Abstract
In pursuit of the anticancer effects of seeds of the rain forest plant Bixa orellana (annatto), we found that its constituent cis-bixin induced cytotoxicity in a wide variety of tumor cell lines (IC50 values from 10 to 50 μM, 24-h exposures) and, importantly, also selectively killed freshly collected patient multiple myeloma cells and highly drug-resistant multiple myeloma cell lines. Mechanistic studies indicated that cis-bixin–induced cytotoxicity was greatly attenuated by co-treatment with glutathione or N-acetylcysteine (NAC); whereas fluorescence-activated cell sorting (FACS) assays using the cell-permeable dyes 5-(and-6) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA), or dihydroethidium demonstrated that cis-bixin rapidly induced cellular reactive oxygen species (ROS) in dose- and time-dependent fashions, collectively implicating ROS as contributory to cis-bixin–induced cytotoxicity. In pursuit of potential contributors to ROS imposition by cis-bixin, we found that cis-bixin inhibited both thioredoxin (Trx) and thioredoxin reductase (TrxR1) activities at concentrations comparable to those required for cytotoxicity, implicating the inhibition of these redox enzymes as potentially contributing to its ability to impose cellular ROS and to kill cancer cells. Collectively, our studies indicate that the annatto constituent cis-bixin has intriguing selective antimyeloma activity that appears to be mediated through effects on redox signaling. Antioxid. Redox Signal. 13, 987–997.
Introduction
Extracts of the seeds of the of the rain forest plant Bixa orellana (annatto) (Fig. 1A) have been used for centuries by indigenous peoples in Mexico, the Caribbean, and Central/South America in traditional body paints, fabric dyes, foodstuffs, and herbal remedies for ailments including inflammatory gastrointestinal, prostate, and skin conditions (7, 8, 10, 15, 17, 26). Presently, annatto is extensively used as a colorant in Western foods including margarine, candies, and cheeses (10), with >8,000 tons produced annually for use as a food colorant, and an additional 3,000 tons used in the cosmetics industry (8). The Joint Federal Agricultural Organization/ World Health Organization Committee on Food Additives (JECFA) has established an acceptable daily intake (ADI) of annatto extracts as 0–12 mg/kg body weight (15), with the average North American consuming approximately 0.38–0.63 mg of annatto extract per day (17). The intake of annatto is much higher in some Latin American countries, where annatto is commonly used as a condiment (26).
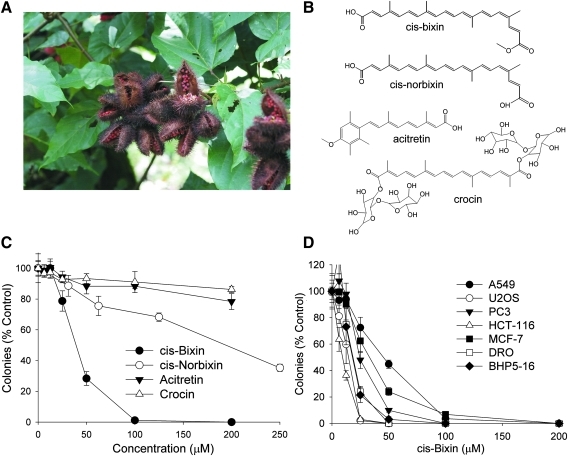
FIG. 1.
Cis-bixin has antineoplastic effects in multiple cancer cell lines. (A) Photo of seed pods of the rain forest plant Bixa orellena, or annatto (Alan J. Wolf, Ph.D. ©2006, University of Wisconsin-Madison). (B) Chemical structures of cis-bixin and several related carotenoids. (C) Colony-forming assay for cytotoxicity using A549 cells shows that cis-bixin is more cytotoxic than are tested related carotenoids. (D) Colony-forming assays in a variety of cancer cell lines, including A549 (lung), U2OS (osteosarcoma), PC3 (prostate), HCT-118 (colon), MCF-7 (breast), DRO (anaplastic thyroid), and BHP5-16 (papillary thyroid) indicate broad anticancer effects of cis-bixin. All drug exposures were for 24 h. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
Based on the knowledge that annatto extracts have been used in herbal medicines by indigenous peoples, we hypothesized that annatto extracts might have interesting biologic and even anticancer properties. Further, because annatto has been found safe when administered to mammals at high levels (≤2 g/kg/day) (3, 11), has cancer chemopreventive effects (1), and has been deemed safe for human consumption as a food additive (3, 7, 8, 10, 11), we further hypothesized that any identified bioactive annatto constituents might have sufficiently favorable safety profiles to allow their development as anticancer therapeutics in humans.
In initial bioactivity studies, we evaluated the anticancer activities of annatto seed extracts to find that methanol and dimethylsulfoxide (DMSO) extracts displayed promising in vitro anticancer effects in A549 human non–small cell lung cancer cells (data not shown). By using high-performance liquid chromatography (HPLC) to fractionate annatto constituents, we subsequently identified cis-bixin as a major component of the organic extracts of annatto and a contributor to observed anticancer activity, prompting the present studies.
Materials and Methods
Reagents
Dried annatto seeds (from Peru) were obtained from Penzey's Spices (Brookfield, WI). Reduced glutathione, N-acetylcysteine (NAC), tert-butyl hydroperoxide (t-BHP), aphidicolin, cycloheximide, 5,6-dichloro-β-d-ribofuranosylbenzimidazole (DRB), acitretin, NADPH, rat liver thioredoxin reductase, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), oxidized glutathione (GSSG), bovine insulin, crocin, dihydroethidium, CelLytic M lysis reagent, and thioredoxin reductase assay kits were purchased from Sigma (St. Louis, MO). Cis-bixin was purchased from Spectrum Chemicals (Gardena, CA); 5-(and-6) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester (CM-H2DCFDA), and 4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid (AMS) were purchased from Invitrogen (Carlsbad, CA); C18 PrepSep columns and trypan blue were purchased from Fisher Scientific; yeast glutathione reductase and Complete Protease Inhibitor Tablets from Roche (Indianapolis, IN); BCA protein assay from Pierce (Rockford, IL) oxidized E. coli thioredoxin and dithiothreitol (DTT) from Promega (Madison, WI), and Coomassie blue and SDS-PAGE gels from BioRad (Hercules, CA). Antibodies to PARP and actin were obtained from BD PharMingen (San Jose, CA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively.
Chemical synthesis of norbixin
A saponification reaction was used to convert cis-bixin to norbixin (10). In brief, 50 mg (0.127 mmol) cis-bixin was added to 10 ml (80 mM) aqueous sodium hydroxide, and the reaction was agitated at 37°C until the solution turned from cloudy to clear. Complete conversion to norbixin was confirmed with HPLC. The compound was then purified on a C18 PrepSep column (Fisher Scientific, Pittsburgh, PA), washing with 10 column volumes of water, and finally eluting with methanol. The solvent was removed by evaporation, and 29.0 mg (0.076 mmol; 60% yield) of purified norbixin was obtained (structure confirmed by using mass spectroscopy and proton nuclear magnet resonance spectroscopy).
Cell culture
Cells were cultured in the following media: A549 in RPMI 1640 containing 5% FBS; U2OS, HCT116, OCI-My-5, RPMI8226, and MM1 in RPMI 1640 containing 10% FBS; PC-3 cells in F12 medium containing 10% FBS; DRO and BHP5-16 in RPMI 1640 containing 10% FBS, nonessential amino acids, sodium pyruvate, sodium bicarbonate, and HEPES buffer; and MCF7 cells in DMEM containing 10% FBS. All media contained 100 U/ml penicillin G and 100 μg/ml streptomycin. Cell lines were passaged 2 to 3 times weekly and maintained at 37°C in an atmosphere containing 95% air and 5% CO2 unless otherwise indicated. OCI-My-5 myeloma cells were kindly provided by Dr. Diane Jelinek; patient marrow cells were attained by an active Mayo IRB–approved protocol, MM.1S/R (dexamethasone sensitive and resistant myeloma cell lines) were kindly provided by Dr. S. T. Rosen, University of Chicago, and RPMI 8226S/RL (doxorubicin sensitive and resistant late-passage myeloma cell lines) were kindly provided by Dr. William Dalton, Moffit Cancer Center. DRO and BHP5-16 were kindly provided by Dr. J. A. Copland, Mayo Clinic. All other cell lines were from ATCC (Manassas, VA).
Assessment of colony formation
In brief, cells obtained from trypsinizing stock flasks of subconfluent cell cultures were deposited in suspensions of 500–1,000 cells per plate into triplicate sets of 35-mm tissue-culture plates and allowed to adhere overnight. Cells were then treated for 24 h with diluent or drugs or both, as indicated in the text and the figures. When indicated, cells were preincubated with inhibitors or antioxidants for 30 min before drug addition. After drug removal and washing, cells were allowed to proliferate in drug-free medium for 7 to 10 days, and thereafter washed and stained with Coomassie blue, with colonies manually counted, as previously described (4).
Assessment of the effects of cis-bixin on patient myeloma/nonmyeloma cells and in chemoresistant myeloma cell lines
To assess effects in patient-derived hematopoietic cells, patient bone marrow cells were collected via posterior superior iliac crest bone marrow aspiration under local anesthesia, in accord with approved Mayo Clinic IRB protocols. Patient bone marrow leukocytes were divided into myeloma (CD138+) and non-myeloma/normal leukocyte (CD138-) fractions with sorting by using magnetic bead technology in kit form (MACS CD138 microbeads; Miltenyi Biotech, Auburn, CA). Sorted patient CD138+ or− cells were suspended in MEM containing 20% FBS and were plated in 96-well tissue-culture plates at a concentration of 5 × 105 cells and dosed with cis-bixin concentrations for 24 h. Survival was assessed by using a trypan blue exclusion assay, by mixing a culture sample with trypan blue in a 1:2 ratio, followed by manual counting by using a light microscope of cells, excluding (live) versus cells including (dead) trypan.
Effects of cis-bixin in doxorubicin- or dexamethasone-resistant myeloma cell lines (RPMI 8226/8226R and MM1RL/MM1 cell line pairs respectively) (6, 9) were assessed by continuously exposing the indicated cell lines to the specified agents in suspension culture, with aliquots of cells removed at indicated time points for assessment of cell viability by using a trypan blue exclusion assay as described in the preceding paragraph.
Electron microscopy
Cells subject to transmission electron microscopy were washed twice with PBS, fixed for l h with Trump's fixative [1% glutaraldehyde and 4% formaldehyde in 0.1 M phosphate buffer (pH 7.2)], treated with phosphate-buffered 1% 0s04, stained en bloc with 2% uranyl acetate for 30 min at 60°C, and embedded in Spurr's resin. Sections (90 nm) were cut on a Reichert Ultracut E or S ultramicrotome (Leica, Inc., Vienna, Austria), collected on 200-mesh copper grids, stained with lead citrate, and examined and photographed with a JOEL 1200 EXII electron microscope (Tokyo, Japan) operating at 60 kV.
Assessment of cellular oxidative stress
Cellular oxidative stress was assessed by using 5,6-chloromethyl-2′, 7′-dichlorodihydrofluorescein diacetate (CM-H2DCFDA) or dihydroethidium as cell-permeable fluorescent probes with FACS analysis. For CM-H2DCFDA, cells were treated with the indicated drug for the indicated time, the medium was aspirated, and the cells were then incubated in warm PBS containing a 6 μM probe at 37°C for 1 h. The probe was then removed, and warm medium was added back to the cells for 10 min. The cells were then collected and resuspended in cold PBS before flow microfluorometry. For dihydroethidium, cells were washed with warm media after treatments, then incubated with a 2 μM probe diluted in warm media for 15 min at 37°C, and finally transferred to chilled tubes on ice. All samples were then analyzed on a FACScan flow cytometer (Becton Dickinson, Mountain View, CA) with a 488-nm laser excitation. Fluorescence emission was observed through a 530/30-nm filter in the case of CM-H2DCFDA experiments and a 585/42-nm filter in the case of the dihydroethidium experiments, and 20,000 events were analyzed for peak shift by using CellQuest software (Verity Software House, Topsham, ME). In separate experiments, tested compounds were found not to interact directly with the probe in vitro, as no fluorescence emission was observed with any tested compound at a 1:10 dye-to-compound ratio.
Assessment of intracellular glutathione
Reduced glutathione was quantitated by using a kit (BioAssay Systems, Hayward, CA) that uses colorimetric detection of the breakdown product of DTNB. In brief, treated A549 cells were washed in PBS and lysed in CellLytic M buffer (Sigma) and then processed with reagents from the kit and read at 405 nm; Beckman AD340 plate reader; Beckman Coulter, Fullerton, CA). The obtained OD value was compared with the included standard to calculate μmolar GSH per microgram protein lysate, and the percentage of control was graphed.
Assessment of apoptosis under normoxic and hypoxic conditions
OCI-MY-5 myeloma cells were plated and treated with cis-bixin and grown in either a tissue-culture incubator with atmospheric oxygen or a hypoxic chamber (Heraeus HERA cell 150; 1% oxygen) for 24 h. At the 24-h time point, cells were assessed for viability by using a trypan blue exclusion assay and in parallel for apoptosis by using Hoechst 33258 through fluorescence microscopy. For PARP immunoblotting, total cellular protein lysates were run on a Tris-SDS PAGE gel, transferred to nitrocellulose, and blotted with antibodies to PARP and actin.
Thioredoxin reductase activity assay (DTNB method)
Cell-free thioredoxin reductase activity was assayed in 100 mM potassium phosphate (pH 7.0), 10 mM EDTA, according to the Sigma kit protocol. Final concentrations were 0.0005 U (Units)/μl of enzyme and 0.24 mM NADPH in the presence of chaetocin as indicated in a 100-μl reaction. The reaction was started by the addition of DTNB (3 mM), and the change in absorbance at 405 nm was monitored in a plate reader. Activity was calculated as the increase in absorbance between 2 and 5 min after DTNB addition.
Thioredoxin activity assay (insulin substrate/precipitation method)
The 100-μl reaction contained 100 mM potassium phosphate (pH 7.0), 2 mM EDTA, 0.13 mM bovine insulin, 3.9 μM E. coli thioredoxin, and cis-bixin, as indicated. The reaction was initiated by addition of 0.33 mM DTT, and turbidity was monitored at 620 nm in a plate reader. The initial linear rate was calculated based on the slope of the line after an increase in absorbance was observed, indicating precipitation of reduced insulin (12).
Glutathione reductase activity assay
Cell-free glutathione reductase activity was assayed in 100 mM potassium phosphate (pH 7.0), 10 mM EDTA. The 200-μl reaction mixture comprised 0.00006 U/μl glutathione reductase, 0.75 mM DTNB, 0.1 mM NADPH, and varying concentrations of cis-bixin or other agents, as indicated. The reaction was started by addition of oxidized glutathione (1 mM) and was monitored in a plate reader at 405 nm. Activity was calculated as the increase in absorbance between 1 and 3 min after glutathione addition.
Thioredoxin reductase activity assay (gel-based oxidation state of thioredoxin method)
Reduction of thioredoxin by thioredoxin reductase was measured by the decrease in electrophoretic mobility caused by covalent modification of thioredoxin by a thiol-reactive probe, AMS, when the disulfide is reduced. The reaction mix contained 100 mM potassium phosphate (pH 7.0), 10 mM EDTA, 0.24 mM NADPH, cis-bixin or diluent, 50 μM oxidized thioredoxin, and 0.0002 U/μl thioredoxin reductase. At the indicated time, a 5-μl sample was removed and immediately added to 5 μl of 30 mM AMS in TE buffer (pH 7.5). The AMS was allowed to react (15 min at 22°C) with reduced thioredoxin sulfhydryl groups (5), and then the samples were mixed with nonreducing sample buffer and electrophoresed on 18% Tris-HCl SDS-PAGE gels. The gels were stained with Coomassie blue, and bands were imaged and quantitated by using a Syngene InGenius gel-documentation system (Frederick, MD).
Statistics
Differences between quantitated ROS values were assessed by using two-sided t tests and pooled estimates of variance.
Results
Cis-bixin induces cytotoxicity in a variety of solid tumor cell lines
Having determined that methanol and DMSO extracts of ground annatto seeds displayed anticancer activity in A549 human lung cancer cells that, on HPLC fractionation, co-eluted with cis-bixin (data not shown), the cytotoxic activities of authentic cis-bixin and the structurally related comparison compounds cis-norbixin, acitretin, and crocin (see Fig. 1B for chemical structures) were evaluated in vitro in A549 cells by using colony-forming assays (Fig. 1C). Cis-bixin exhibited an IC50 of 38.5 ± 3 μM, whereas cis-norbixin displayed an IC50 of 203.3 ± 86 μM, whereas acitretin and crocin displayed no appreciable activity in this assay. Cis-bixin was then tested in a variety of other cancer cell lines, including U2OS (osteosarcoma), PC3 (prostate), HCT-116 (colon), MCF7 (breast), DRO (anaplastic thyroid), and BHP5-16 (papillary thyroid) and found to be similarly active against all assayed lines (Fig. 1D).
Cis-bixin selectively kills neoplastic cells in an ex vivo myeloma model system
As nonselective cytotoxins have little therapeutic interest, we next assessed whether cis-bixin might kill neoplastic cells in preference to normal cells. As one means of assessing this, we compared the effects of cis-bixin in sorted patient multiple myeloma cells and corresponding paired normal leukocytes. Impressively, ex vivo–treated myeloma cells were markedly more sensitive to cis-bixin than were matched normal bone marrow leukocytes (Fig. 2A; the results obtained from cells of three different myeloma patients shown are representative of seven total similarly assessed patient myeloma samples).
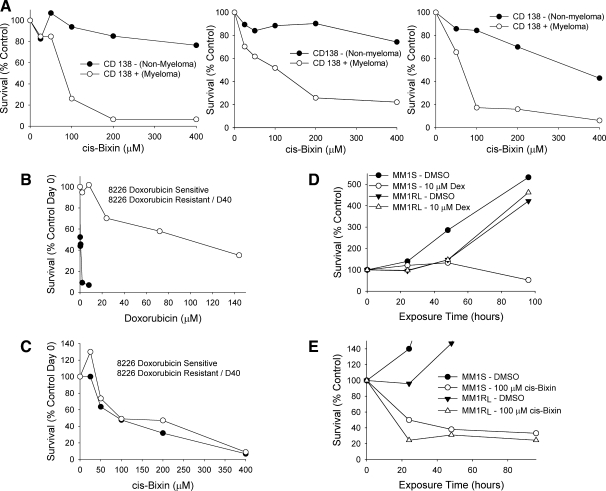
FIG. 2.
Cis-bixin selectively kills freshly collected ex vivo–treated patient myeloma cells and also highly drug-resistant myeloma cell lines. (A) A 24-h cis-bixin treatment kills CD138+ cells while largely sparing paired normal CD138− marrow cells (results from three representative patient samples shown). (B, C) Doxorubicin-resistant 8226R cells are killed as readily as doxorubicin-sensitive 8226S cells by cis-bixin, indicating non–cross resistance. (D, E) Dexamethasone-resistant MM1RL cells are killed equally as well as parental dexamethasone-sensitive MM1S cells by cis-bixin, indicating non–cross resistance.
Because we observed cis-bixin to have antimyeloma selectivity even in myeloma cells derived from patients with therapy-refractory disease, we hypothesized that cis-bixin might also have efficacy in drug-resistant myeloma cell lines. To assess this preliminarily, we used paired RPMI 8226 doxorubicin-sensitive (8226S) and P-glycoprotein–overexpressing doxorubicin-resistant (8226R) myeloma cell lines (6) to find that doxorubicin-resistant 8226R cells were, remarkably, not cross-resistant to cis-bixin (Fig. 2B and C). We further used a dexamethasone-resistant MM1RL myeloma cell line that over expresses the glycocorticoid receptor (9) to assess the effects of cis-bixin in a model of dexamethasone-resistant myeloma. Encouragingly also, dexamethasone-resistant MM1RL cells were as sensitive to cis-bixin–induced cytotoxicity as were dexamethasone-sensitive MM1S cells (Fig. 2D and E). These preliminary findings indicating that cis-bixin is not subject to cross-resistance in several known myeloma MDR models suggested to us that cis-bixin might have novel molecular targets as well as potential in treating drug-resistant myeloma and perhaps other cancers.
Cis-bixin–induced cytotoxicity in myeloma cells is associated with apoptosis induction
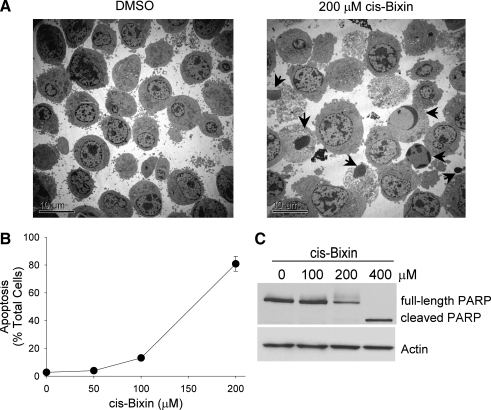
To examine whether cis-bixin–induced cell death in myeloma cells might be associated with apoptotic or necrotic morphologic changes, we assessed the effects of cis-bixin on OCI-My-5 myeloma cells by using a variety of approaches, including electron microscopy, Hoechst 33258 fluorescence staining, and immunoblotting for PARP. We reasoned that the lipophylic nature of cis-bixin might have potential to integrate into and disrupt cellular membranes, thereby potentially inducing necrosis rather than apoptosis. Instead, we observed induction of typical apoptotic morphologic changes as assessed by both electron microscopy (Fig. 3A) and Hoechst 33258 fluorescence staining (Fig. 3B). Further, PARP cleavage was observed in parallel (Fig. 3C), as anticipated for the induction of apoptotic, as opposed to necrotic, cell death.
FIG. 3.
Cis-bixin–induced cytotoxicity in myeloma cells is associated with apoptosis. (A) Electron microscopic images (×2,500) of OCI-MY-5 myeloma cells treated with DMSO diluent or 200 μM cis-bixin, demonstrating induction of morphologic apoptosis (arrows). (B) Concentration-dependence of apoptosis induction by cis-bixin in OCI-MY-5 myeloma cells. Apoptosis was assessed with Hoechst 33258 nuclear staining after 24-h exposures to indicated cis-bixin concentrations; error bars indicate ±1 standard deviation. (C) Cis-bixin induces PARP cleavage in OCI-MY-5 cells (24-h drug exposures).
Cis-bixin–induced cytotoxicity is markedly attenuated by the antioxidants N-acetylcysteine and glutathione
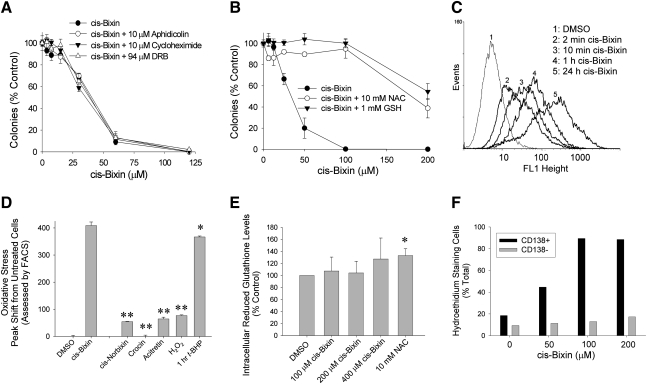
In exploratory studies examining potential contributors to the cytotoxic effects of cis-bixin, we next conducted colony-forming assays in the presence and absence of inhibitors of protein (cycloheximide), RNA (5,6-dichloro-β-d-ribofuranosylbenzimidazole; DRB), or DNA (aphidicolin) synthesis (Fig. 4A), or in the presence of the cell-permeable glutathione precursor N-acetyl cysteine (NAC) or reduced glutathione (GSH; Fig. 4B). Of these inhibitors, NAC and GSH dramatically attenuated cis-bixin–induced cytotoxicity (Fig. 4B), whereas other tested inhibitors had no effect, raising the possibility that cis-bixin–induced cytotoxicity might be mediated primarily through the imposition of cellular oxidative stress otherwise reversed by NAC or GSH, as opposed to through other means dependent on protein, RNA, or DNA synthesis. As a precaution, we also tested whether the inhibitory effects of NAC or GSH might be due to direct reduction of the cis-bixin molecule by the agents (rather than consequent to their abilities to ameliorate ROS) by using HPLC-mass spectroscopy and found that neither NAC nor GSH could reduce cis-bixin under the conditions of the experiments.
FIG. 4.
Cis-bixin–induced cytotoxicity can be overcome by the antioxidants NAC and GSH, but not by inhibitors of DNA, RNA, or protein synthesis, and is associated with the potent induction of oxidative stress in cells. (A) Inhibitors of DNA (aphidicolin), RNA, (5,6-dichloro-β-d-ribofuranosyl-benzimidazole; DRB), and protein (cycloheximide) synthesis do not alter cis-bixin–associated cytotoxicity, as assessed with colony-forming assays (24-h cis-bixin exposures in A549 cells, indicated inhibitors added 30 min before cis-bixin addition). (B) N-acetyl cysteine (NAC) and reduced glutathione (GSH) rescue cis-bixin–treated cells (colony-forming assays, 24-h cis-bixin exposures in A549 cells; indicated inhibitors added 30 min before cis-bixin addition). (C) Representative FACS data showing that cis-bixin shifts the fluorescence intensity of an ROS-indicator (CMH2DCFDA) in A549 cells, inducing high levels of ROS, starting at very short exposure times (2 min, cis-bixin concentration 200 μM). (D) Quantitated oxidative stress levels induced in A549 cells by cis-bixin, cis-norbixin, crocin, acitretin, hydrogen peroxide, or tert-butyl hydroperoxide (24-h treatments, unless otherwise noted; all drug concentrations were 200 μM). All values were statistically significant from DMSO (p < 0.001) except for t-BHP, which was not statistically different. Levels of oxidative stress induced by other agents were also significantly different from cis-bixin, as follows: *p < 0.01 and **p < 0.001. Error bars indicate mean ± 1 sample standard deviation of triplicate samples. (E) Effects of cis-bixin on intracellular reduced glutathione levels in A549 cells (24-h treatments; indicated statistical significances are in comparison to DMSO control). (F) Levels of oxidative stress in CD138+/- patient myeloma cells treated with cis-bixin for 24 h as assessed by dihydroethidium staining for superoxide by using FACS (results expressed as percentage of total cells; results from one myeloma patient of three examined are shown).
Cis-bixin induces cellular oxidative stress
To examine directly the possibility that cis-bixin might impose cellular oxidative stress, a fluorometric-based FACS assay using the cell-permeable dye 5-(and-6) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate acetyl ester (CM-H2DCFDA) was used with peak shifts indicative of induced intracellular oxidative species (Fig. 4C). As hypothesized, based on the preceding experiments, cis-bixin rapidly and dramatically induced cellular oxidative stress in a time-dependent fashion and of a similar magnitude to that of the potent oxidative stressor tert-butyl hydroperoxide (t-BHP; Fig. 4D). In contrast, the less-cytotoxic comparison compounds cis-norbixin and acitretin induced oxidative stress much less potently, and crocin, not at all (Fig. 4D). Cis-bixin treatment did not, however, affect cellular glutathione levels (Fig. 4E), excluding the possibility that glutathione depletion might contribute to observed cytotoxic effects of cis-bixin. Further, cis-bixin assayed in the absence of tumor cells did not induce any peak shift in the CM-H2DCFDA ROS assay (data not shown), indicating that the observed results were not directly attributable to any prooxidant effects of cis-bixin itself.
Cis-bixin induces higher levels of ROS in CD138+ patient myeloma cells in comparison to matched CD138− patient nonmyeloma cells
To assess preliminarily whether the observed selectivity of cis-bixin in killing patient myeloma cells in comparison to matched normal bone marrow cells (e.g., Fig. 2A) might be attributable to differential levels of induced cellular oxidative stress, we examined the induction of ROS in a series of patient CD138+ myeloma cells in comparison to the paired CD138− nonmyeloma cells. Dihydroethidium staining with FACS analyses was used in these experiments to assess ROS induction, based on our prior experience indicating the unsuitability of CM-H2DCFDA for this application. As shown in representative paired patient sample results (Fig. 4F), ROS induction was greater in patient CD138+ myeloma cells in comparison to paired CD138− nonmyeloma bone marrow cells, bolstering our hypothesis that ROS induction by cis-bixin might relate not only to its cytotoxic effects in cancer cell lines but also to its antimyeloma selectivity.
Cis-bixin–induced cytotoxicity and apoptosis are equivalent under normoxic and hypoxic tissue-culture conditions
Based on these results implicating the imposition of cellular oxidative stress by cis-bixin as potentially causative in its ability to kill tumor cells, we next examined whether cis-bixin–induced cytotoxicity or apoptosis might be altered under hypoxic conditions. We reasoned that the less-oxidative environment of hypoxic tissue-culture conditions (and by analogy, patient tumors) might lead to attenuation of the cytotoxic effects of cis-bixin, potentially attenuating enthusiasm for its further development as a candidate anticancer therapeutic. However, 24-h exposure of OCI-MY-5 myeloma cells to 200 μM cis-bixin resulted in 80.3 ± 5.3% apoptotic cells under normoxic versus 81.5 ± 7.2% apoptotic cells under hypoxic conditions (NS), and 39.2 ± 6.8% versus 41.6 ± 13.1% trypan blue inclusive cells under normoxic versus hypoxic tissue-culture conditions (NS), indicating that the cytotoxic effects of cis-bixin are encouragingly unaffected by hypoxic conditions.
Cis-bixin inhibits thioredoxin and thioredoxin reductase activities, but not glutathione reductase or superoxide dismutase activities
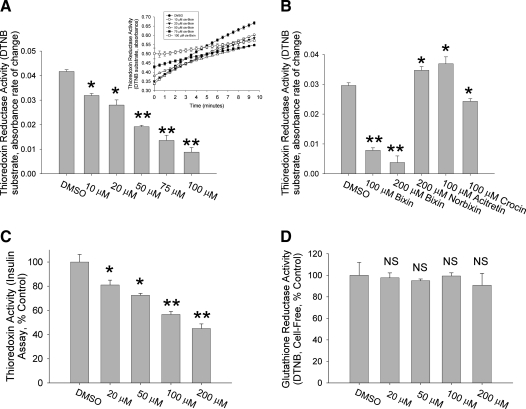
As we found no evidence that cis-bixin might impose cellular oxidative stress through depletion of the primary cellular reductant, glutathione (Fig. 4E), we next undertook studies of its effects on the principal cellular redox enzymes. In contrast, cis-bixin inhibited thioredoxin reductase (TrxR1)-mediated turnover of the synthetic substrate DTNB (Reaction 1) in a cell-free assay in a dose-dependent fashion, with an IC50 of ∼50 μM (Fig. 5A and B).
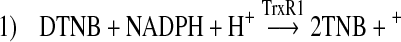 |
FIG. 5.
Cis-bixin inhibits thioredoxin and thioredoxin reductase activities, but not glutathione reductase activity. (A, B) Effects of cis-bixin on thioredoxin reductase activity. DTNB was used as the substrate, and the rate of change in absorbance at 405 nm, as shown in the inset in (A), reflects activity of thioredoxin reductase. (B) Effects of cis-bixin compared with those of related carotenoids cis-norbixin, acitretin, and crocin on thioredoxin reductase activity. (C) Effects of cis-bixin on thioredoxin activity as assessed by the insulin precipitation assay, with rate of change in absorbance at 620 nm shown as a percentage of diluent control activity. (D) Effects of cis-bixin on glutathione reductase activity. DTNB was used as the substrate; absorbance was monitored at 405 nm and plotted as percentage of control of diluent activity. Error bars, +1 standard deviation; statistical significance, *p < 0.02 and **p < 0.001; each panel contains representative results of one of three distinct experiments conducted in triplicate, except for reference drug experiments in (B), performed in duplicate.
We next examined the effects of cis-bixin on the reductase activity of thioredoxin (Trx), as Trx is a major downstream effector substrate of TrxR1, and because Trx is a disulfide-containing reductase structurally related to TrxR1. Because we had established that cis-bixin inhibits TrxR1 activity, however, we necessarily used an activity assay based on insulin reduction (Reaction 2) that did not rely on the coupled TrxR1/Trx reaction.
 |
Interestingly, Trx activity was also inhibited by cis-bixin (albeit somewhat less potently than TrxR1), with an IC50 of ∼150 μM (Fig. 5C).
The activity of a related reductase family member glutathione reductase (GR; Reaction 3), however, was unaffected by ≤200 μM cis-bixin (Fig. 5D), as was the activity of superoxide dismutase 1 and 2 in cellular assays using ≤400 μM cis-bixin (SODs; 4-h exposures, data not shown).
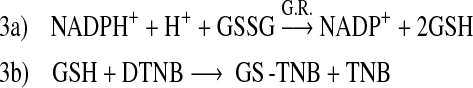 |
Collectively, these results indicate that cis-bixin inhibits TrxR1 and Trx activity with selectivity when compared with at least some other related redox enzymes, indicating that cis-bixin is therefore not an indiscriminate inhibitor of all disulfide-containing reductases and other redox enzymes.
Cis-bixin inhibits the reduction of thioredoxin by thioredoxin reductase
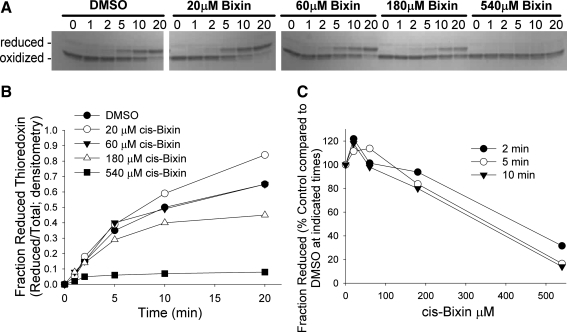
As the small molecule DTNB used in the preceding assay is not the native substrate for thioredoxin reductase, we next investigated whether cis-bixin might also impair the ability of TrxR1 to reduce its native substrate, thioredoxin (Reaction 4).
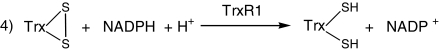 |
We used a gel-based kinetics assay to resolve the oxidized and reduced forms of Trx by rapid covalent modification of the free sulfhydryl groups of Trx with AMS. With this assay, cis-bixin indeed inhibited the reduction of Trx by TrxR1 (Fig. 6A, gel data) in a time- and dose-dependent fashion, with an IC50 of ∼400 μM (Fig. 6B and C), thereby validating previous TrxR1 DTNB reduction assay results with the TrxR1 native substrate. In coordination with these experiments, we also used mass spectroscopy to examine whether cis-bixin might itself be reduced by TrxR1, to test the hypothesis that cis-bixin might inhibit TrxR1 by serving as a competitive substrate for the enzyme. However, unlike some other natural product TrxR1 inhibitors (24), cis-bixin was not reduced by TrxR1 when tested under the same conditions in which it served as a TrxR1 inhibitor (assessed by HPLC–mass spectroscopy, data not shown).
FIG. 6.
Cis-bixin inhibits the reduction of thioredoxin by thioredoxin reductase. (A) A gel-based assay was devised by using AMS to trap reduced thioredoxin and induce a gel motility shift in this species relative to the migration of oxidized thioredoxin. Gel bands from enzyme reaction product stopped at time points from 0 to 20 min are shown for each of five concentrations of cis-bixin, with densitometry quantitation of gel bands used to construct (B) showing the fraction of reduced thioredoxin produced over time. (C) The fraction of reduced thioredoxin relative to control is shown as a function of cis-bixin concentration at three time points, to allow the determination of IC50 values for the inhibition of reduction of Trx by TrxR1. Data shown are representative of three separate experiments.
Discussion
Although annatto has previously been found to have chemopreventive properties, we report now that annatto seed extracts also have promising anticancer effects mediated, at least in part, by its constituent cis-bixin. Presented results are potentially significant in several respects.
First, it is intriguing that we have recently identified two structurally dissimilar natural products, chaetocin (24), and now cis-bixin, both with selective antimyeloma activity attributable to imposition of cellular oxidative stress and both associated with inhibition of the thioredoxin/thioredoxin reductase redox pathway. These observations point to the possibility that the Trx/TrxR1 pathway may have particular importance as an antineoplastic therapeutic target, especially in multiple myeloma. In recent studies of others (reviewed in refs. 18, 19, 25) have also brought to light the potential utility of the Trx/TrxR1 pathway as a novel target for cancer therapy, as several anticancer agents are already known to attenuate the ROS mitigation activity of this pathway. In particular, the TrxR1 inhibitor PX-916 has recently been found to have promising in vivo activity in several solid tumors (20) and is advancing to human clinical trials.
Second, it is interesting that carotenoids have most commonly been found to have antioxidant properties—and generally not prooxidant effects, as reported herein. In particular, because of their conjugated structures (e.g., Fig. 1B), carotenoids have been implicated as reactive oxygen species (ROS) scavengers (reviewed in ref. 23), whereas other evidence points instead to their abilities to chelate iron as contributory to their antioxidant properties (16). In a cell-free system, cis-bixin itself was even previously demonstrated to scavenge hydroxyl radicals (27) and has also been reported to have the in vitro antioxidant property of inhibiting cyclooxygenases (Cox-1 and Cox-2), albeit to a much lesser extent than the related compounds lycopene, β-carotene, and chlorophyll (21). Norbixin has additionally been reported to protect DNA against oxidative damage in an in vitro assay (14). Other studies have, in contrast, pointed to the prooxidant properties of some carotenoids, including β-carotene and N-(4-hydroxyphenyl) retinamide in cancer cells (5, 13, 22), more consistent with our present studies. Interestingly, studies of the related retinoids fenretinide and all trans-retinoic acid (ATRA) in lymphoma cells indicated that, whereas fenretinide induced ROS, ATRA did not, yet both were cytotoxic (2). These apparently conflicting reports point to the possibility that the pro- versus antioxidant properties of carotenoids may be very compound or dosage specific or both.
Also, at present, the relation between chemical structure and cytotoxicity or ROS induction by carotenoids remains largely obscure. It has been our experience that the existence of one, rather than two or no, nonesterified carboxylic acid (as in the case of cis-bixin or retinoic acid relative to cis-norbixin and crocin) seems important to the cytotoxic properties of carotenoids as a class. However, other factors are also involved in the prooxidant and cytotoxic effects of carotenoids, and the situation may be further complicated by the presence of retinoic acid receptors and whether a particular compound might bind to these receptors. Illustrative of the complexity of the situation is that acitretin, with one carboxylic acid like cis-bixin and retinoic acid, remains largely noncytotoxic, despite its single carboxylic acid and otherwise similar chemical structure to retinoic acid.
Third, our studies point to the possibility that cis-bixin may represent a promising candidate anticancer therapeutic worthy of further study. In particular, myeloma cells from heavily pretreated patients and dexamethasone- or doxorubicin-resistant myeloma cell lines were found not to be cross resistant to cis-bixin (Fig. 2). Moreover, not only does cis-bixin exert anticancer effects across many tumor cell lines (Fig. 1C and D), but it also has dramatic anticancer selectivity, as assessed in our ex vivo studies of neoplastic CD138+ myeloma versus paired normal CD138− bone marrow cells (Fig. 2A). Moreover, available evidence suggests that the cytotoxic effects of cis-bixin are maintained even in hypoxic environments, a favorable property for candidate anticancer therapeutics. Taken together, available evidence provides a compelling rationale for pursuing further studies of cis-bixin as a candidate anticancer therapeutic.
In summary, having noted promising anticancer activity in organic extracts of annatto seeds, we established that cis-bixin contributes to this activity, and moreover, that cis-bixin exerts its cytotoxic effects via imposition of cellular ROS, mediated at least in part by inhibition of the thioredoxin/thioredoxin reductase redox pathway. Given impressive antimyeloma selectivity in ex vivo assays of patient myeloma samples and non–cross resistance in highly drug-resistant myeloma cell lines, further studies of cis-bixin as a candidate anticancer therapeutic seem warranted. Furthermore, our studies importantly point to the possibility that the thioredoxin/thioredoxin reductase and other ROS pathways may harbor promising antineoplastic therapeutic targets and may represent a sort of Achilles heel for some cancers.
Abbreviations Used
- AMS
4-acetamido-4′-maleimidylstilbene-2,2′-disulfonic acid
- CM-H2DCFDA
5-(and-6) chloromethyl-2′,7′-dichlorodihydrofluorescein diacetate, acetyl ester
- DMSO
dimethylsulfoxide
- DRB
5,6-dichloro-β-d-ribofuranosylbenzimidazole
- DTNB
5,5′-dithiobis(2-nitrobenzoic acid)
- DTT
dithiothreitol
- FACS
fluorescence-activated cell sorting
- HPLC
high-performance liquid chromatography
- NAC
N-acetylcysteine
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- t-BHP
tert-butyl hydroperoxide
- Trx
thioredoxin
- TrxR1
thioredoxin reductase-1
Acknowledgments
The authors are indebted to the staffs of the Mayo Clinic FACS and Mass Spectroscopy facilities (especially to Linda Benson) for critical technical assistance, and to Dr. Yuan-Ping Pang for providing nuclear magnetic resonance spectroscopy support. We are also grateful for helpful insights provided by Mr. Robert A. Rodriguez; and to Alan J. Wolf, Ph.D. (University of Wisconsin-Madison, Madison, WI), for permission to use his photo of annatto seed pods (Fig. 1A). We also thank Drs. Philip Greipp and Angela Dispenzieri of the Mayo Clinic Myeloma and Dysproteinemia Group and for kindly additionally providing patient bone marrow cells, with technical assistance from Kim Henderson, Kristy Finke, and Roberta DeGoey. Thyroid cell lines were also kindly provided by J. A. Copland, Ph.D. (Mayo Clinic, Jacksonville, FL). Dr. William Dalton (H. Lee Moffit Cancer Center, Tampa, Florida) further kindly provided RPMI 8226 doxorubicin-resistant myeloma cells, and Dr. S. T. Rosen (Northwestern University, Chicago, Illinois) kindly provided MM1 dexamethasone-resistant and parental myeloma cells.
This work was supported in part by grants from the National Cancer Institute (CA97129, CA98118, CA125750; KCB), by a Multiple Myeloma Research Foundation (MMRF) Postdoctoral Fellowship (JDT), and by an Eagle's Fellowship (JDT).
Author Disclosure Statement
The authors have no conflicts of interest to disclose related to this manuscript.
References
- 1.Agner AR. Bazo AP. Ribeiro LR. Salvadori DM. DNA damage and aberrant crypt foci as putative biomarkers to evaluate the chemopreventive effect of annatto (Bixa orellana L.) in rat colon carcinogenesis. Mutat Res. 2005;582:146–154. doi: 10.1016/j.mrgentox.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Barna G. Sebestyén A. Weischede S. Peták I. Mihalik R. Formelli F. Kopper L. Different ways to induce apoptosis by fenretinide and all-trans-retinoic acid in human B lymphoma cells. Anticancer Res. 2005;25:4179–4185. [PubMed] [Google Scholar]
- 3.Bautista AR. Moreira EL. Batista MS. Miranda MS. Gomes IC. Subacute toxicity assessment of annatto in rat. Food Chem Toxicol. 2004;42:625–629. doi: 10.1016/j.fct.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Bible KC. Kaufmann SH. Flavopiridol: cytotoxic flavone that induces cell death in human lung carcinoma cells. Cancer Res. 1996;56:4856–4861. [PubMed] [Google Scholar]
- 5.Cui Y. Lu Z. Bai L. Shi Z. Zhao WE. Zhao B. Beta-carotene induces apoptosis and up-regulates peroxisome proliferator-activated receptor gamma expression and reactive oxygen species production in MCF-7 cancer cells. Eur J Cancer. 2007;43:2590–2601. doi: 10.1016/j.ejca.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 6.Dalton WS. Durie BG. Alberts DS. Gerlach JH. Cress AE. Characterization of a new drug-resistant human myeloma cell line that expresses P-glycoprotein. Cancer Res. 1986;46:125–130. [PubMed] [Google Scholar]
- 7.Evans WC. Annatto: a natural choice. Biologist (London) 2000;47:181–184. [PubMed] [Google Scholar]
- 8.Green CL. Rubini E. Chandrasekharan C. Natural colorants and dyestuffs. In: Schmincke K-H, editor. Non-wood Forest Products 4. Rome: FAO, Food and Agriculture Organization of the United Nations; 1995. pp. 1–37. [Google Scholar]
- 9.Greenstein S. Krett NL. Ma C. Chauhan D. Hideshima T. Anderson KC. Rosen ST. Characterization of the MM.1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol. 2003;31:271–282. doi: 10.1016/s0301-472x(03)00023-7. [DOI] [PubMed] [Google Scholar]
- 10.Guiliano G. Rosati C. Bramly PM. To dye or not to dye: the biochemistry of annatto unveiled. Trends Biotech. 2003;21:513–516. doi: 10.1016/j.tibtech.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Hagiwara A. Imai N. Doi Y. Nabae K. Hirota T. Yoshino H. Kawabe M. Tsushima Y. Aoki H. Yasuhara K. Koda T. Nakamura M. Shirai T. Absence of liver tumor promoting effects of annatto extract (norbixin), a natural carotenoid food color, in a medium-term liver carcinogenesis bioassay using male F344 rats. Cancer Lett. 2003;199:9–17. doi: 10.1016/s0304-3835(03)00339-2. [DOI] [PubMed] [Google Scholar]
- 12.Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979;254:9627–9632. [PubMed] [Google Scholar]
- 13.Kadara H. Lacroix L. Lotan D. Lotan R. Induction of endoplasmic reticulum stress by the pro-apoptotic retinoid N-(4-hydroxyphenyl) retinamide via a reactive oxygen species-dependent mechanism in human head and neck cancer cells. Cancer Biol Ther. 2007;6:705–711. doi: 10.4161/cbt.6.5.3963. [DOI] [PubMed] [Google Scholar]
- 14.Kovary K. Louvain TS. Costa e Silva MC. Albano F. Pires BB. Laranja GA. Lage CL. Felzenszwalb I. Biochemical behaviour of norbixin during in vitro DNA damage induced by reactive oxygen species. Br J Nutr. 2001;85:431–440. doi: 10.1079/bjn2000287. [DOI] [PubMed] [Google Scholar]
- 15.Kroes R. Verger P. Annatto extracts. In: Mattock H, editor. WHO Food Additive Series 52: Safety evaluations of certain food additives and contaminants. Geneva: World Health Organization; 2004. pp. 13–53. [Google Scholar]
- 16.Lopes GKB. Schulman HM. Hermes-Lim M. Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta. 1999;1472:142–152. doi: 10.1016/s0304-4165(99)00117-8. [DOI] [PubMed] [Google Scholar]
- 17.Kroes R. Munro IC. Walker R. Annatto extracts (addendum) In: Mattock H, editor. WHO Food Additive Series 58: Safety Evaluations of Certain Food Additives and Contaminants. Geneva: World Health Organization; 2007. pp. 3–13. [Google Scholar]
- 18.Nguyen P. Awwad RT. Smart DD. Spitz DR. Gius D. Thioredoxin reductase as a novel molecular target for cancer therapy. Cancer Lett. 2006;236:164–174. doi: 10.1016/j.canlet.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 19.Powis G. Kirkpatrick DL. Thioredoxin signaling as a target for cancer therapy. Curr Opin Pharmacol. 2007;7:392–397. doi: 10.1016/j.coph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 20.Powis G. Wipf P. Lynch SM. Birmingham A. Kirkpatrick DL. Molecular pharmacology and antitumor activity of palmarumycin-based inhibitors of thioredoxin reductase. Mol Cancer Ther. 2006;5:630–636. doi: 10.1158/1535-7163.MCT-05-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reddy MK. Alexander-Lindo RL. Nair MG. Relative inhibition of lipid peroxidation, cyclooxygenase enzymes, and human tumor cell proliferation by natural food colors. J Agric Food Chem. 2005;53:9268–9273. doi: 10.1021/jf051399j. [DOI] [PubMed] [Google Scholar]
- 22.Samuel W. Kutty RK. Nagineni S. Vijayasarathy C. Chandraratna RA. Wiggert B. N-(4-hydroxyphenyl) retinamide induces apoptosis in human retinal pigment epithelial cells: retinoic acid receptors regulate apoptosis, reactive oxygen species generation, and the expression of heme oxygenase-1 and Gadd153. J Cell Physiol. 2006;209:854–865. doi: 10.1002/jcp.20774. [DOI] [PubMed] [Google Scholar]
- 23.Tapiero H. Townsend DM. Tew KD. The role of carotenoids in the prevention of human pathologies. Biomed Pharmacother. 2004;58:100–110. doi: 10.1016/j.biopha.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibodeau JD. Benson LM. Isham CR. Owen WG. Bible KC. The anticancer agent chaetocin is a competitive substrate and inhibitor of thioredoxin reductase. Antioxid Redox Signal. 2009;11:1097–1106. doi: 10.1089/ars.2008.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Urig S. Becker K. On the potential of thioredoxin reductase inhibitors for cancer therapy. Semin Cancer Biol. 2006;16:452–465. doi: 10.1016/j.semcancer.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Verger P. Evaluation of national assessments of intake of annatto extracts (Bixin) In: Eriksen GS, editor; Pennington J, editor; Alexander J, editor; Thuvander A, editor. Safety Evaluation of Certain Food Additives and Contaminants. WHO Food Additive Series 44. Geneva: World Health Organization; 2000. pp. 485–492. [Google Scholar]
- 27.Zhao WE. Han Y. Zhao B. Hirota S. Hou J. Xin W. Effect of carotenoids on the respiratory burst of rat peritoneal macrophages. Biochim Biophys Acta. 1998;1381:77–88. doi: 10.1016/s0304-4165(98)00013-0. [DOI] [PubMed] [Google Scholar]