRF-1 | 5′-GGCGGGAGGATCTTTTATATGCTTTTGA-3′ | 5′-GGCCTCTGATGCTTGCGTCGTCT-3′ |
| mtTFA | 5′-GTCCATAGGCACCGTATTGC-3′ | 5′-CCCATGCTG GAA AAA CACTT-3′ |
| Prx3 | 5′-CTGAGTGTCAACGACCTTCCG-3′ | 5′-ACTGGAACGCCTTTACCAAACG-3′ |
| Prx5 | 5′-CCAAGTTCACCTTCTTTCCCG-3′ | 5′-GGAGATGCCATTCCCTCAGTG-3′ |
| TrxR2 | 5′-GAGGATTTCCCA AAGAGCCG-3′ | 5′-ATGCCATTGGAGATGTTGCTGA-3′ |
| Trx2 | 5′-GACTCTGGTGGTGTGTACTGTCCG-3′ | 5′-GGCTTCCCTCACCTCTAAGACC-3′ |
| SOD2 | CCTACGTGAACAATCTCA ACG-3′ | 5′-GGCTGAAGAGCGACCTGAGTT-3′ |
Histological analysis
Tissue sections (6 μm) from the central portion of the LV were stained with Sirius Red (Sigma) for fibrosis, and FITC-conjugated wheat germ agglutinin (AF488, Invitrogen, Carlsbad, CA) to evaluate myocyte size. For mean myocyte size, the cross sectional area of at least 120 cells/sample and four samples/group were averaged. The percent fibrosis was determined as described previously (20).
Citrate synthase activity assay
Total citrate synthase activity was measured by citrate synthase assay kit (Sigma).
Data and statistical analysis
All values are expressed as mean ± standard error. Statistical significance was defined as p < 0.05. Two-way analysis of variance (ANOVA) was used to test each variable for differences among the treatment groups with StatView (SAS Institute Inc, New York, NY). If ANOVA demonstrated a significant effect, pairwise post hoc comparisons were made with Fisher's least significant difference test.
Results
PGC-1α-/- exacerbated TAC-induced ventricular hypertrophy and fibrosis
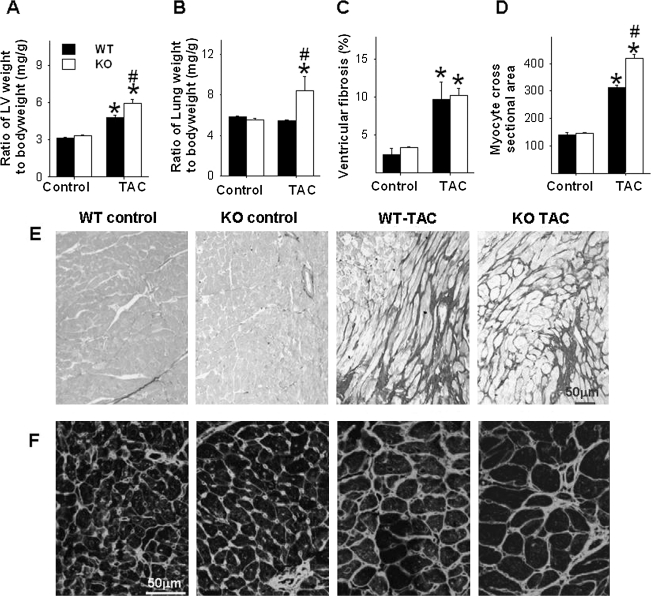
Under control conditions, ventricular weight and the ratio of ventricular weight to body weight were not different between PGC-1α−/− and wild-type mice (Fig. 1A). Histological staining of LV tissue showed no difference in cardiac myocyte size or relative fibrosis between PGC-1α−/− and Wt mice (Figs. 1C–1F). After 6 weeks of TAC, the LV weight and the ratio of LV weight to body weight were significantly higher in the PGC-1α−/− mice than in the wild-type mice (Fig. 1A), indicating that loss of PGC-1α exacerbated the TAC-induced myocardial hypertrophy. Histological staining showed that TAC caused significantly greater increases of cardiac myocyte cross-sectional area in PGC-1α−/− mice than in wild-type mice (Figs. 1D, 1F). TAC caused similar increases of LV fibrosis in both PGC-1α−/− and wild-type mice. The total mortality during the 6-week period following TAC was not different between PGC-1α−/− (2 out of 13 mice) and wild-type mice (3 out of 14 mice).
FIG. 1.
PGC-1α−/− significantly exacerbates chronic TAC-induced LV hypertrophy and fibrosis. Chronic TAC caused greater LV hypertrophy (A) and pulmonary congestion (B) in PGC-1α−/− as compared with wild-type mice. Chronic TAC causes similar degree of LV fibrosis (C, E), but more cardiac myocyte hypertrophy (D, F) in PGC-1α−/− as compared with wild-type mice. *p < 0.05 compared to the corresponding control; #p < 0.05 compared to Wt-TAC.
PGC-1α−/− exacerbated TAC-induced LV dysfunction
Under control conditions, the tissue weight of lung and its ratio to body weight were not different between PGC-1α−/− and wild-type mice. However, TAC resulted in a significantly greater increase of lung weight and its ratio to body weight in PGC-1α−/− mice as compared with wild-type mice, indicating that more severe LV dysfunction in the PGC-1α−/− mice after TAC (Fig. 1B).
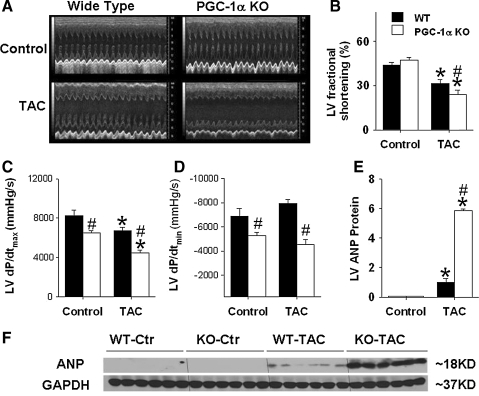
Echocardiographic imaging showed similar LV dimensions and function between PGC-1α−/− mice and wild-type mice under unstressed control conditions. Echocardiography 6 weeks after TAC demonstrated significant increases of LV end-systolic diameter (ESD) and LV end-diastolic diameter (EDD) in both PGC-1α−/− and wild type mice (Fig. 2A). However, the degree of LV dilatation, assessed as LV end-diastolic diameter, was significantly greater in PGC-1α−/− mice than in wild-type mice. The increase in LV end-diastolic wall thickness caused by TAC was also greater in PGC-1α−/− mice than in wild-type mice (data not shown). Systolic dysfunction was more severe in the PGC-1α−/− mice, as demonstrated by greater decreases of LV systolic fractional shortening following TAC in the PGC-1α−/− mice (Figs. 2A and 2B), and a greater increase in end-systolic diameter, as compared to the wild-type mice (Fig. 2A).
FIG. 2.
PGC-1α−/− significantly exacerbates chronic TAC-induced LV dysfunction. PGC-1α−/− significantly exacerbates chronic moderate TAC-induced decrease of LV fraction shortening (A, B), decrease of LV contractility as demonstrated by the decrease of LV dP/dtmax and LV dP/dtmin (C, D), and increase of myocardial ANP (E, F). *p < 0.05 compared to the corresponding control; #p < 0.05 compared to wild type.
Under control conditions, the mean aortic pressure and LV systolic pressure were not different between wild-type mice and PGC-1α−/− mice, whereas the LV dP/dtmax and LV dP/dtmin were significantly less in PGC-1α−/− mice (Figs. 2C and 2D), suggesting a subtle abnormality of left ventricular contractility in the PGC-1α−/− mice. Six weeks after TAC, significant increases of LV systolic pressure were found in both wild-type mice and PGC-1α−/− mice (data not shown). After TAC, LV peak systolic pressure, LV dP/dtmax and LV dP/dtmin were all significantly less in PGC-1α−/− mice as compared to wild-type mice, consistent with more LV dysfunction in these mice (Figs. 2C and 2D). ANP is a sensitive biochemical marker for LV hypertrophy and/or ventricular remodeling. Myocardial ANP levels were increased in both the wild-type and the PGC-1α−/− mice after TAC, but this increase was significantly greater in the PGC-1α−/− mice (Figs. 2E and 2F).
Collectively, the data indicate that chronic TAC caused significantly more LV dysfunction in PGC-1α−/− mice as compared with their wild type littermates.
PGC-1α−/− attenuated the expression of myocardial SOD2 and other mitochondrial antioxidant enzymes
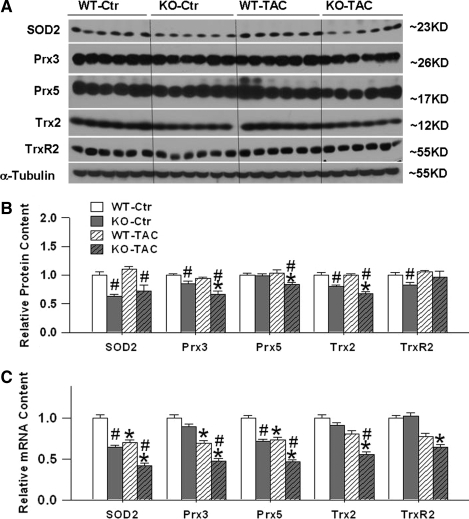
Because of previous reports that PGC-1α can affect the expression of mitochondrial ROS detoxifying enzymes in neuronal cells and vascular endothelial cells (14, 30, 32), we measured protein and mRNA levels of five mitochondrial antioxidant enzymes in left ventricular myocardium (Figs. 3A–3C). Western blots demonstrated that SOD2, Trx2, and TrxR2 protein contents were significantly lower in PGC-1α−/− mice as compared with wild-type mice under basal conditions. Six weeks after TAC there was downregulation of SOD2, Prx3, Prx5, and Trx2 in the PGC-1α−/− mice but not in wild-type mice. Although myocardial SOD2 protein content was still significantly lower in PGC-1α−/− mice as compared with wild-type mice 6 weeks after TAC, TAC caused no significant changes of ventricular SOD2 protein content in either the wild-type or the PGC-1α−/− mice.
FIG. 3.
PGC-1α−/− significantly attenuated expressions of myocardial SOD2, Prx3, Prx5, Trx2, and TrxR2 Protein or mRNA. PGC-1α−/− significantly attenuates mRNA contents (C) and protein expressions of myocardial mitochondrial antioxidants SOD2, Prx3, Prx5, Trx2, and TrxR2 under control conditions or after TAC (A, B). Data are normalized to wild-type control mice. *p < 0.05 compared to the corresponding control; #p < 0.05 compared to wild type.
Real-time RT-PCR demonstrated that left ventricular SOD2 mRNA content was significantly decreased in PGC-1α−/− mice as compared with wild-type mice, both under control conditions and after 6 weeks TAC, suggesting that SOD2 protein expression is at least partially regulated at the transcriptional level. Ventricular Prx5 mRNA content was significantly lower in PGC-1α−/− mice as compared with wild-type mice, both under control conditions and 6 weeks after TAC. Ventricular Prx3, Trx2, and TrxR2 mRNA contents were not significantly different between PGC-1α−/− mice and wild-type mice during control conditions, but 6 weeks after TAC there were significantly greater decreases of mRNA contents of Prx3, Trx2, and TrxR2 in the PGC-1α−/− mice as compared with wild-type mice.
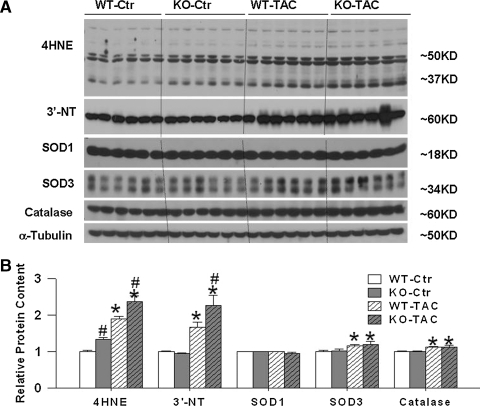
We also determined the protein content of SOD1 (CuZn-SOD, the cytotolic SOD), catalase (cytosolic antioxidant), and SOD3 (an extracellular SOD). Expression levels of these proteins were not statistically different between PGC-1α−/− mice and their wild-type littermates either under control conditions or after TAC. The SOD1, catalase, and SOD3 levels in PGC-1α−/− and wild-type mice under basal conditions and after TAC suggests that PGC-1α may not involved in regulating the expression of these enzymes (Figs. 4A and 4B).
FIG. 4.
PGC-1α−/− has no effect on protein expression of myocardial SOD1, SOD3, and catalase, but significantly exacerbated the TAC-induced increase of LV oxidative stress (A, B). Data are normalized to wild-type control mice. *p < 0.05 compared to the corresponding control; #p < 0.05 compared to wild type.
Taken together, the data indicate that PGC-1α deficiency leads to downregulation of myocardial mitochondrial antioxidant enzymes SOD2, Prx3, Prx5, and Trx2, both in both unstressed hearts and during chronic pressure overload caused by TAC.
PGC-1α-/- exacerbated TAC-induced myocardial oxidative stress
In order to assess the effect of PGC-1α on myocardial oxidative stress, we measured two widely used oxidative stress markers, 3’-nitrotyrosine (3’-NT) and 4-hydroxynonenal (4-HNE). Western blots for 3’-NT revealed a major band at ∼62 KDa; during unstressed conditions, the myocardial 3’-NT content in PGC-1α−/− mice was not different from the content in wild-type mice. TAC caused significant increases of myocardial 3’-NT in both wild-type mice and PGC-1α−/− mice, but the increase was significantly greater in the PGC-1α−/− mice than in the wild-type mice (Figs. 4A and 4B).
Western blots for myocardial 4-HNE revealed two major bands at ∼50 KDa and 37 KDa. Under unstressed conditions, the myocardial 4-HNE protein content (either 37 KDa or 50 KDa) was significantly higher in PGC-1α−/− mice as compared with wild-type mice. TAC caused significant overall increases of myocardial 4-HNE (37 KDa and 50 KDa) in both wild-type and PGC-1α−/− mice, but this increase was significantly greater in the PGC-1α−/− mice than in the wild-type mice (Figs. 4A and 4B). These data suggest that PGC-1α−/− caused a subtle increase of myocardial oxidative stress (as indicated by increase of 4-HNE) under unstressed conditions. PGC-1α−/− exacerbated the TAC-induced increase of LV oxidative stress as demonstrated by significantly greater increases of myocardial 3-NT and 4-HNE than occurred in wild-type mice.
PGC-1α-/- causes decrease of myocardial ERRα expression
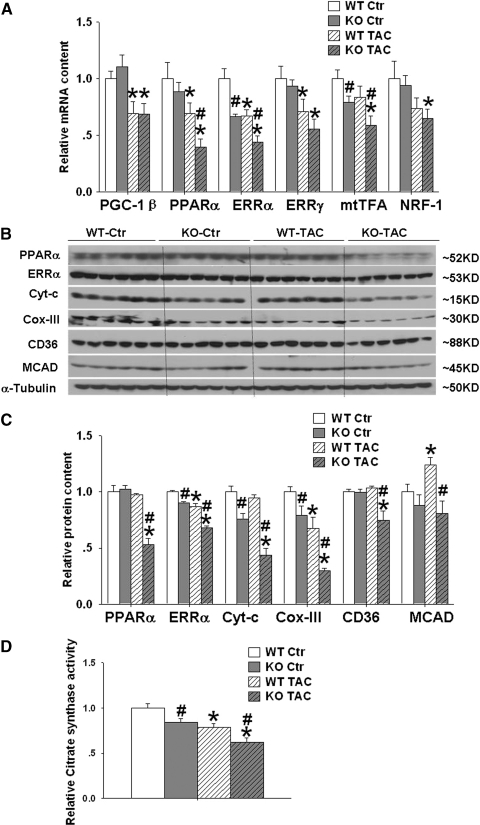
PGC-1( regulates mitochondrial biogenesis through interaction with other metabolic transcriptional factors. Recent evidence suggests that estrogen-related receptor α (ERRα), a specific PGC-1α partner, is essential for PGC-1α-mediated induction of mitochondrial antioxidant genes (25) and ERRα−/− mice develop more severe heart failure (compare to wild-type controls) in response to pressure overload (11). We therefore examined the protein and mRNA levels of ERRα and some other transcriptional factors that interact with PGC-1α or PGC-1β [such as ERRγ, mtTFA, and NRF-1] (Fig. 5A).
FIG. 5.
Effect of PGC-1α−/− on expression of myocardial metabolism related transcriptional factors, metabolic enzymes, and citrate synthase activity. PGC-1α−/− had no effect on myocardial PGC-1β, but significantly attenuated expressions of myocardial ERRα, PPARα, ERRγ, mtTFA, and NRF1 under control conditions or after TAC, which was associated with decrease of myocardial ERRα, PPARα, Cyto C, COX-III, CD36, and MCAD (A–C). PGC-1α−/− also decreased myocardial citrate synthase activity under control condition and after TAC (D). Data are normalized to wild-type control mice. *p < 0.05 compared to the corresponding control; #p < 0.05 compared to wild type.
Under control conditions, myocardial mRNA levels of ERRα were significantly decreased in PGC-1α−/− mice. TAC caused significant downregulation of myocardial mRNA of ERRα in both Wt and PGC-1α−/− mice. The decrease in myocardial ERRα mRNA induced by TAC was significantly greater in PGC-1α−/− mice as compared to wild-type mice. Western blot analysis showed that PGC-1α−/− significantly attenuated the expressions of ERRα protein under both control conditions and after TAC (Figs. 5B and 5C). In PGC-1α−/− mice, the loss of ERRα protein expression in response to TAC was exacerbated compared to wild-type mice. The decrease of ERRα mRNA and protein level under control conditions suggested that PGC-1α regulates myocardial ERRα at the transcriptional level. The further decrease of myocardial ERRα in PGC-1α−/− mice after TAC indicates additional factors other than PGC-1α also contribute to the alteration of myocardial ERRα expression.
PGC-1α−/− effects on other metabolic transcriptional factors
Under control condition, myocardial mRNA levels of PGC-1β, ERRγ, PPARα, mtTFA a, NRF-1, (Fig. 5A) and PPARγ were unaffected, while myocardial the mRNA levels of mtTFA was also significantly decreased in PGC-1α−/− mice (Fig. 5A). TAC caused significant downregulation of myocardial mRNA of PGC-1β, PPARα, and ERRγ in both WT and PGC-1α−/− mice. TAC caused significantly greater decreases of myocardial mRNA of mtTFA and PPARα in PGC-1α−/− mice as compared to wild-type mice. TAC also caused significantly greater decreases of myocardial PPARα protein expression in PGC-1α−/− mice as compared to wild type mice (Fig. 5).
PGC-1α−/− attenuated myocardial Cyt-c and COX-III
Since studies suggested that PGC-1( regulates mitochondrial metabolic enzyme, we determined the protein levels of several previously reported downstream targets of PGC1α such as Cyt-C, COX-III, and medium-chain acyl-CoA dehydrogenases (MCAD). The results showed that Cyto-C and COX-III were significantly decreased in PGC-1α−/− mice under both control conditions and after TAC (Figs. 5B and 5C). As compared with corresponding wild-type mice, myocardial MCAD protein content was unchanged in PGC-1α−/− mice under control conditions, but was significantly decreased in these mice after TAC (Figs. 5B and 5C). CD36 plays an important role to transport fatty acids from the extracellular space into the cytosol for oxidation. We found that myocardial CD36 was unchanged in PGC-1α−/− mice under control conditions, but was significantly decreased in these mice after TAC (Fig. 5B and 5C).
PGC-1α-/- attenuated LV citrate synthase activity
We further measured the citrate synthase activity, a commonly used index for mitochondrial protein content. The results demonstrated that LV citrate synthase activity was reduced in PGC-1α−/− group at baseline and further reduced after TAC (Fig. 5D).
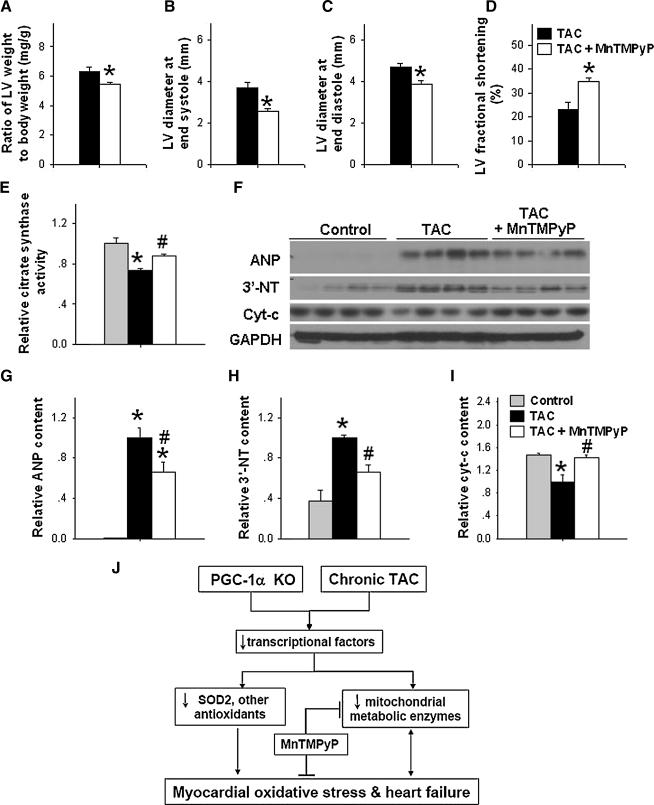
SOD mimetic MnTMPyP partially rescued PGC-1α−/− mice from TAC-induced LV hypertrophy and dysfunction
Since TAC-caused a greater increase of LV oxidative stress in PGC-1α−/− mice, we subsequently determined the rescue effect of SOD mimetic MnTMPyP on TAC-induced LV hypertrophy and dysfunction in PGC-1α−/− mice. SOD mimetic MnTMPyP significantly attenuated TAC-induced LV hypertrophy in PGC-1α−/− mice (Fig. 6A). SOD mimetic MnTMPyP also significantly attenuated TAC-induced increase of LV end-systolic diameter and LV end-diastolic diameter, and decrease of LV fractional shortening in PGC-1α−/− mice (Fig. 6B–6D). MnTMPyP treatment also attenuated TAC-induced decrease of citrate synthase activity (Fig. 6E). Western blot results demonstrated that MnTMPyP also attenuated TAC-induced increases of myocardial level of ANP and 3-NT, and decrease of cyt-c expression (Figs. 6F–6I). These data indicate that increased oxidative stress partially contributed to TAC-induced decreases of mitochondrial proteins and decrease of LV dysfunction in PGC-1α−/− mice.
FIG. 6.
SOD mimetic MnTMPyP significantly attenuated TAC-induced LV hypertrophy, dysfunction, and increase of LV oxidative stress in PGC-1α−/− mice. SOD mimetic MnTMPyP significantly attenuated TAC-induced LV hypertrophy (A) and LV dysfunction (B–D) in PGC-1α−/− mice. *p < 0.05 compared to TAC group; MnTMPyP also attenuated TAC induced the decrease of citrate synthase activity (E) and increase of ANP and 3’-NT content (F–I). The potential signaling pathways for the exacerbated LV myocardial oxidative stress and heart failure in PGC-1α−/− mice are summarized in panel (J). *p < 0.05 compared to the control; #p < 0.05 compared to TAC group.
Discussion
The major findings of this study are that genetic deletion of PGC-1α resulted in (i) reduced expression of myocardial mitochondrial antioxidant enzymes and ERRα in both unstressed and pressure overloaded hearts; (ii) chronic TAC caused a greater increase in myocardial oxidative stress, pulmonary congestion, LV dilation, and a greater reduction of left ventricular fractional shortening and dP/dtmax in PGC-1α −/− hearts than in wild type hearts; and (iii) SOD mimetic MnTMPyP partially rescued PGC-1α−/− mice from chronic TAC-induced decrease of LV mitochondrial protein contents and LV dysfunction. These findings imply that PGC-1α exerts an important role in protecting heart against pressure overload-induced oxidative stress and contractile dysfunction through regulating SOD2 and/or other mitochondrial antioxidants.
Myocardial oxidative stress injury is determined by the balance between ROS generation and ROS elimination by various antioxidants in the tissue. Mitochondrial-derived ROS contributes the majority of myocardial ROS production. Systolic overload increases myocardial mitochondrial oxygen consumption to generate ATP, which increases ROS production at the same time. Under basal conditions, PGC-1α deficiency caused significant decreases of several myocardial mitochondrial antioxidants and metabolic enzymes but caused only a subtle increase of ventricular oxidative stress markers with no apparent LV hypertrophy or dysfunction, indicating that basal ROS metabolism and ATP production were largely maintained in these mice. The significantly greater decrease of LV ejection fraction and contractility (as demonstrated by the depressed LV dP/dtmax), and greater increases of pulmonary congestion and LV dilation in PGC-1α −/− mice after TAC indicate a profound myocardial defect in maintaining cardiac structure and function under this chronic stress condition. The significantly greater increase of myocardial oxidative stress markers in PGC-1α −/− mice after TAC suggests that the levels of mitochondrial antioxidants in these mice were insufficient to handle the increased ROS production under the chronic stress condition (Fig. 4).
SOD2 is the major antioxidant to detoxify superoxide anions into H2O2 in mitochondria. Recent studies reported that PGC-1α plays an important role in expression of SOD2 in neuronal cells (29) and vascular endothelial cells (32). The significant decrease of myocardial SOD2 mRNA and protein in PGC-1α-/- mice demonstrated that PGC-1α is important for SOD2 expression in the heart as well. Because cardiac-specific SOD2 gene deletion causes increased ventricular oxidative stress and congestive heart failure in mice (22), and even the reduced expression of myocardial SOD2 in heterozygous SOD2 gene-deficient mice causes significant LV dysfunction (17), it is possible that the decreased expression of ventricular SOD2 in the PGC-1α−/− mice contributed to the exacerbated LV oxidative stress and dysfunction observed in these mice after TAC.
Interestingly, PGC-1α-/- mice also showed significantly reduced expression of Prx3, Trx2, and TrxR2, a group of mitochondrial proteins that form a critical protein complex involved in removal of toxic H2O2 produced by SOD2 in the mitochondria. It has been shown that global Trx2 gene deletion causes mitochondrial injury and embryonic lethality (23), while cardiac-specific ablation of TrxR2 results in mitochondrial injury and fatal dilated cardiomyopathy(4). Conversely, cardiac specific overexpression of Prx3-protected hearts against myocardial infarction induced LV oxidative stress and heart failure (21). It is thus likely that the significant reduction in ventricular Prx3, Prx5, and Trx2 expression is a contributing factor to the increased ventricular oxidative stress, LV hypertrophy, and dysfunction in PGC-1α −/− mice in response to chronic TAC. The rescue effect of MnTMPyP on the TAC-induced LV hypertrophy and dysfunction in PGC-1α-/- mice supports the notion that increased myocardial oxidative stress contributed to the exacerbated LV remodeling in these mice.
In addition to reduced expression of proteins that remove ROS, PGC-1α−/− mice also exhibited decreased expression of mitochondrial respiratory chain components Cyto-C and COX-III. Chronic heart failure is also often associated with reduced expression of genes encoding electron transport chain components. Abnormal expression of mitochondrial respiratory chain components has been shown to increase mitochondrial ROS production and myocardial oxidative stress in the failing heart (12). Therefore, the increased myocardial oxidative stress and ventricular dysfunction in PGC-1α−/− mice after TAC is likely a collective effect of not only the decreased expression of mitochondrial antioxidants, but also increased ROS generation as a result of deficiencies in electron transport chain components.
The mechanism by which PGC-1α regulates expression of antioxidants in the heart is of considerable interest. Recent evidence shows that ERRα, a specific PGC-1α partner, is essential for PGC-1α-mediated induction of mitochondrial antioxidant genes (25). Indeed, like PGC-1α−/− mice, ERRα−/− mice develop more severe heart failure as compared to wild-type controls in response to pressure overload (11). Data extracted from the original microarray data generated by Huss et al. showed reduced expression of SOD2 (20%–28% decrease), Trx2 (20% decrease), and TrxR2 (28% decrease) in ERRα−/− mice under control conditions (11). PPARα is also an important PGC-1α partner.
Previous studies found that cardiac PPARα deficiency led to significant decrease of LV SOD2 expression (8). Further studies demonstrated that the absence of PPARα results in a more pronounced hypertrophic growth response and signs of functional deterioration (28). A similar relationship between PPARα deficiency and increased oxidative stress was also found in an alcoholic liver disease model (24). As TAC caused a greater decrease of LV PPARα in PGC-1α−/− mice than in wild-type mice, the decreased LV PPARα expression in these mice might have contributed to the greater increase of LV oxidative stress in these mice. However, as LV PPARα expression was unchanged in PGC-1α−/− mice under control conditions, the decrease of LV SOD2 and other antioxidants in these mice under control conditions appears not related to PPARα. Together, these data suggest that PGC-1α, ERRα, and PPARα form a well-regulated network for the control of adaptive mitochondrial biogenesis, which enables the heart to respond to the increased energy demand and oxidative stress imposed by hemodynamic overload.
A previous study demonstrated that PGC-1α−/− caused deficiencies in cardiac energy reserve and LV dysfunction when the hearts were stimulated with adrenergic agents (15). In addition, abnormalities in energy transduction mediated by reduced PGC-1α activity have been implicated in the evolution of heart disease (2, 5, 6). For example, disruption of sarcolemmal ATP-sensitive potassium channel activity decreased the expression of PGC-1α and a group of energy metabolism-related genes and impaired the cardiac response to systolic overload (10). Chronic activation of cardiac Cdk9 in mice confers a predisposition to heart failure via repression of PGC-1α transcription (27). Mice with generalized loss of PGC-1α develop accelerated cardiac dysfunction after 2 months of TAC that is accompanied by clinical signs of heart failure (2). Using a different PGC-1α−/− line mice, we also found that PGC-1α−/− caused more severe LV dilation and contractile dysfunction (Fig. 2), as well as greater myocardial hypertrophy after 6 week TAC (Fig. 1). These findings demonstrate that endogenous levels of PGC-1α are critical for the heart to defend against stress. The protective effect of PGC-1α may be related to its effect in regulating the expression of other metabolic nuclear factors such as ERRα.
Limitations
PGC-1α gene deletion caused decreases of several myocardial mitochondrial antioxidants as well as enzymes involved in ATP production. The greater LV hypertrophy and dysfunction in the PGC-1α−/− mice was likely to be a collective effect of these changes, but the present data cannot differentiate their individual contributions. In addition, PGC-1α gene deletion caused changes of ERRα and several metabolism-related nuclear factors that have the potential to affect the expression of mitochondrial antioxidants and metabolic enzymes. The relative contributions of these individual metabolism-related nuclear factors on the development of heart failure is beyond the scope of the present study. In addition, a significant limitation of the present study is that we were unable to examine the effect of MnTMPyP on TAC-induced LV hypertrophy and CHF in littermate controls of the PGC-1α−/− mice. Nevertheless, as oxidative stress is a general feature of pressure overload hypertrophy and heart failure (18, 19, 20, 34) and we recently demonstrated that the SOD mimetic M40401 can attenuate TAC-induced LV hypertrophy and dysfunction (19), it is perceivable that MnTMPyP would protect both PGC-1α KO mice and wild-type mice from TAC-induced LV hypertrophy and heart failure.
In summary, the finding that PGC-1α−/− suppressed myocardial mitochondrial antioxidant expression and exacerbated LV oxidative stress, hypertrophy, dilation, and contractile dysfunction in response to TAC indicates that endogenous PGC-1α plays an important protective role against stress-induced myocardial oxidative stress by regulating the expression of mitochondrial antioxidants (Fig. 6J).
Acknowledgments
We are grateful to Dr. Daniel Kelly for the supply of PGC-1α−/− mouse strain. This study was supported by NHLBI Grants HL079168, HL102597 (YC) from NIH, grant 09GRNT2260175 (YC) from American Heart Association-Midwest Affiliate, Beijing Natural Science Foundation No.5042006 (YH), No.7072012 (YH), and a grant from K.C. Wong Education Foundation (BZ).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Arany Z. He H. Lin J. Hoyer K. Handschin C. Toka O. Ahmad F. Matsui T. Chin S. Wu PH. Rybkin II. Shelton JM. Manieri M. Cinti S. Schoen FJ. Bassel–Duby R. Rosenzweig A. Ingwall JS. Spiegelman BM. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Arany Z. Novikov M. Chin S. Ma Y. Rosenzweig A. Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-gamma coactivator 1alpha. Proc Natl Acad Sci USA. 2006;103:10086–10091. doi: 10.1073/pnas.0603615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balaban RS. Nemoto S. Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Conrad M. Jakupoglu C. Moreno SG. Lippl S. Banjac A. Schneider M. Beck H. Hatzopoulos AK. Just U. Sinowatz F. Schmahl W. Chien KR. Wurst W. Bornkamm GW. Brielmeier M. Essential role for mitochondrial thioredoxin reductase in hematopoiesis, heart development, and heart function. Mol Cell Biol. 2004;24:9414–9423. doi: 10.1128/MCB.24.21.9414-9423.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finck BN. Kelly DP. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation. 2007;115:2540–2548. doi: 10.1161/CIRCULATIONAHA.107.670588. [DOI] [PubMed] [Google Scholar]
- 6.Finck BN. Kelly DP. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J Clin Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano FJ. Oxygen, oxidative stress, hypoxia, and heart failure. J Clin Invest. 2005;115:500–508. doi: 10.1172/JCI200524408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guellich A. Damy T. Lecarpentier Y. Conti M. Claes V. Samuel JL. Quillard J. Hebert JL. Pineau T. Coirault C. Role of oxidative stress in cardiac dysfunction of PPARalpha-/- mice. Am J Physiol Heart Circ Physiol. 2007;293:H93–H102. doi: 10.1152/ajpheart.00037.2007. [DOI] [PubMed] [Google Scholar]
- 9.Hu P. Zhang D. Swenson L. Chakrabarti G. Abel ED. Litwin SE. Minimally invasive aortic banding in mice: Effects of altered cardiomyocyte insulin signaling during pressure overload. Am J Physiol Heart Circ Physiol. 2003;285:H1261–H1269. doi: 10.1152/ajpheart.00108.2003. [DOI] [PubMed] [Google Scholar]
- 10.Hu X. Xu X. Huang Y. Fassett J. Flagg TP. Zhang Y. Nichols CG. Bache RJ. Chen Y. Disruption of sarcolemmal ATP-sensitive potassium channel activity impairs the cardiac response to systolic overload. Circ Res. 2008;103:1009–1017. doi: 10.1161/CIRCRESAHA.107.170795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huss JM. Imahashi K. Dufour CR. Weinheimer CJ. Courtois M. Kovacs A. Giguere V. Murphy E. Kelly DP. The nuclear receptor ERRalpha is required for the bioenergetic and functional adaptation to cardiac pressure overload. Cell Metab. 2007;6:25–37. doi: 10.1016/j.cmet.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 12.Ide T. Tsutsui H. Kinugawa S. Utsumi H. Kang D. Hattori N. Uchida K. Arimura K. Egashira K. Takeshita A. Mitochondrial electron transport complex I is a potential source of oxygen free radicals in the failing myocardium. Circ Res. 1999;85:357–363. doi: 10.1161/01.res.85.4.357. [DOI] [PubMed] [Google Scholar]
- 13.Kelly DP. Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 14.Kukidome D. Nishikawa T. Sonoda K. Imoto K. Fujisawa K. Yano M. Motoshima H. Taguchi T. Matsumura T. Araki E. Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes. 2006;55:120–127. [PubMed] [Google Scholar]
- 15.Lehman JJ. Boudina S. Banke NH. Sambandam N. Han X. Young DM. Leone TC. Gross RW. Lewandowski ED. Abel ED. Kelly DP. The transcriptional coactivator PGC-1alpha is essential for maximal and efficient cardiac mitochondrial fatty acid oxidation and lipid homeostasis. Am J Physiol Heart Circ Physiol. 2008;295:H185–H196. doi: 10.1152/ajpheart.00081.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leone TC. Lehman JJ. Finck BN. Schaeffer PJ. Wende AR. Boudina S. Courtois M. Wozniak DF. Sambandam N. Bernal-Mizrachi C. Chen Z. Holloszy JO. Medeiros DM. Schmidt RE. Saffitz JE. Abel ED. Semenkovich CF. Kelly DP. PGC-1alpha deficiency causes multi-system energy metabolic derangements: Muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biol. 3:e101–2005. doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loch T. Vakhrusheva O. Piotrowska I. Ziolkowski W. Ebelt H. Braun T. Bober E. Different extent of cardiac malfunction and resistance to oxidative stress in heterozygous and homozygous manganese-dependent superoxide dismutase-mutant mice. Cardiovasc Res. 2009;82:448–457. doi: 10.1093/cvr/cvp092. [DOI] [PubMed] [Google Scholar]
- 18.Lu Z. Fassett J. Xu X. Hu X. Zhu G. French J. Zhang P. Schnermann J. Bache RJ. Chen Y. Adenosine A3 receptor deficiency exerts unanticipated protective effects on the pressure-overloaded left ventricle. Circulation. 2008;118:1713–1721. doi: 10.1161/CIRCULATIONAHA.108.788307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu Z. Xu X. Hu X. Lee S. Traverse JH. Zhu G. Fassett J. Tao Y. Zhang P. Dos RC. Pritzker M. Hall JL. Garry DJ. Chen Y. Oxidative stress regulates left ventricular PDE5 expression in the failing heart. Circulation. 2010;121:1474–1483. doi: 10.1161/CIRCULATIONAHA.109.906818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Z. Xu X. Hu X. Zhu G. Zhang P. van Deel ED. French JP. Fassett JT. Oury TD. Bache RJ. Chen Y. Extracellular superoxide dismutase deficiency exacerbates pressure overload-induced left ventricular hypertrophy and dysfunction. Hypertension. 2008;51:19–25. doi: 10.1161/HYPERTENSIONAHA.107.098186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushima S. Ide T. Yamato M. Matsusaka H. Hattori F. Ikeuchi M. Kubota T. Sunagawa K. Hasegawa Y. Kurihara T. Oikawa S. Kinugawa S. Tsutsui H. Overexpression of mitochondrial peroxiredoxin-3 prevents left ventricular remodeling and failure after myocardial infarction in mice. Circulation. 2006;113:1779–1786. doi: 10.1161/CIRCULATIONAHA.105.582239. [DOI] [PubMed] [Google Scholar]
- 22.Nojiri H. Shimizu T. Funakoshi M. Yamaguchi O. Zhou H. Kawakami S. Ohta Y. Sami M. Tachibana T. Ishikawa H. Kurosawa H. Kahn RC. Otsu K. Shirasawa T. Oxidative stress causes heart failure with impaired mitochondrial respiration. J Biol Chem. 2006;281:33789–33801. doi: 10.1074/jbc.M602118200. [DOI] [PubMed] [Google Scholar]
- 23.Nonn L. Williams RR. Erickson RP. Powis G. The absence of mitochondrial thioredoxin 2 causes massive apoptosis, exencephaly, and early embryonic lethality in homozygous mice. Mol Cell Biol. 2003;23:916–922. doi: 10.1128/MCB.23.3.916-922.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Okiyama W. Tanaka N. Nakajima T. Tanaka E. Kiyosawa K. Gonzalez FJ. Aoyama T. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J Hepatol. 2009;50:1236–1246. doi: 10.1016/j.jhep.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rangwala SM. Li X. Lindsley L. Wang X. Shaughnessy S. Daniels TG. Szustakowski J. Nirmala NR. Wu Z. Stevenson SC. Estrogen-related receptor alpha is essential for the expression of antioxidant protection genes and mitochondrial function. Biochem Biophys Res Commun. 2007;357:231–236. doi: 10.1016/j.bbrc.2007.03.126. [DOI] [PubMed] [Google Scholar]
- 26.Rockman HA. Ross RS. Harris AN. Knowlton KU. Steinhelper ME. Field LJ. Ross J., Jr Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sano M. Wang SC. Shirai M. Scaglia F. Xie M. Sakai S. Tanaka T. Kulkarni PA. Barger PM. Youker KA. Taffet GE. Hamamori Y. Michael LH. Craigen WJ. Schneider MD. Activation of cardiac Cdk9 represses PGC-1 and confers a predisposition to heart failure. EMBO J. 2004;23:3559–3569. doi: 10.1038/sj.emboj.7600351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smeets PJ. Teunissen BE. Willemsen PH. van Nieuwenhoven FA. Brouns AE. Janssen BJ. Cleutjens JP. Staels B. van d V. van BM. Cardiac hypertrophy is enhanced in PPAR alpha-/- mice in response to chronic pressure overload. Cardiovasc Res. 2008;78:79–89. doi: 10.1093/cvr/cvn001. [DOI] [PubMed] [Google Scholar]
- 29.St-Pierre J. Drori S. Uldry M. Silvaggi JM. Rhee J. Jager S. Handschin C. Zheng K. Lin J. Yang W. Simon DK. Bachoo R. Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 30.St-Pierre J. Lin J. Krauss S. Tarr PT. Yang R. Newgard CB. Spiegelman BM. Bioenergetic analysis of peroxisome proliferator-activated receptor gamma coactivators 1alpha and 1beta (PGC-1alpha and PGC-1beta) in muscle cells. J Biol Chem. 2003;278:26597–26603. doi: 10.1074/jbc.M301850200. [DOI] [PubMed] [Google Scholar]
- 31.Takimoto E. Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension. 2007;49:241–248. doi: 10.1161/01.HYP.0000254415.31362.a7. [DOI] [PubMed] [Google Scholar]
- 32.Valle I. varez-Barrientos A. Arza E. Lamas S. Monsalve M. PGC-1alpha regulates the mitochondrial antioxidant defense system in vascular endothelial cells. Cardiovasc Res. 2005;66:562–573. doi: 10.1016/j.cardiores.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 33.Wu Z. Puigserver P. Andersson U. Zhang C. Adelmant G. Mootha V. Troy A. Cinti S. Lowell B. Scarpulla RC. Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 34.Zhang P. Xu X. Hu X. van Deel ED. Zhu G. Chen Y. Inducible nitric oxide synthase deficiency protects the heart from systolic overload-induced ventricular hypertrophy and congestive heart failure. Circ Res. 2007;100:1089–1098. doi: 10.1161/01.RES.0000264081.78659.45. [DOI] [PMC free article] [PubMed] [Google Scholar]