Abstract
Disulfide-bond formation is important for the correct folding of a great number of proteins that are exported to the cell envelope of bacteria. Bacterial cells have evolved elaborate systems to promote the joining of two cysteines to form a disulfide bond and to repair misoxidized proteins. In the past two decades, significant advances have occurred in our understanding of the enzyme systems (DsbA, DsbB, DsbC, DsbG, and DsbD) used by the gram-negative bacterium Escherichia coli to ensure that correct pairs of cysteines are joined during the process of protein folding. However, a number of fundamental questions about these processes remain, especially about how they occur inside the cell. In addition, recent recognition of the increasing diversity among bacteria in the disulfide bond–forming capacity and in the systems for introducing disulfide bonds into proteins is raising new questions. We review here the marked progress in this field and discuss important questions that remain for future studies. Antioxid. Redox Signal. 13, 1231–1246.
Introduction
Disulfide bonds contribute to the stability, activity, and folding of many secretory or extracytoplasmic domains of membrane proteins. Chemically, formation of a protein disulfide bond is an oxidation reaction, in which two electrons are removed from the protein. In contrast, breakage of a protein disulfide bond is a reduction reaction, in which two electrons are donated to the protein. In vitro, formation of protein disulfide bonds can occur spontaneously in the presence of an appropriate electron acceptor, although such a spontaneous process often requires hours or days of incubation. In contrast, formation of disulfide bonds in a folding protein in vivo is a much more rapid process, occurring within seconds or minutes after the synthesis of the proteins or even during their synthesis, thus necessitating enzyme catalysts for the process (10).
The enzymes that interact directly with folding proteins to introduce or break disulfide bonds are, in most cases, oxidoreductases belonging to the thioredoxin superfamily. The oxidoreductases of this superfamily often have an active site containing a CXXC motif (cysteines separated by two amino acids) embedded in a thioredoxin-fold that is observed in the three-dimensional structure of thioredoxin (6). In both prokaryotes and eukaryotes, the members of the thioredoxin superfamily exist in many cellular compartments and promote the oxidation or reduction of protein's thiols (97).
For the members of the thioredoxin superfamily to perform their functions, the cysteines of their active site must be maintained in the appropriate redox state. Increasingly, enzymes are being identified that are responsible for these maintenance functions. These enzymes, which usually do not belong to the thioredoxin superfamily, act on the direct catalysts of oxidative protein folding, providing them with oxidizing or reducing equivalents. These enzymes thereby connect the oxidative folding pathways to cellular metabolism (55, 97).
In Escherichia coli, approximately 300 proteins containing multiple cysteines are exported to the periplasm (27, 38). These proteins are potential substrates for disulfide bond–forming enzymes. Despite the large number of potential substrates, the components involved in disulfide-bond formation (Dsb proteins) are not essential for viability, although mutants exhibit a variety of defects (55). The nonessentiality of the components, the availability of strong genetic tools, the deep biologic knowledge of this organism available, and the ease with which the relevant proteins can be produced have greatly facilitated the analysis of disulfide-bond formation in this organism. In the past two decades, we have learned that a surprising variety of structures, mechanisms, and factors are required to carry out the process. However, the detailed mechanisms by which disulfide bonds are introduced into proteins by these systems, especially in vivo, are still unclear. Unless otherwise stated, we discuss the systems of E. coli that are responsible for introducing correct disulfide bonds into cell envelope proteins. We also describe some advances in our understanding of the disulfide bond–forming systems of other bacteria.
DsbA: A Disulfide Bond–Forming Enzyme
DsbA is a primary oxidant of many extracytoplasmic proteins (Fig. 1). In the absence of DsbA, many cell envelope proteins of E. coli acquire their disulfides much more slowly or fail to acquire them (10, 57). Thus, mutants that are missing DsbA show pleiotropic phenotypes, such as loss of motility and increased sensitivity to dithiothreitol and metal ions like Cd2+ and Hg2+ (55).
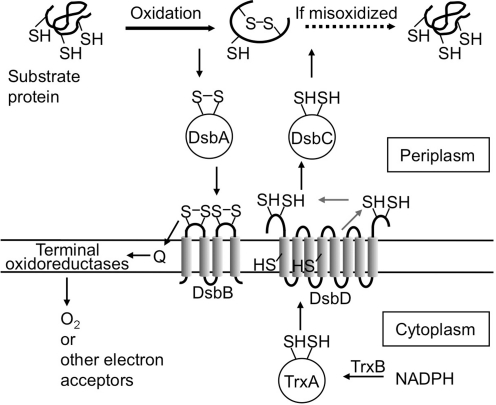
FIG. 1.
Models for disulfide-bond formation in the periplasm of E. coli. The solid black rightward arrow indicates the oxidative folding reaction catalyzed by DsbA. In the instance in which the first folding reaction resulted in a misoxidized protein, DsbC may repair it by acting as a reductase (dotted arrow) or an isomerase (not shown in this figure) of the incorrect disulfide bond. The thinner arrows indicate the flow of reducing equivalents. Q, quinones (ubiquinone or menaquinone). For simplicity, DsbC, a dimeric molecule, is depicted as a monomer.
DsbA is a monomeric, periplasmic enzyme (21 kDa) having a redox active sequence, Cys30-Pro31-His32-Cys33, embedded in a thioredoxin fold (10, 74) (Fig. 2A). These two cysteines are kept oxidized (disulfide bonded) in vivo (60). DsbA rapidly oxidizes proteins secreted into the periplasm by donating its disulfide bonds to a pair of cysteines of the substrate. This process likely occurs through formation of an unstable mixed-disulfide complex between DsbA and its substrate (Fig. 3). Recently, such a DsbA-substrate complex, an apparent intermediate during oxidative protein folding, was detected and characterized in vivo (53).
FIG. 2.
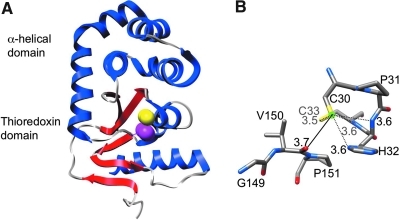
The three-dimensional structure of DsbA. (A) The structure of the oxidized form of DsbA (PDB:1FVK) (74). DsbA has a thioredoxin-like fold with the insertion of an α-helical domain. The α-helices (blue), β-sheets (red), cis-Pro151 (magenta), and the sulfur atoms of the active-site cysteines (yellow) are indicated. Only the sulfur of Cys30 is surface-exposed. (B) The close-up view of the active site of the reduced form of DsbA (PDB: 1A2L) (33) in stick presentation. Possible hydrogen-bond interactions stabilizing the thiolate anion of Cys30 are indicated by dotted lines, with their distances in ångströms. A green ball represents the sulfur of Cys30. The distance between the sulfur of Cys30 and the main-chain oxygen of Val150 in the cis-Pro loop is also shown in ångströms with a solid line. Molecular graphics images were produced by using the UCSF Chimera (85). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
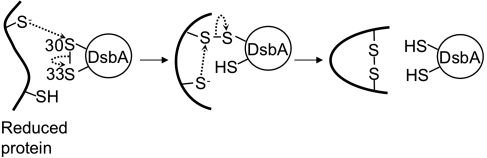
FIG. 3.
Disulfide-bond formation by DsbA. Substrate oxidation by DsbA likely proceeds through two steps. First, a deprotonated cysteine of a substrate attacks the sulfur atom of Cys30 of the oxidized DsbA, leading to the formation of a disulfide-linked complex between DsbA and the substrate. In the next step, one of the remaining cysteines of the substrate is deprotonated and attacks the sulfur atom of the substrate cysteine that is disulfide bonded with Cys30 of DsbA. This reaction results in the formation of a disulfide bond in the substrate and reduction of DsbA.
The thioredoxin-fold, such as that found in DsbA, is described as an N-terminal βαβ motif and a C-terminal ββα motif, connected by a loop of residues that incorporates an α-helix (75). After the N-terminal βαβ motif, the thioredoxin-fold of DsbA has an insertion of a 65-residues α-helical domain that consists of four α-helices (Fig. 2A). It has been suggested that the α-helical domain protects the redox-active cysteines of DsbA from misreduction by DsbD (35) (for the description of DsbD, see later section).
Among the known enzymes that promote the exchange of thiols and disulfides, DsbA, with a standard redox potential of ∼−120 mV, is one of the strongest thiol oxidants (42). This enzyme can introduce disulfide bonds into its substrates quickly in vivo and in vitro. For comparison, thioredoxin has a much lower standard redox potential of −270 mV and reduces disulfide bonds in the cytoplasm (5).
The DsbA catalytic cysteine residues, Cys30 and Cys33, are located at the N-terminus of the first α-helix of the thioredoxin fold. Of the two cysteines, only Cys30 is surface exposed. The activity of DsbA as a strong oxidant derives in part from its biophysical properties. The Cys30 of DsbA has an unusually low pKa value of ∼3.3 (the normal pKa of cysteine is ∼9) (42, 81). Thus, Cys30 is in the thiolate anion state at physiologic pH. The structure of DsbA explains how the thiolate anion of Cys30 is stabilized (33). First, this residue is located at the N-terminus of the first helix of the thioredoxin-fold, allowing the partial positive charge from the dipole of the helix to stabilize the thiolate. Second, His32, which lies between Cys30 and Cys33, is hydrogen-bonded to Cys30 in the reduced but not in the oxidized DsbA, providing additional stabilization of the thiolate anion of Cys30 (Fig. 2B). In addition, the structure of reduced DsbA reveals a number of potential hydrogen bonds that stabilize the thiolate anion of Cys30 (Fig. 2B). One of them is a hydrogen bond between the thiolate anion of Cys30 and the thiol of Cys33. The other two hydrogen-bond interactions are between the thiolate anion of Cys30 and the backbone amides of His32 and Cys33 (33). These electrostatic interactions, which are unique to the reduced form of this protein, cause the reduced form of this protein to be more stable than the oxidized form (109). In contrast, most proteins that are substrates of DsbA are stabilized by formation of disulfide bonds. This difference in the stabilities of oxidized and reduced forms provides a thermodynamic driving force for the transfer of disulfide bonds from DsbA to its substrates.
The in vitro redox properties of DsbA as well as other thioredoxin-fold proteins are strongly influenced by the amino acid sequence between the active-site cysteines (31, 87), as seen with His32 of DsbA (see earlier). However, the CXXC motif is not the only structural feature that greatly affects the function of DsbA. Most of the thioredoxin-superfamily members, including DsbA, have a highly conserved cis-proline residue in their structures (75) (Fig. 2A). The loop containing this residue is close in three-dimensional space to the CXXC motif but distant in sequence (Fig. 2B). Growing evidence suggests that the cis-proline loop plays important roles in the function of thioredoxin and its relatives.
First, biophysical studies of DsbA and thioredoxin indicate that the conserved cis-proline contributes to the stability and structure of these proteins (16). Second, a mutation that alters the Pro151 of DsbA, which corresponds to the highly conserved cis-proline in this enzyme, to threonine causes retardation of the resolution of intermediate complexes between DsbA and its substrates in vivo, suggesting that this residue is important structurally or functionally for substrate release (56). Third, in the crystal structure of a complex between DsbA and its model substrate peptide, a few residues preceding the cis-proline form an anti-parallel β-sheet with a part of the substrate, indicating that the cis-proline loop is important for substrate recognition (83). Finally, recent studies show that the residue just N-terminal to the cis-proline residue, Val150 in E. coli DsbA, strongly modifies the redox properties and substrate specificities of some thioredoxin-superfamily members (66, 89). Thus, in addition to the CXXC motif, the cis-proline loop is crucial in defining the properties of DsbA and other enzymes.
In E. coli, more than 300 potential substrates exist of DsbA (27, 38). This estimate is based on a bioinformatics analysis showing that that many cell envelope proteins occur with at least two cysteine residues. Some of these have been verified to be substrates of DsbA (38, 56, 67, 103). How is it possible for this enzyme to interact with so many different substrates? First, studies on the protease-accessible sites on the surface of DsbA revealed that the surface of the oxidized form is more flexible than that of the reduced form (104). Thus, the greater flexibility of the oxidized form may facilitate the accommodation of various substrates, and the more-rigid nature of the reduced form may expedite the release of the oxidized substrates. Second, in the crystal structures of a complex between DsbA and its polypeptide (see earlier), amino acids in the substrate polypeptide were hydrogen-bonded to the backbone atoms of the residues of the cis-proline loop in the anti-parallel β-sheet structure. The use of the backbone atoms, but not the side chains, for binding may allow the enzyme to interact with a greater variety of substrates (83).
Most structural disulfide bonds in the substrates of DsbA are buried and not solvent accessible in their final folded structures. Thus, DsbA must introduce disulfide bonds into its substrates before they are folded into their native structures. Conversely, DsbA avoids interaction with a subset of folded proteins, even if they contain cysteines that can be oxidized (e.g., DsbC and DsbD; see later) (110). Consistent with its physiologic roles, DsbA appears preferentially to interact with unfolded proteins. For example, DsbA reacts about 10 to 25 times faster with unfolded proteins than it does with DTT (108). Further, in a complex between DsbA and unfolded RNase T1 (stably formed by mutating Cys33 of DsbA), the DsbA moiety is stabilized by 4.7 kJ/mol when it is linked with the unfolded RNase T1 (30). This strongly suggests that DsbA interacts noncovalently with its substrates. In addition, in the complex formed between DsbA and its model peptide (see earlier), the peptide was bound at the interface of the thioredoxin and α-helical domains of DsbA with multiple contacts (83). Such numerous interactions would not be available if the protein were already folded. These findings are consistent with the role of DsbA as a catalyst of disulfide-bond formation in folding proteins.
The surface structure of DsbA has a relatively deep hydrophobic groove running alongside the active site. Because of its hydrophobic nature, this groove has been postulated to be the binding site for the unfolded substrates (34). The recent crystal structure of a DsbA–DsbB complex shows that this groove constitutes a part of the binding site for DsbB (see later for DsbB) (44). However, no direct evidence indicates that this groove is actually used for the recognition of its unfolded substrates (83).
As in the case of other members of thioredoxin superfamily, thiol-disulfide exchange by DsbA likely proceeds by two steps (30, 53) (Fig. 3). In the first step, a deprotonated cysteine of a substrate attacks the sulfur atom of Cys30 in the Cys30–Cys33 disulfide bond. This reaction leads to the formation of an intermolecular disulfide bond between the cysteine of the substrate and Cys30 of DsbA and reduction of Cys33 of DsbA. In the next step, one of the remaining cysteines of the substrate is deprotonated and attacks the sulfur atom of the substrate cysteine that is disulfide bonded with Cys30 of DsbA. This reaction results in the formation of a stable disulfide bond in the substrate and in the reduction of both cysteines of DsbA.
How are these reactions facilitated on the enzyme? Studies on DsbL, a uropathogen-specific DsbA homologue, have provided some insights into this question. This protein is one of the most oxidizing thioredoxin superfamily members known to date (32, 105). By using molecular dynamics simulation, Narzi et al. (80) studied the active-site protonation characteristics of DsbL. Their characterization revealed that the buried active-site cysteine (Cys32) alternates between protonated and deprotonated states, which is achieved by proton shuffling between Cys32 and the neighboring Lys23. This proton shuffling avoids the negative-charge collision that would otherwise occur between the two active-site cysteines (Cys29 and Cys32) of DsbL, facilitating the smooth electron transfer from the substrate cysteines to the disulfide bond of DsbL. It is suggested that a residue with a similar function may also exist in other proteins sharing a CXXC active-site motif (80).
Good evidence indicates that the thiol-disulfide exchange reaction of thioredoxin-superfamily members, including DsbA, uses an SN2 mechanism (20, 30, 53, 84, 106, 107). A reaction that adopts an SN2 mechanism is predicted to be highly directional, proceeding through a transition state in which the three involved atoms form an ∼180-degree angle (29, 107). For example, in the first step of DsbA-catalyzed protein oxidation reaction, the three involved atoms are the sulfur atom of the attacking cysteine of the substrate, and the two sulfur atoms (Cys30, and Cys33) of DsbA. Thus, the SN2 reaction theory predicts that these three sulfur atoms must be linearly arranged at the transition state. Therefore, relative positions of these sulfur atoms must be important for efficient catalysis. Because an open space exists in front of Cys30 along the S-S axis of active-site cysteines of the oxidized DsbA, an attacking thiolate of a substrate will, at least, be able to access the sulfur atom of Cys30 in an SN2 manner. To perform the reaction efficiently, DsbA must have evolved to bind its substrates in a manner that allows the attacking thiolate to be linearly placed at an ∼180-degree angle from the Cys30–Cys33 disulfide of DsbA.
DsbB: A Membrane Protein That Maintains DsbA in the Oxidized Active State
After transfer of its disulfide bond to a target protein, DsbA must be reoxidized to repeat another catalytic cycle. This reoxidation is performed by a membrane protein DsbB (9). In wild-type cells, DsbA is maintained exclusively in the oxidized state. However, in dsbB-null mutants, DsbA fails to be reoxidized and remains in the reduced state. Thus, the dsbB mutants are strongly defective in disulfide-bond formation of many cell-envelope proteins. However, the extent of disulfide defect in the dsbB mutants is slightly less than that of the dsbA mutants, especially in rich medium containing a large amount of cystine or the oxidized form of glutathione, as DsbA can be oxidized, albeit much less efficiently, by these small-molecule oxidants (9).
DsbB restores the disulfide bond to DsbA by using the oxidizing power of the electron-transport chain (Fig. 1) (7, 63). Aerobically, ubiquinone (an aerobic quinone) receives electrons from DsbB. The reduced ubiquinone is then reoxidized by terminal oxidases, which pass electrons to O2. Anaerobically, electrons are transferred from DsbB to menaquinone (an anaerobic quinone). The reduced menaquinone is then reoxidized by an anaerobic electron-transport system. The ability of DsbB to use both ubiquinone and menaquinone as electron acceptors allows the DsbA–DsbB system to work under both aerobic and anaerobic conditions (7).
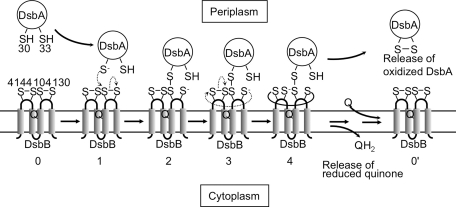
DsbB (20 kDa) spans the membrane 4 times and has four essential cysteines. In the oxidized state of DsbB, the cysteines form disulfide bonds (Cys41–Cys44, and Cys104–Cys130). They are located in its first and second periplasmic loop, respectively, and are redox active (48, 62) (Fig. 4, stage 0). The in vivo and in vitro analysis of mutants in which these cysteines are altered has revealed the following picture for the function of each cysteine in oxidizing DsbA (54, 61). The Cys104–Cys130 pair undergoes an electron-transfer process with DsbA. This exchange is initiated by the attack of the reduced DsbA Cys30 on Cys104 in the Cys104–Cys130 disulfide bond of DsbB, resulting in the formation of an interprotein disulfide bond between Cys30(DsbA) and Cys104(DsbB) (54). Because the Cys41–Cys44 pair is required to maintain the Cys104–Cys130 pair in the oxidized state (61, 62), electrons are transferred from the Cys104–Cys130 pair to the Cys41–Cys44 pair. Further, because Cys41 and Cys130 can form an interloop disulfide linkage in vivo (54) and in vitro (45), the transfer of electrons from the second periplasmic loop to the first periplasmic loop is likely mediated by the attack of Cys130 on Cys41 in the Cys41–Cys44 disulfide bond (45, 54) (Fig. 4, stage 3). Finally, the Cys41–Cys44 pair is directly oxidized by quinones (62, 88). Thus, electrons from DsbA flow first to the Cys104–Cys130 pair, then to the Cys41–Cys44 pair, and from there to quinones in the respiratory chain.
FIG. 4.
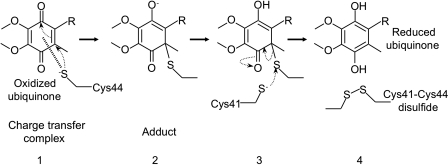
A model for early steps in the reoxidation of DsbA by DsbB. DsbA is highly oxidizing. Thus, it tends to be reduced. The mechanism by which DsbB oxidizes such a highly oxidizing protein is a fundamental question. In this model, the interloop disulfide bond formed between Cys130 and Cys41 (stage 4) prevents the back reaction (stage 2 to stage 0) that would tend to occur because of the highly oxidizing nature of DsbA. Thus, DsbB coordinates the action of its four cysteines to oxidize DsbA. In this model, the DsbB–DsbA complex in stage 4 corresponds to the charge-transfer complex that is depicted in Fig. 5. In the alternative model, electron transfer from DsbA to quinone proceeds through a sequential set of two thiol-disulfide exchange reactions, and then reduction of quinones through a charge-transfer complex (not shown). Q indicates the position of ubiquinone bound to DsbB.
The three-dimensional structures of DsbB show four transmembrane helices (TM1–TM4) arranged in a left-handed bundle (44, 73, 112). TM1 is bent slightly outside the bundle, creating a groove between TM1 and TM4 to accommodate ubiquinone (112). The redox-active ring structure of ubiquinone is located in close proximity to the Cys41–Cys44 disulfide and to Arg48 (73, 112). This arrangement of the ubiquinone ring is consistent with the model outlined earlier, in which quinones directly oxidize the Cys41–Cys44 pair of DsbB and also with the observation that Arg48 is important for the transfer of electrons from DsbB to quinone (52).
Then how is the Cys41–Cys44 pair of DsbB reoxidized by quinones? Quantum chemical simulation by Inaba et al. (46) suggested the formation of a charge-transfer complex between ubiquinone and DsbB. In this model, a thiolate anion of the reduced Cys44 transfers partial charge to the benzoquinone ring of ubiquinone (Fig. 5, stage 1). This charge-transfer complex formation may be followed by the nucleophilic attack on the ubiquinone by Cys44, resulting in the formation of a covalent adduct between ubiquinone and DsbB (Fig. 5, stage 2). Subsequently, Cys41 may attack Cys44 of DsbB (Fig. 5, stage 3), generating the Cys41–Cys44 disulfide bond and the reduced form of ubiquinone (Fig. 5, stage 4) (46).
FIG. 5.
A model for the oxidation of the N-terminal cysteine pair of DsbB by DsbB-bound ubiquinone. See text for the description of the model.
The side chain of Arg48 likely stabilizes the charge-transfer complex by interaction with the Cys44 thiolate anion or the benzoquinone ring. The existence of a transient charge-transfer complex between the Cys44 thiolate and ubiquinone is supported by the observation that DsbB manifests a characteristic purple color on interaction with reduced DsbA (45). A similar absorbance in the visible region has been observed in a charge-transfer complex between a cysteine-thiolate and a flavin redox ring in glutathione reductase (58, 73). This charge-transfer model is consistent with the structures of DsbB, in which Cys44 is well positioned to form the charge-transfer complex (43, 44, 73, 112).
Paradoxically, the standard redox potential measurements indicate that the cysteine pairs of DsbB are significantly less oxidizing than the cysteine pair of DsbA, with redox potentials of −207 mV for Cys41–Cys44, and −224 mV for Cys104–Cys130 (47). The latter value indicates that transfer of reducing equivalent from DsbA to the Cys104–Cys130 pair of DsbB is energetically unfavorable.
Two models have been proposed that may explain how this energetic barrier is overcome (47, 54). In both models, the reaction is initiated by the nucleophilic attack of Cys30 of DsbA on the Cys104–Cys130 disulfide in DsbB, resulting in a Cys30(DsbA)–Cys104(DsbB) interprotein disulfide bond. The two models diverge after this step. In one model (Fig. 4), the reduced Cys130 immediately attacks Cys41 in the Cys41–Cys44 disulfide to form the Cys41–Cys130 disulfide bond (Fig. 4, stage 3). This interloop Cys41–Cys130 disulfide bond would function as a lock on Cys130, preventing it from attacking the disulfide bond between DsbB and DsbA (54). This attack would initiate the unwanted backreaction, yielding released reduced DsbA. This lock on Cys130 may be a necessary step because the redox potentials of the cysteine pairs involved would strongly favor this backreaction. After the formation of the interloop disulfide bond, the reduced Cys44 thiolate and ubiquinone would form a charge-transfer complex (46). This concerted model is supported by kinetic measurements by Tapley et al. (102), which showed that the rate of DsbA oxidation is the same as the rate of ubiquinone reduction. Such a concerted reaction may become possible because of the close proximity of all six involved cysteines in a model of DsbA–DsbB structure (112).
In the alternative model, electron transfer from DsbA to quinone proceeds through a sequential set of two thiol-disulfide exchange reactions and then reduction of quinones (47). Importantly, in this model, release of oxidized DsbA from the DsbA–DsbB complex occurs before the interloop electron transfer. In this model, the backreaction is prevented by relocation of Cys130 away from Cys104 in the DsbA–DsbB complex (44). This nonconcerted model is supported by in vitro analysis using quinone-free DsbB (47) (under steady-state conditions, DsbB has a bound quinone). The structures of DsbB are consistent with both of the two models (44, 112). These two models are still currently under debate.
DsbC: A Periplasmic Disulfide-Bond Isomerase
For a protein with two cysteines, only one possible disulfide bond can form within that protein. However, in a protein with three or more cysteines, two cysteines can be joined in a disulfide bond that does not appear in the native structure. Such nonnative disulfide bonds do occur in the process of disulfide-bond formation in vivo. In E. coli, a periplasmic protein DsbC repairs proteins with such incorrect disulfide bonds (12, 38, 90, 99, 110) (Figs. 1 and 6).
FIG. 6.
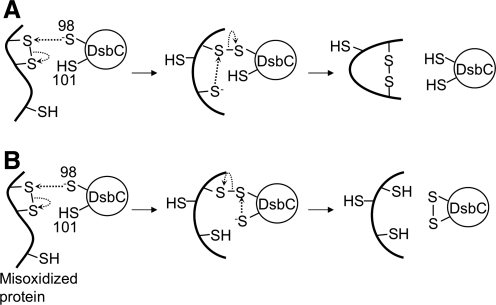
Disulfide-bond isomerization (A) and reduction (B) by DsbC. Two mechanisms have been proposed for the repair of a misoxidized cysteine pair by DsbC. After the reduction of the substrate protein in sequence (B), DsbA can reoxidize the substrate, potentially generating the correct disulfide bond (not shown).
In E. coli mutants lacking DsbC, some disulfide-bonded proteins are reduced in activity or are degraded. These proteins include periplasmic endoribonuclease RNase I, penicillin-insensitive murine endopeptidase MepA, periplasmic acid phosphatase AppA, and periplasmic endonuclease-1 End1 (12, 38, 78, 103). A common feature of these proteins is that they have or likely have, in their native structure, at least one nonconsecutive disulfide bond, a disulfide bond formed between two cysteines that do not appear in sequence in the protein. When a protein contains, in its native structure, one or more nonconsecutive disulfide bonds, it appears that the incorrect disulfide bonds are often made. By using AppA as a model substrate, Berkmen et al. (12) showed that DsbC is important for the folding of proteins with such nonconsecutive disulfide bonds. Another evident feature of dsbC-null mutants is that they show an increased sensitivity to copper. Because copper causes misoxidation of proteins in the presence of molecular oxygen, it has been proposed that the copper sensitivity of dsbC mutants is due to the inability of the mutants to repair the misoxidized proteins in the periplasm that have accumulated in the presence of copper (39).
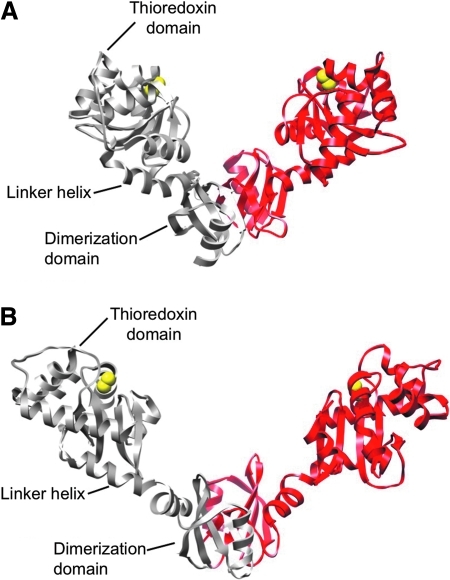
DsbC is a homodimeric molecule with a V-shaped structure (Fig. 7A). Each monomer (23 kDa) of DsbC consists of a C-terminal thioredoxin domain with a CXXC active site and an N-terminal dimerization domain connected by a linker α-helix (77). Two cysteines (Cys98 and Cys101) in the active site from each monomer face the inside of the V-shape and are in the reduced state in vivo (49, 91). The dimeric structure of DsbC is crucial for its function: in contrast to DsbA, which is monomeric and oxidized by DsbB, the active site of DsbC is sterically protected from such unwanted oxidation by its dimeric state. A mutation in the dimer interface of DsbC, which results in the inability to form a dimer, allows DsbB to oxidize DsbC. The oxidized mutant DsbC can now act as an oxidant of exported proteins in the periplasm (8) (see later). DsbC has a standard redox potential of −130 mV (110), which is slightly lower than that of DsbA (−120 mV).
FIG. 7.
The crystal structures of DsbC (PDB:1EEJ) (A) and DsbG (PDB:1V58) (B). DsbC and DsbG are V-shaped homodimers; each arm is a monomer containing an N-terminal dimerization domain and a C-terminal thioredoxin domain. The two domains are connected by a linker helix. The yellow spheres represent the sulfurs of active-site cysteines. The dimer form of DsbG was generated by applying crystallographic symmetry (85). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at www.liebertonline.com/ars).
How can DsbC repair incorrect disulfide bonds of its substrates? Two models have been proposed (55, 91) (Fig. 6). In both cases, the reaction starts with the nucleophilic attack by Cys98 of DsbC on the incorrect disulfide bond. This reaction results in a mixed-disulfide bond between DsbC and its substrate, which can then be resolved in two ways. In the first model (Fig. 6A), the mixed disulfide is attacked by a third cysteine of the substrate, resolving the complex, allowing a more stable disulfide bond to form in the substrate and restoring a reduced DsbC. In this case, DsbC is acting as a true disulfide isomerase. In the second (Fig. 6B), the mixed disulfide is attacked by Cys101 of DsbC, generating a reduced substrate and an oxidized DsbC. The reduced substrate will then have a chance to be oxidized again by DsbA to give the correct disulfide bond. In the latter case, DsbC is acting as a reductase of disulfide bonds rather than as an isomerase. In both models, the active site of DsbC must be in the reduced state for the enzyme to carry out the first step in the repair of a nonnative disulfide bond.
Theoretically, nonnative disulfide bonds can be repaired by repeating the isomerization or reduction–oxidation reaction until the native disulfide connectivity is established in the substrate protein. Although distinguishing between these two possibilities for the repair of the nonnative disulfide bonds in vivo has remained elusive, a recent study with TrxP, a thioredoxin-fold protein from Bacteroides fragilis, showed that one of the mechanisms can work in vivo. TrxP has strong disulfide reductase activity but very weak isomerase activity. By expressing TrxP in the periplasm of E. coli cells lacking DsbC, Shouldice et al. (100) showed that the reduction–oxidation mechanism can indeed operate in vivo to repair nonnative disulfide bonds.
It should be noted that other possible mechanisms exist for the isomerization of disulfide bonds. For example, oxidized DsbC generated after the reduction of a misoxidized substrate (Fig. 6B) may, in turn, act to introduce a disulfide bond into the reduced substrate. It may also be possible that a free cysteine on a misoxidized protein may attack a nonnative disulfide bond on the protein to isomerize, by itself, the disulfide bond (internal rearrangement). However, currently no experiments have tested these mechanisms in vivo.
In addition to the isomerase or reductase activity, DsbC has chaperone activity that allows this protein to bind unfolded proteins. For this activity, the active-site cysteine residues are not required (70). The chaperone activity of DsbC may allow this enzyme to capture its misoxidized (thus likely unfolded) substrates efficiently.
The recognition site in DsbC for unfolded structures likely exists in the cleft formed by the inside of the V-shape (Fig. 7A). First, this cleft is large enough (40 Å × 40 Å × 25 Å) to bind small or unstructured/unfolded proteins. Second, the inner surface is covered with uncharged and hydrophobic residues, a feature often seen in domains that bind misfolded or unfolded proteins (77). Third, fusion of the N-terminal dimerization domain of DsbC to other oxidoreductases, like DsbA, that do not exhibit chaperone activity, confers both chaperone and disulfide isomerase activities (see later) (96, 111). Finally, disruption of the dimerization domain, by a mutation in the dimer interface, results in the loss of both chaperone and disulfide isomerase activities (8). Thus, the dimeric nature of the bottom of the cleft is necessary for the chaperone activity.
Because of the dimerization, DsbC has two catalytic domains, raising the question of whether both catalytic sites are required for its activity. To answer this question, Arredondo et al. (4) engineered a DsbC variant, in which the DsbC sequence is tandemly duplicated on a single polypeptide. Surprisingly, a sufficiently long linker sequence placed between the two monomer sequences allowed the polypeptide to fold into an enzyme that displayed essentially identical catalytic and biochemical properties to the authentic dimeric DsbC. Intriguingly, because two monomer sequences are on the same polypeptide, it is possible to design heterodimer-like DsbC variants that have mutations only on the one of the two monomer sequences. With this system, the authors showed that, even if DsbC is missing one of the two catalytic domains, the protein still exhibits the isomerase activity. However, such a mutant was oxidized by DsbB and acted also as an oxidant in vivo. Thus, the possession of two catalytic domains is not essential for the activity but is required to protect the active site from misoxidation by DsbB.
DsbG: A DsbC Homologue That Is Able to Protect Single Cysteines from Hyperoxidation
DsbG is a periplasmic thiol-oxidoreductase that is homologous to DsbC (2, 14). DsbG shares 24% amino acid sequence identity with DsbC. The size of the monomer is 26 kDa. Like DsbC, DsbG is dimeric and assumes a V-shaped structure with its N-terminal dimerization domain and its C-terminal thioredoxin domain connected by a linker helix (36). Two cysteines (Cys109 and Cys112) in its active-site CXXC motif are maintained in the reduced state in vivo by DsbD (14). DsbG has a redox potential of −126 mV, which is similar to that of DsbC (14).
Although the overall structure of DsbG resembles that of DsbC (Fig. 7A and B), significant differences exist. Most strikingly, the size of the cleft formed by the V-shaped structure in DsbG is almost twice the size of that of DsbC. Additionally, DsbG has several acidic residues lining the cleft, which form a negatively charged surface that is absent in DsbC. These properties of DsbG led Heras et al. (36) to propose that DsbG can interact with proteins that are larger and have less hydrophobic surfaces than do the substrates of DsbC.
Then what is the role of DsbG in vivo? DsbG exhibits molecular chaperone and protein disulfide isomerase activity in vitro (14, 98), although its protein disulfide isomerase activity was poor as compared with that of DsbC (14, 40). Consistently, overexpression of DsbG suppressed some of the defects of dsbC-null mutations (12, 14). Yet, the in vivo function of DsbG had remained unknown because of the inability to define its physiologic substrates (38, 103).
Recently, Depuydt et al. (23) identified the substrates of DsbG, results that led to the discovery of an unexpected function for both DsbG and DsbC. This group found that DsbG preferentially interacts with three periplasmic proteins. All of them belong to the same family of l,d-transpeptidases, which are responsible for cross-linking the major outer-membrane lipoprotein to the peptidoglycan of E. coli (71). Importantly, they possess a sole cysteine, essential for activity. By using dyes that specifically detect sulfenic acid (Cys-S-OH), Depuydt et al. (23) showed that such a cysteine can be oxidized to a sulfenic acid in the periplasm and that both DsbG and DsbC can protect the cysteine from sulfenylation. The level of sulfenylation was highest in cells lacking both DsbG and DsbC. However, even the cells lacking DsbG alone, but not those lacking DsbC alone, showed increased sulfenylation of sole cysteines over the wild-type cells. Thus, DsbG appears to be more proficient than DsbC in protecting free cysteines in proteins from sulfenylation.
As discussed earlier, the cleft formed by the V-shape of DsbG is much larger and more hydrophilic than that of DsbC (36). Because the preferred substrates of DsbG are folded proteins with an oxidized single cysteine, these unique features of DsbG may allow this enzyme to bind its substrates more efficiently than DsbC.
DsbD: A Membrane Protein That Maintains Both DsbC and DsbG in the Reduced Active State
Both DsbC and DsbG must be maintained in the reduced state to be active. This task is performed by a membrane protein, DsbD (14, 79, 91). In dsbD mutants, both DsbC and DsbG fail to be reduced and, thus, cannot act as isomerases or reductases of misoxidized proteins. As a result of reducing DsbC or DsbG, DsbD becomes oxidized. The oxidized DsbD is, in turn, reduced by cytoplasmic thioredoxin (TrxA), which itself is reduced by thioredoxin reductase (TrxB). Thus, reducing power to maintain DsbC and DsbG in their reduced state originated from NADPH (91).
DsbD (59 kDa) has three domains: an N-terminal periplasmic domain (DsbDα) with an immunoglobulin-like fold (35), a hydrophobic core domain (DsbDβ) with eight transmembrane segments (18), and a C-terminal periplasmic domain (DsbDγ) with a thioredoxin-like fold (94). Each domain has a pair of cysteines that are essential for the protein's activity (59).
How does DsbD catalyze the transmembrane electron transfer from TrxA in the cytoplasm to DsbC in the periplasm? Katzen and Beckwith (59) reconstituted DsbD activity by expressing three domains of DsbD separately in vivo. With this system, they revealed that this electron-transfer reaction is carried out through a sequential reduction and oxidation of the three structural domains: DsbDα directly reduces oxidized DsbC; oxidized DsbDα, thus generated, is then reduced by DsbDγ; the resulting oxidized DsbDγ is reduced by DsbDβ; and finally, oxidized DsbDβ is reduced by thioredoxin in the cytoplasm. Subsequent in vitro results are consistent with this cascade model (19, 35, 94).
The analyses described revealed that electrons from TrxA in the cytoplasm are passed through DsbD to the oxidized DsbC or DsbG in the periplasm in the following order: TrxA, DsbDβ, DsbDγ, DsbDα, to DsbC/DsbG. The standard redox potentials of their active-site cysteine pairs are −270 mV(TrxA), −246 mV (DsbDβ), −241 mV (DsbDγ), −229 mV (DsbDα), −130 mV (DsbC), and −126 mV(DsbG) (19, 93). Thus, electron-transfer reactions between every two active-site cysteine pairs along the DsbD-mediated electron flow are thermodynamically favorable (19, 93). This is in contrast to the oxidation of DsbA by the second periplasmic domain of DsbB (see earlier).
Two cysteines of DsbDβ receive electrons from TrxA in the cytoplasm and transfer them to DsbDγ in the periplasm (59). How is it possible for the two cysteines of DsbDβ to perform the reactions on both sides of the membrane? Recent studies by Cho et al. (17, 18) suggest that DsbDβ exhibits hourglass-like openings on both sides of the membrane, having its two active-site cysteines at the constriction site. This physical arrangement appears to allow two cysteines in DsbDβ to interact with the cysteines of thioredoxin on the cytoplasmic side and with the cysteines of DsbDγ on the periplasmic side of the membrane, facilitating the electron-transfer reaction across the membrane.
DsbD represents a unique type of two-electron transporter. Still unclear is the mechanism that would allow the passage of electrons across the membrane without perturbing the function of the membrane as a permeation barrier. Determination of the three-dimensional structure of DsbDβ may be necessary to arrive at an answer to this question.
Factors Influencing DsbA-mediated Oxidative Protein Folding
Despite considerable knowledge of the structure and biochemical properties of the enzymes involved in disulfide bond formation, we know very little about how each disulfide bond forms in a protein that is translocated into the bacterial periplasm in vivo. When during the process of translation, membrane translocation, and folding does disulfide bond formation take place? How important are the properties of the cysteines themselves in determining which cysteines are covalently joined? Here we discuss factors that may affect the process of oxidative protein folding.
Vectorality of protein export and oxidative folding in the periplasm
For the protein substrates of DsbA to cross the membrane through the Sec channel, the polypeptides must be maintained in the cytoplasm in an unfolded state (41, 69). As the proteins pass through the Sec channel, the N-terminus of the protein appears in the periplasm first (51, 82). These mechanistic features of protein export appear to affect the mechanisms of oxidative protein folding. Both indirect and direct evidence support a model in which disulfide bonds are often formed sequentially between two cysteines of a protein as they appear in the periplasm.
If disulfide bond formation does proceed vectorially, it would cause a problem for those proteins that have nonconsecutive disulfide bonds in their native structure. Such a mechanism would lead to the formation of incorrect disulfide bonds in these proteins, necessitating the action of an isomerase. As described earlier, the disulfide isomerase DsbC is required for the efficient folding of such proteins (12, 38), consistent with the vectorial model.
This model also predicts that the formation of a disulfide bond between a correct pair of cysteines would be compromised if there were an unpaired cysteine before or between cysteines that are meant to be joined in the native structure. Interestingly, bioinformatics analysis by Dutton et al. (27) revealed that, for those organisms that make disulfide bonds, the majority of bacterial cell envelope proteins appear to have evolved to avoid an unpaired cysteine, apparently to prevent the formation of incorrect disulfide bonds (see later), consistent with the vectorial model.
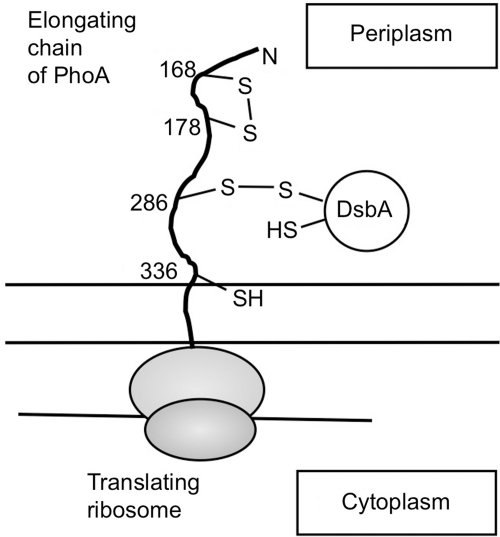
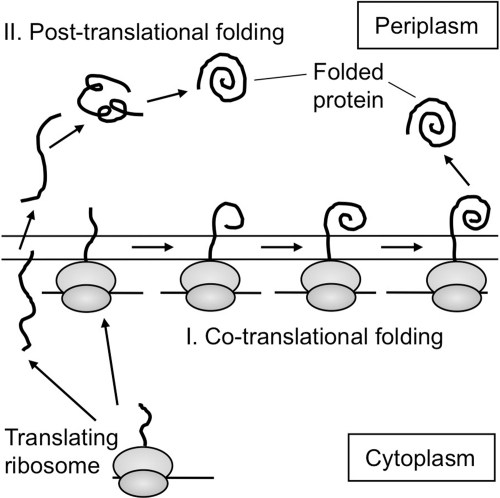
More-direct evidence for this model came from a recent study of oxidative folding of PhoA, a periplasmic protein of E. coli having two disulfide bonds (53). This protein crosses the membrane by a mixed co-translational/posttranslational mechanism in which some of the protein is exported during its synthesis on the ribosome, and some is exported after completion of its synthesis (51). During cotranslational folding of PhoA, the formation of the N-terminal disulfide bond took place before that of the C-terminal disulfide bond, consistent with the order in which each cysteine pair appears in the periplasm. In addition, steps of electron transfer leading to the formation of the C-terminal disulfide bond began by Cys286 attacking DsbA when the PhoA peptide elongated to ∼43 kDa in length, thus forming a mixed-disulfide complex between DsbA and the nascent chain of PhoA (Fig. 8). Further elongation of the peptide allowed Cys336 to attack the mixed disulfide, resulting in the resolution of the complex and formation of a Cys286–Cys336 disulfide in the nascent chain, completing the process. Thus, two cysteines that form a disulfide bond also acted sequentially in the order in which they appear in the periplasm (53), further supporting the vectorial model.
FIG. 8.
A model for the disulfide-linked DsbA-substrate complex that forms during the cotranslational folding of PhoA. PhoA has two pairs of disulfide bonds. During cotranslational folding of PhoA, the PhoA Cys286 attacked DsbA to form a disulfide-linked complex (this model) between DsbA and the elongating chain of PhoA. This reaction took place when the PhoA polypeptide was elongated to ∼43 kDa in length. Further elongation of the polypeptide allowed Cys336 to attack the mixed-disulfide bond, completing the formation of the C-terminal disulfide bond of PhoA (not shown) (53).
These results indicate that the vectorality in the appearance of nascent polypeptide from the Sec machinery can be an important factor affecting oxidative protein folding in the periplasm. However, recent evidence indicates the existence of nonvectorial folding and other factors that may affect the oxidative protein folding.
Partially correct folding of RNase I even in the absence of DsbC
RNase I of E. coli has four disulfide bonds, one of which is formed between a nonconsecutive cysteine pair. Messens et al. (78) studied the DsbA-catalyzed formation of disulfide bonds in this protein in vivo and in vitro in the presence or absence of DsbC. They found that DsbA activity alone is capable of yielding some of the protein containing its correct disulfide bonds. This finding suggests that a factor, other than vectorial export, must be influencing the interaction between DsbA and its substrate, leading to the fairly correct oxidative folding of this protein despite the protein's nonconsecutive disulfide bond. One possible explanation is that the speed with which DsbA acts on its emerging substrates is slow enough so that some fraction of the polypeptide chains achieve a sufficient degree of folding to make it possible for DsbA to join the correct cysteines.
Disulfide-bond formation guided by the structural changes of a protein
Elastase of Pseudomonas aeruginosa is synthesized as a preproenzyme. The mature protein has two disulfide bonds. Interestingly, disulfide-bond formation in this protein takes place nonvectorially by three steps (15). In the first step, DsbA introduces the C-terminal disulfide bond into the proenzyme. This step is required for the autocatalytic processing of its propeptide. In the final step, DsbA introduces the N-terminal disulfide bond before translocation of the mature protein across the outer membrane. Thus, it appears that disulfide-bond formation in this protein by DsbA in vivo is an ordered process, guided by structural changes of the protein.
The activity of the ribosome can affect the extracytoplasmic protein folding
Analysis of the oxidative folding process of PhoA during cotranslational or posttranslational folding revealed an unexpected effect of the ribosome in the folding process of a protein in the bacterial periplasm (53) (Fig. 9).
FIG. 9.
Cotranslational and posttranslational oxidative folding of PhoA. Results (53) suggest that the mode of translocation (cotranslational versus posttranslational) can affect the folding process of a protein in the periplasm.
As we described earlier, during the cotranslational formation of the Cys286–Cys336 disulfide bond of PhoA, Cys286 was the cysteine used to form the mixed-disulfide complex with DsbA. However, surprisingly, during posttranslational folding of PhoA, both Cys286 and Cys336 were used to form the disulfide-linked complexes between DsbA and PhoA (53), suggesting that the process of disulfide-bond formation during posttranslational folding may be different from that of cotranslational folding. Further, in a PhoA variant lacking its second cysteine, Cys178, the first cysteine, Cys168, is unpaired because it does not have its native partner for the formation of the Cys168–Cys178 disulfide bond. When PhoA was exported cotranslationally by using an SRP-dependent signal sequence, the folding of PhoA was more sensitive to the presence of the unpaired cysteine than was the same protein exported both cotranslationally and posttranslationally with its authentic signal sequence. These results suggest that oxidative folding of PhoA occurs more vectorially during its cotranslational folding than that of the same protein during posttranslational folding (53).
It has been estimated that the rate of posttranslational export is more than 10 times greater than that of cotranslational export (86). Thus, when the polypeptide is crossing the membrane in a cotranslational manner, it may be the slowness of the appearance of the polypeptide in the periplasm that results in polypeptide folding more vectorially from the N-terminus to the C-terminus in this experiment. In contrast, the more rapid posttranslational export may cause the protein folding to outcompete the early action of DsbA on a substrate even more significantly.
Increasing evidence suggests that the ribosome plays an important role in the folding of proteins in the cytoplasm (65). This finding on the unexpected link between the activity of ribosome and the extracytoplasmic protein folding may expand our understanding regarding the role of the ribosome in protein folding.
Is the oxidative-folding process regulated by the reactivity of cysteines?
As described earlier, during cotranslational formation of the C-terminal disulfide bond of PhoA, Cys286 attacked DsbA to initiate the reaction. The resulting DsbA–PhoA complex was resolved by Cys336, completing the reaction (53). This finding indicates that two cysteines of a folding protein can play different roles in the formation of their disulfide bond in a reaction catalyzed by a thioredoxin-superfamily member.
Two factors could be important in determining which cysteine will attack DsbA during disulfide-bond formation. First, the cysteine on a substrate must be sterically available to DsbA to initiate the process. Second, as the deprotonation of its sulfhydryl group is essential for a cysteine to attack a disulfide bond (29, 106), the reactivity of a substrate cysteine may also be affected by its pKa. During the cotranslational formation of the C-terminal disulfide bond of PhoA, the availability of the cysteine (the order of appearance in the periplasm) most likely determined which cysteine attacked DsbA (53).
Can difference in the reactivity of cysteines, if it exists during oxidative folding, regulate this process in vivo? Before addressing this question, we should consider whether DsbA is able to choose a proper pair of cysteines from among others to form a correct disulfide bond. DsbA often makes mistakes. This protein can introduce disulfide bonds into proteins that do not have disulfide bonds in their native state. For example, when a cytoplasmic enzyme, β-galactosidase, is artificially exported to the periplasm, the cysteines of this protein, normally in the reduced state, are joined by DsbA to form disulfide bonds, resulting in the misfolding of this protein (10). In addition, as we have seen in the section for DsbC, DsbA tends to introduce incorrect disulfide bonds into proteins that have one or more nonconsecutive disulfide bonds in their native structure (12). These observations have led to a view that DsbA is an indiscriminating enzyme that does not distinguish among the cysteines it joins in forming disulfide bonds (100). However, as we saw previously, some evidence suggests that this is not necessarily the case. For example, a significant proportion of RNase I is oxidized by DsbA to give the correct disulfide bonds, even in the absence of DsbC in vivo and in vitro, although a nonconsecutive disulfide bond exists in this protein (78). Further, disulfide-bond formation in elastase of Pseudomonas aeruginosa by DsbA of this organism is a regulated process occurring in a manner guided by structural changes of this protein (15). These findings suggest that DsbA may, at least in some cases, be able to discriminate between a proper pair of cysteines and others to form a correct disulfide bond.
At least two ways exist to imagine that DsbA is able to discriminate in the recognition of substrate cysteines. First, the substrate-binding site of DsbA may have evolved to recognize a specific set of substrate cysteines (e.g., by their ionic state or the local conformation in their vicinity). In this regard, the residue preceding the conservative cis-proline of DsbA was recently suggested to be important for the redox properties and substrate specificities of this enzyme (89). Second, some substrates of DsbA may have evolved to allow the reactivity of each of their cysteines toward DsbA to be regulated during oxidative folding, as discussed earlier. Although the substrate cysteine's reactivity could be potentially important in understanding the mechanisms of oxidative protein folding, our knowledge on this aspect is greatly limited because of the difficulty in detection and characterization of the intermediate complexes.
Diversity among Bacteria in Disulfide Bond–Forming Capacity and Mechanism
Searches for enzymes involved in pathways leading to disulfide-bond formation in bacteria other than E. coli have largely been based on seeking homologues of the DsbA and DsbB proteins. However, given the vast range of environments in which bacteria live, one might expect to find variability in seeking answers to the following questions: (a) Do all bacteria make proteins with structural disulfide bonds? (b) Do those bacteria that make disulfide bonds all use the DsbA and DsbB enzymes? and (c) Is it the case for all bacteria that proteins with disulfide bonds are absent from their cytoplasms and are found only in proteins translocated through the cytoplasmic membrane?
In recent years, a number of approaches have been used to answers these questions. These studies reveal diversity in the capacity and mechanism for disulfide-bond formation among bacterial species. Bioinformatic approaches for analyzing genome sequences have yielded predictions for whether and how an organism carries out disulfide-bond formation. Biochemical and genetic studies of individual organisms have revealed unexpected variants on the E. coli system. Finally, the novel mechanisms for achieving disulfide-bond formation in bacteria in laboratory-based evolution experiments have provided plausible scenarios for what may be found in bacterial species that have yet to be examined in detail.
Disulfide-bond formation in the cytoplasm
Until recently, it has generally been assumed that structural disulfide bonds are not present in cytoplasmic proteins. Two lines of evidence have challenged that assumption. First, E. coli strains have been mutationally altered in their reductive pathways, such that disulfide bonds are formed in certain proteins with high efficiency (13, 24). The proteins that did acquire disulfide bonds in these strains were those that were ordinarily exported from the cell but, in these studies, were expressed in the cytoplasm by deleting their signal sequences. Second, combining a computational approach with structural characterization of the proteins of a collection of microbial hyperthermophiles, Yeates and co-workers (72) discovered that some of these organisms, particularly the Archaea, do contain cytoplasmic proteins with disulfide bonds (11, 72). This unexpected finding was explained as being due to the evolutionary adaptation of these organisms to the harsh environments in which they live, requiring the added stability conferred on their proteins by disulfide bonds.
Proteins in cells or compartments in which disulfide bonds are made tend to have even numbers of cysteines
The computational approach used by Yeates' group assumed that cellular compartments that had many disulfide-bonded proteins would be enriched for proteins with even numbers of cysteines (11, 72). This assumption is reasonable, given the properties of a protein's cysteines in environments that are oxidizing or in which an enzyme catalyzing disulfide-bond formation is present, or both. The assumption comes, in part, from the knowledge that the prototypical disulfide bond–forming enzyme, DsbA, is relatively indiscriminate in forming disulfide bonds. That is, DsbA often catalyzes formation of the wrong disulfide bonds and even catalyzes disulfide-bond formation in proteins that do not ordinarily have such bonds (1, 10). Incorrect disulfide-bond formation can be a particular problem for proteins that contain an odd number of cysteines, in which the process may involve sequential joining of cysteines as they appear in the periplasm during the translocation process (12, 53). A second relevant property of cysteine is that it is the amino acid most sensitive to oxidation, often being converted to sulfenic acid and higher oxidation states in the presence of such oxidants as hydrogen peroxide. As a result, proteins with odd numbers of cysteines, even if some of those cysteines were joined in disulfide bonds, would still have a free cysteine that could have oxidative damage or be joined in a disulfide bond with a cysteine in another protein. Considering these problems, a preference in evolution may have been to avoid odd numbers of cysteines in those proteins destined for export to compartments that are oxidative or where disulfide bonds are made or both. With hyperthermophilic Archaea, Yeates et al. (72) translated all of the open reading frames of these organisms into protein sequences and found that their entire proteome showed a strong preference for proteins containing even numbers of cysteines. This finding was in contrast to the same analysis applied to the entire E. coli proteome, in which no marked preference for even numbers of cysteines was found. Because the bulk of the cell's proteins are cytoplasmic, the overall preference for even numbers of cysteines in the hyperthermophiles suggested that disulfide-bonded proteins were made within the cell's cytoplasm. This presumption was confirmed by the finding of cytoplasmic disulfide-bonded proteins in these organisms. These results are in contrast to the findings with E. coli in which structural disulfide bonds in proteins are made only in the periplasm.
An extended computational approach to diversity
The even/odd computational approach pioneered by Yeates and co-workers (72) has been modified by us to extend it beyond the analysis of the entire proteome of the bacterial cell to an analysis that distinguishes between cytoplasmic and exported proteins. The modification requires separate calculations of the overall amount of cysteine present in proteins in the cytoplasm and of cysteine present in proteins of extracellular compartments and secreted proteins. Distinguishing between the locations of proteins required bioinformatic means of predicting from sequences which proteins are exported and which domains of membrane proteins extend outside the cytoplasmic membrane. In addition, the analysis included a search for homologues of the DsbA and DsbB proteins in the sequenced genomes. This approach has been applied to 374 sequenced bacterial genomes. The data obtained from this combined approach leads to the following predictions (27):
Many bacteria incorporate disulfide bonds into a sizable fraction of their exported proteins, by using the same DsbA/DsbB system as does E. coli. These organisms include most of the α-, β-, and γ-proteobacteria. This prediction is consistent with structural studies on proteins from many of these organisms.
Certain other classes of bacteria, including many Actinobacteria, Cyanobacteria, and aerobic δ-proteobacteria, also make disulfide bonds in many of their proteins by using DsbA, but instead of DsbB, they use a homologue of vertebrate vitamin K epoxide reductase (VKOR) to reoxidize DsbA (27, 101). Vertebrate VKOR catalyzes reduction of vitamin K, a step in the set of enzymatic reactions that leads to blood coagulation (68, 92). Studies so far indicate that the mechanism of action of bacterial VKOR is quite similar to that of DsbB, that structural similarities exist between the two proteins, but that bacterial VKOR evolved separately and is not a homologue of DsbB. Remarkably, the VKOR of Mycobacterium tuberculosis, when expressed in E. coli, can substitute for DsbB and is inhibited by warfarin, a blood thinner that is used to prevent blood clotting in humans (26).
Many bacteria do contain homologues of DsbB and DsbA, but the cysteine-counting analysis predicts that they either do not make disulfide bonds in proteins at all or make them only in a small fraction of their exported proteins. In these cases, evidence suggests that some of these organisms may use relatively specialized DsbA homologues to introduce disulfide bonds into a very limited number of proteins. For example, genes encoding a DsbA and a DsbB were found in an operon with a gene for an arylsulfate sulfotransferase in a number of bacterial species, including Geobacter metallireducens and a uropathogenic E. coli (27, 32). (Some of these bacteria, including the latter organism, also contain genes encoding prototypical DsbA and DsbB proteins.) Evidence suggested that these DsbAs do not have the broad specificity of the prototypical DsbA and may have been designed to catalyze disulfide bond formation efficiently in the arylsulfate sulfotransferase. In another example, the gram-positive firmicute Bacillus subtilis encodes two DsbA/DsbB-like pairs (the Bdb proteins), for each of which only one known disulfide-bonded substrate has been found (64).
Many bacteria appear to have neither DsbA nor DsbB (nor VKOR) and from cysteine-counting are predicted not to make disulfide bonds. Many of these are obligate anaerobes, such as Bacteroides fragilis, or grow obligately only intracellularly (e.g., Rickettsia) (27). These organisms, because the extracellular space available to them or their periplasm represents a strongly reducing environment, may always maintain their cysteines in the reduced state. This may be an active process promoted by extracytoplasmic reductants or simply due to the absence of any oxidative catalysts of disulfide-bond formation. Evidence supporting the proposal that these organisms do not allow disulfide-bond formation comes from the finding that cysteines in proteins that are ordinarily disulfide bonded in their native host, E. coli, when expressed in Bacteroides fragilis, remain in the reduced state. Nevertheless, other organisms such as these may not encounter oxygen, yet can use electron acceptors such as nitrate or fumarate to permit disulfide-bond formation.
Some organisms exist for which the results of the various bioinformatic analyses may appear inconsistent. For example, organisms such as Staphylococcus aureus have a DsbA but no apparent DsbB, nor do they have a VKOR. In this case, based on the structure and the unusual stability of the oxidized form of this DsbA, Glockshuber and co-workers (37) proposed that the SaDsbA is more effective than the prototypical DsbA at using small-molecule oxidants as a replacement for DsbB.
Bioinformatic analysis in a number of laboratories has revealed added variability in the biology of the Dsb proteins. Although the DsbA of gram-negative bacteria is usually a soluble protein localized to the periplasmic space, gram-positive bacteria express DsbA homologues that are membrane bound either as lipoproteins or by transmembrane-spanning segments (64). This membrane attachment may be necessitated by the absence of the outer-membrane barrier found in gram-negative bacteria. Finally, some bacteria contain several homologues of Dsb proteins. For example, Neisseria meningitides expresses three DsbA homologues that may play different roles in the interaction of the bacteria with the host, again suggesting the possibility of differing specificities of the homologues (66).
Laboratory-generated evolution of alternative disulfide bond–forming systems in bacteria
After the discovery that the DsbA and DsbB proteins were responsible for disulfide bond formation in E. coli in the early 1990s, much of the research in this area has focused on the mechanisms of action of these two enzymes and on their presence in many other bacteria. However, as described in the previous section, work published in recent years has revealed a more complex and diverse picture of mechanisms of disulfide-bond formation in bacteria. These findings raise the possibility that there exist even more diverse mechanisms for disulfide-bond formation than have been detected. One approach to asking what other pathways for disulfide-bond formation might exist in bacteria is to evolve new pathways in the laboratory. Bioinformatic approaches can then be used to ask whether any evidence for such laboratory-evolved pathways can be detected among the array of bacteria species.
A number of studies have reported instances in which the requirement for DsbA for disulfide-bond formation has been bypassed. For example, when signal sequences are attached to cytoplasmic thioredoxin, it is exported to the periplasm of E. coli, where it is oxidized by DsbB (21, 22, 50). Even though thioredoxin has a very low redox potential and is a powerful reductant, it still catalyzed a measurable degree of disulfide-bond formation. This oxidizing activity was greatly enhanced by altering the two amino acids between the active-site cysteines of thioredoxin, so as to increase its redox potential to favor oxidation.
In another example, the disulfide isomerase DsbC, a thioredoxin family member, which ordinarily does not serve as an oxidant, can be converted by mutation or genetic engineering to an effective catalyst of disulfide-bond formation. Ordinarily, the homodimeric DsbC cannot act as an oxidant, as its active sites are protected from oxidation by DsbB, and it is efficiently reduced by DsbD. However, mutations of dsbC that interfere with dimerization cause DsbC monomers to accumulate, which can then be oxidized by DsbB, allowing the protein to catalyze disulfide-bond formation (see earlier) (8). Furthermore, deletions of amino acid residues from the DsbC linker helix that cause the DsbC active site to be more exposed also allow it to be oxidized by DsbB (95). Conversely, fusions of DsbA to dimerization domains result in a dimeric DsbA that now acts like DsbC as a disulfide isomerase (3, 96, 111). These manipulations hardly point to a potential new mechanism of disulfide-bond formation but, rather, may simply be recapitulating the evolution of thioredoxin family members, such as DsbA and DsbC.
However, two novel pathways for disulfide-bond formation have been generated in the laboratory. In the first case, mutagenesis and forced export of thioredoxin yielded a version of the protein in which the CXXC motif has been replaced with a CCXC motif (76). This version of thioredoxin formed a dimeric complex in which a [2Fe-2S] bridge linked the monomers via the cysteines. This dimeric complex was itself an effective catalyst of disulfide-bond formation in the absence of DsbB and DsbA and appeared to depend simply on oxygen. A second novel pathway involved the forced export of a glutaredoxin (glutaredoxin 3) of E. coli to the periplasm. In this case, disulfide-bond formation was also achieved without a requirement for DsbB. Rather, the ability of periplasmic glutaredoxin 3 to oxidize substrate proteins in the periplasm depended on the presence of the glutathione biosynthetic pathway in the cytoplasm (28). These results were interpreted to mean that the periplasmic glutaredoxin was able to promote disulfide-bond formation by using oxidized glutathione exported by cells. Although no evidence exists that either of these novel pathways is used normally by any bacterial species, their discovery should alert us to the possibility that they may be found as either the primary or a secondary pathway for disulfide-bond formation in some organisms.
Conclusion and Perspectives
In the past two decades, we have learned that a surprising variety of structures, mechanisms, and factors are involved in carrying out the process of disulfide-bond formation. However, fundamental questions remain about the molecular mechanism by which each component accomplishes its task. For example, DsbA introduces disulfide bonds into proteins in the periplasm. The proteins presumably begin to fold when a portion of the polypeptide appears in the periplasm. We do not know at what point of protein folding each disulfide bond is introduced into the protein by DsbA. Knowing how substrate cysteines attack DsbA to initiate the process will certainly increase our understanding of oxidative protein folding (53). In addition to DsbA, DsbC, and DsbG, E. coli possess a number of proteins that may affect protein folding in the periplasm. It will be important to determine the coordination between the disulfide catalysts and other factors such periplasmic molecular chaperones, peptidyl-prolyl cis-trans isomerases, export machinery proteins, and the ribosome (25, 53). Important features of the mechanisms by which the membrane components DsbB and DsbD carry out electron-transfer processes also remain largely unknown. How the C-terminal cysteine pair of DsbB oxidizes DsbA is still under debate. DsbB has a bound quinone, which is apparently reduced after oxidation of DsbA by DsbB. However, it is unclear at this point how the oxidized quinone is restored on DsbB. The mechanism by which the DsbD β domain promotes transmembrane electron transfer also remains unclear. Furthermore, many questions remain about the newly discovered system for repair of hyperoxidized single cysteines by DsbG and DsbC (23). How do these enzymes recognize and reduce the modified cysteines on folded proteins? Recent bioinformatic analysis reveals that some bacteria evolved multiple or totally different enzyme systems to form disulfide bonds (27). For many bacteria that are the causative agents of human disease, disulfide-bonded proteins, toxins, adherence factors, and others are essential for pathogenesis. Thus, the disulfide-bond–formation systems may serve as therapeutic targets, making it important to understand the mechanisms involved in disulfide-bond formation. Answering all of these questions will require a variety of genetic, biochemical, structural, and bioinformatic approaches.
Abbreviations Used
- DTT
dithiothreitol
- SRP
signal-recognition particle
- TrxA
thioredoxin
- TrxB
thioredoxin reductase
- VKOR
vertebrate vitamin K epoxide reductase
Acknowledgments
We thank Fredric Åslund and Seung-Hyun Cho for discussions, Jean-François Collet for communicating his group's results before publication, and Rachel Dutton for comments on the manuscript. J.B. is an American Cancer Society Professor. This work was supported by NIH grant GM41883 (to J.B.) and by an international research fellowship from the Global COE program in NAIST from MEXT of Japan and Grant-in-Aid for Scientific Research C (21580092) (to H.K.).
References
- 1.Alksne LE. Rasmussen BA. Dsb-insensitive expression of CcrA, a metallo-β-lactamase from Bacteroides fragilis, in Escherichia coli after amino acid substitution at two cysteine residues within CcrA. J Bacteriol. 1996;178:4306–4309. doi: 10.1128/jb.178.14.4306-4309.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen CL. Matthey-Dupraz A. Missiakas D. Raina S. A new Escherichia coli gene, dsbG, encodes a periplasmic protein involved in disulphide bond formation, required for recycling DsbA/DsbB and DsbC redox proteins. Mol Microbiol. 1997;26:121–132. doi: 10.1046/j.1365-2958.1997.5581925.x. [DOI] [PubMed] [Google Scholar]
- 3.Arredondo S. Segatori L. Gilbert HF. Georgiou G. De novo design and evolution of artificial disulfide isomerase enzymes analogous to the bacterial DsbC. J Biol Chem. 2008;283:31469–31476. doi: 10.1074/jbc.M803346200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arredondo SA. Chen TF. Riggs AF. Gilbert HF. Georgiou G. Role of dimerization in the catalytic properties of the Escherichia coli disulfide isomerase DsbC. J Biol Chem. 2009;284:23972–23979. doi: 10.1074/jbc.M109.010199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Åslund F. Berndt KD. Holmgren A. Redox potentials of glutaredoxins and other thiol-disulfide oxidoreductases of the thioredoxin superfamily determined by direct protein-protein redox equilibria. J Biol Chem. 1997;272:30780–30786. doi: 10.1074/jbc.272.49.30780. [DOI] [PubMed] [Google Scholar]
- 6.Atkinson HJ. Babbitt PC. An atlas of the thioredoxin fold class reveals the complexity of function-enabling adaptations. PLoS Comput Biol. 2009;5:e1000541. doi: 10.1371/journal.pcbi.1000541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bader M. Muse W. Ballou DP. Gassner C. Bardwell JC. Oxidative protein folding is driven by the electron transport system. Cell. 1999;98:217–227. doi: 10.1016/s0092-8674(00)81016-8. [DOI] [PubMed] [Google Scholar]
- 8.Bader MW. Hiniker A. Regeimbal J. Goldstone D. Haebel PW. Riemer J. Metcalf P. Bardwell JC. Turning a disulfide isomerase into an oxidase: DsbC mutants that imitate DsbA. EMBO J. 2001;20:1555–1562. doi: 10.1093/emboj/20.7.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bardwell JC. Lee JO. Jander G. Martin N. Belin D. Beckwith J. A pathway for disulfide bond formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardwell JC. McGovern K. Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 11.Beeby M. O'Connor BD. Ryttersgaard C. Boutz DR. Perry LJ. Yeates TO. The genomics of disulfide bonding and protein stabilization in thermophiles. PLoS Biol. 2005;3:e309. doi: 10.1371/journal.pbio.0030309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berkmen M. Boyd D. Beckwith J. The nonconsecutive disulfide bond of Escherichia coli phytase (AppA) renders it dependent on the protein-disulfide isomerase, DsbC. J Biol Chem. 2005;280:11387–11394. doi: 10.1074/jbc.M411774200. [DOI] [PubMed] [Google Scholar]
- 13.Bessette PH. Åslund F. Beckwith J. Georgiou G. Efficient folding of proteins with multiple disulfide bonds in the Escherichia coli cytoplasm. Proc Natl Acad Sci U S A. 1999;96:13703–13708. doi: 10.1073/pnas.96.24.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bessette PH. Cotto JJ. Gilbert HF. Georgiou G. In vivo and in vitro function of the Escherichia coli periplasmic cysteine oxidoreductase DsbG. J Biol Chem. 1999;274:7784–7792. doi: 10.1074/jbc.274.12.7784. [DOI] [PubMed] [Google Scholar]
- 15.Braun P. Ockhuijsen C. Eppens E. Koster M. Bitter W. Tommassen J. Maturation of Pseudomonas aeruginosa elastase: formation of the disulfide bonds. J Biol Chem. 2001;276:26030–26035. doi: 10.1074/jbc.M007122200. [DOI] [PubMed] [Google Scholar]
- 16.Charbonnier JB. Belin P. Moutiez M. Stura EA. Quéméneur E. On the role of the cis-proline residue in the active site of DsbA. Protein Sci. 1999;8:96–105. doi: 10.1110/ps.8.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho SH. Beckwith J. Two snapshots of electron transport across the membrane: insights into the structure and function of DsbD. J Biol Chem. 2009;284:11416–11424. doi: 10.1074/jbc.M900651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho SH. Porat A. Ye J. Beckwith J. Redox-active cysteines of a membrane electron transporter DsbD show dual compartment accessibility. EMBO J. 2007;26:3509–3520. doi: 10.1038/sj.emboj.7601799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collet JF. Riemer J. Bader MW. Bardwell JC. Reconstitution of a disulfide isomerization system. J Biol Chem. 2002;277:26886–26892. doi: 10.1074/jbc.M203028200. [DOI] [PubMed] [Google Scholar]
- 20.Darby NJ. Creighton TE. Catalytic mechanism of DsbA and its comparison with that of protein disulfide isomerase. Biochemistry. 1995;34:3576–3587. doi: 10.1021/bi00011a012. [DOI] [PubMed] [Google Scholar]
- 21.Debarbieux L. Beckwith J. On the functional interchangeability, oxidant versus reductant, of members of the thioredoxin superfamily. J Bacteriol. 2000;182:723–727. doi: 10.1128/jb.182.3.723-727.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Debarbieux L. Beckwith J. The reductive enzyme thioredoxin 1 acts as an oxidant when it is exported to the Escherichia coli periplasm. Proc Natl Acad Sci U S A. 1998;95:10751–10756. doi: 10.1073/pnas.95.18.10751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Depuydt M. Leonard SE. Vertommen D. Denoncin K. Morsomme P. Wahni K. Messens J. Carroll KS. Collet JF. A periplasmic reducing system protects single cysteine residues from oxidation. Science. 2009;326:1109–1111. doi: 10.1126/science.1179557. [DOI] [PubMed] [Google Scholar]
- 24.Derman AI. Prinz WA. Belin D. Beckwith J. Mutations that allow disulfide bond formation in the cytoplasm of Escherichia coli. Science. 1993;262:1744–1747. doi: 10.1126/science.8259521. [DOI] [PubMed] [Google Scholar]
- 25.Duguay AR. Silhavy TJ. Quality control in the bacterial periplasm. Biochim Biophys Acta. 2004;1694:121–134. doi: 10.1016/j.bbamcr.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Dutton RJ. Wayman A. Wei JR. Rubin EJ. Beckwith J. Boyd D. Inhibition of bacterial disulfide bond formation by the anti-coagulant warfarin. Proc Natl Acad Sci U S A. 2010;107:297–301. doi: 10.1073/pnas.0912952107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dutton RJ. Boyd D. Berkmen M. Beckwith J. Bacterial species exhibit diversity in their mechanisms and capacity for protein disulfide bond formation. Proc Natl Acad Sci U S A. 2008;105:11933–11938. doi: 10.1073/pnas.0804621105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eser M. Masip L. Kadokura H. Georgiou G. Beckwith J. Disulfide bond formation by exported glutaredoxin indicates glutathione's presence in the E. coli periplasm. Proc Natl Acad Sci U S A. 2009;106:1572–1577. doi: 10.1073/pnas.0812596106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandes PA. Ramos MJ. Theoretical insights into the mechanism for thiol/disulfide exchange. Chemistry. 2004;10:257–266. doi: 10.1002/chem.200305343. [DOI] [PubMed] [Google Scholar]
- 30.Frech C. Wunderlich M. Glockshuber R. Schmid FX. Preferential binding of an unfolded protein to DsbA. EMBO J. 1996;15:392–398. [PMC free article] [PubMed] [Google Scholar]
- 31.Grauschopf U. Winther JR. Korber P. Zander T. Dallinger P. Bardwell JC. Why is DsbA such an oxidizing disulfide catalyst? Cell. 1995;83:947–955. doi: 10.1016/0092-8674(95)90210-4. [DOI] [PubMed] [Google Scholar]
- 32.Grimshaw JP. Stirnimann CU. Brozzo MS. Malojcic G. Grütter MG. Capitani G. Glockshuber R. DsbL and DsbI form a specific dithiol oxidase system for periplasmic arylsulfate sulfotransferase in uropathogenic Escherichia coli. J Mol Biol. 2008;380:667–680. doi: 10.1016/j.jmb.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 33.Guddat LW. Bardwell JC. Martin JL. Crystal structures of reduced and oxidized DsbA: Investigation of domain motion and thiolate stabilization. Structure. 1998;6:757–767. doi: 10.1016/s0969-2126(98)00077-x. [DOI] [PubMed] [Google Scholar]
- 34.Guddat LW. Bardwell JC. Zander T. Martin JL. The uncharged surface features surrounding the active site of Escherichia coli DsbA are conserved and are implicated in peptide binding. Protein Sci. 1997;6:1148–1156. doi: 10.1002/pro.5560060603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haebel PW. Goldstone D. Katzen F. Beckwith J. Metcalf P. The disulfide bond isomerase DsbC is activated by an immunoglobulin-fold thiol oxidoreductase: crystal structure of the DsbC-DsbDα complex. EMBO J. 2002;21:4774–4784. doi: 10.1093/emboj/cdf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heras B. Edeling MA. Schirra HJ. Raina S. Martin JL. Crystal structures of the DsbG disulfide isomerase reveal an unstable disulfide. Proc Natl Acad Sci U S A. 2004;101:8876–8881. doi: 10.1073/pnas.0402769101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heras B. Kurz M. Jarrott R. Shouldice SR. Frei P. Robin G. Čemažar M. Thöny-Meyer L. Glockshuber R. Martin JL. Staphylococcus aureus DsbA does not have a destabilizing disulfide: a new paradigm for bacterial oxidative folding. J Biol Chem. 2008;283:4261–4271. doi: 10.1074/jbc.M707838200. [DOI] [PubMed] [Google Scholar]
- 38.Hiniker A. Bardwell JC. In vivo substrate specificity of periplasmic disulfide oxidoreductases. J Biol Chem. 2004;279:12967–12973. doi: 10.1074/jbc.M311391200. [DOI] [PubMed] [Google Scholar]
- 39.Hiniker A. Collet JF. Bardwell JC. Copper stress causes an in vivo requirement for the Escherichia coli disulfide isomerase DsbC. J Biol Chem. 2005;280:33785–33791. doi: 10.1074/jbc.M505742200. [DOI] [PubMed] [Google Scholar]
- 40.Hiniker A. Ren G. Heras B. Zheng Y. Laurinec S. Jobson RW. Stuckey JA. Martin JL. Bardwell JC. Laboratory evolution of one disulfide isomerase to resemble another. Proc Natl Acad Sci U S A. 2007;104:11670–11675. doi: 10.1073/pnas.0704692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber D. Cha MI. Debarbieux L. Planson AG. Cruz N. López G. Tasayco ML. Chaffotte A. Beckwith J. A selection for mutants that interfere with folding of Escherichia coli thioredoxin-1 in vivo. Proc Natl Acad Sci U S A. 2005;102:18872–18877. doi: 10.1073/pnas.0509583102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huber-Wunderlich M. Glockshuber R. A single dipeptide sequence modulates the redox properties of a whole enzyme family. Fold Des. 1998;3:161–171. doi: 10.1016/S1359-0278(98)00024-8. [DOI] [PubMed] [Google Scholar]
- 43.Inaba K. Murakami S. Nakagawa A. Iida H. Kinjo M. Ito K. Suzuki M. Dynamic nature of disulphide bond formation catalysts revealed by crystal structures of DsbB. EMBO J. 2009;28:779–791. doi: 10.1038/emboj.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inaba K. Murakami S. Suzuki M. Nakagawa A. Yamashita E. Okada K. Ito K. Crystal structure of the DsbB-DsbA complex reveals a mechanism of disulfide bond generation. Cell. 2006;127:789–801. doi: 10.1016/j.cell.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 45.Inaba K. Takahashi YH. Fujieda N. Kano K. Miyoshi H. Ito K. DsbB elicits a red-shift of bound ubiquinone during the catalysis of DsbA oxidation. J Biol Chem. 2004;279:6761–6768. doi: 10.1074/jbc.M310765200. [DOI] [PubMed] [Google Scholar]
- 46.Inaba K. Takahashi YH. Ito K. Hayashi S. Critical role of a thiolate-quinone charge transfer complex and its adduct form in de novo disulfide bond generation by DsbB. Proc Natl Acad Sci U S A. 2006;103:287–292. doi: 10.1073/pnas.0507570103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Inaba K. Takahashi YH. Ito K. Reactivities of quinone-free DsbB from Escherichia coli. J Biol Chem. 2005;280:33035–33044. doi: 10.1074/jbc.M506189200. [DOI] [PubMed] [Google Scholar]
- 48.Jander G. Martin NL. Beckwith J. Two cysteines in each periplasmic domain of the membrane protein DsbB are required for its function in protein disulfide bond formation. EMBO J. 1994;13:5121–5127. doi: 10.1002/j.1460-2075.1994.tb06841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joly JC. Swartz JR. In vitro and in vivo redox states of the Escherichia coli periplasmic oxidoreductases DsbA and DsbC. Biochemistry. 1997;36:10067–10072. doi: 10.1021/bi9707739. [DOI] [PubMed] [Google Scholar]
- 50.Jonda S. Huber-Wunderlich M. Glockshuber R. Mössner E. Complementation of DsbA deficiency with secreted thioredoxin variants reveals the crucial role of an efficient dithiol oxidant for catalyzed protein folding in the bacterial periplasm. EMBO J. 1999;18:3271–3281. doi: 10.1093/emboj/18.12.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Josefsson LG. Randall LL. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981;25:151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- 52.Kadokura H. Bader M. Tian H. Bardwell JC. Beckwith J. Roles of a conserved arginine residue of DsbB in linking protein disulfide-bond-formation pathway to the respiratory chain of Escherichia coli. Proc Natl Acad Sci U S A. 2000;97:10884–10889. doi: 10.1073/pnas.97.20.10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kadokura H. Beckwith J. Detecting folding intermediates of a protein as it passes through the bacterial translocation channel. Cell. 2009;138:1164–1173. doi: 10.1016/j.cell.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kadokura H. Beckwith J. Four cysteines of the membrane protein DsbB act in concert to oxidize its substrate DsbA. EMBO J. 2002;21:2354–2363. doi: 10.1093/emboj/21.10.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kadokura H. Katzen F. Beckwith J. Protein disulfide bond formation in prokaryotes. Annu Rev Biochem. 2003;72:111–135. doi: 10.1146/annurev.biochem.72.121801.161459. [DOI] [PubMed] [Google Scholar]
- 56.Kadokura H. Tian H. Zander T. Bardwell JC. Beckwith J. Snapshots of DsbA in action: detection of proteins in the process of oxidative folding. Science. 2004;303:534–537. doi: 10.1126/science.1091724. [DOI] [PubMed] [Google Scholar]
- 57.Kamitani S. Akiyama Y. Ito K. Identification and characterization of an Escherichia coli gene required for the formation of correctly folded alkaline phosphatase, a periplasmic enzyme. EMBO J. 1992;11:57–62. doi: 10.1002/j.1460-2075.1992.tb05027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Karplus PA. Schulz GE. Substrate binding and catalysis by glutathione reductase as derived from refined enzyme: substrate crystal structures at 2 Å resolution. J Mol Biol. 1989;210:163–180. doi: 10.1016/0022-2836(89)90298-2. [DOI] [PubMed] [Google Scholar]
- 59.Katzen F. Beckwith J. Transmembrane electron transfer by the membrane protein DsbD occurs via a disulfide bond cascade. Cell. 2000;103:769–779. doi: 10.1016/s0092-8674(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 60.Kishigami S. Akiyama Y. Ito K. Redox states of DsbA in the periplasm of Escherichia coli. FEBS Lett. 1995;364:55–58. doi: 10.1016/0014-5793(95)00354-c. [DOI] [PubMed] [Google Scholar]
- 61.Kishigami S. Ito K. Roles of cysteine residues of DsbB in its activity to reoxidize DsbA, the protein disulphide bond catalyst of Escherichia coli. Genes Cells. 1996;1:201–208. doi: 10.1046/j.1365-2443.1996.d01-233.x. [DOI] [PubMed] [Google Scholar]
- 62.Kobayashi T. Ito K. Respiratory chain strongly oxidizes the CXXC motif of DsbB in the Escherichia coli disulfide bond formation pathway. EMBO J. 1999;18:1192–1198. doi: 10.1093/emboj/18.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kobayashi T. Kishigami S. Sone M. Inokuchi H. Mogi T. Ito K. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci U S A. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kouwen TR. van der Goot A. Dorenbos R. Winter T. Antelmann H. Plaisier MC. Quax WJ. van Dijl JM. Dubois JY. Thiol-disulphide oxidoreductase modules in the low-GC Gram-positive bacteria. Mol Microbiol. 2007;64:984–999. doi: 10.1111/j.1365-2958.2007.05707.x. [DOI] [PubMed] [Google Scholar]
- 65.Kramer G. Boehringer D. Ban N. Bukau B. The ribosome as a platform for co-translational processing, folding and targeting of newly synthesized proteins. Nat Struct Mol Biol. 2009;16:589–597. doi: 10.1038/nsmb.1614. [DOI] [PubMed] [Google Scholar]
- 66.Lafaye C. Iwema T. Carpentier P. Jullian-Binard C. Kroll JS. Collet JF. Serre L. Biochemical and structural study of the homologues of the thiol-disulfide oxidoreductase DsbA in Neisseria meningitidis. J Mol Biol. 2009;392:952–966. doi: 10.1016/j.jmb.2009.07.056. [DOI] [PubMed] [Google Scholar]
- 67.Leichert LI. Jakob U. Protein thiol modifications visualized in vivo. PLoS Biol. 2004;2:e333. doi: 10.1371/journal.pbio.0020333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li T. Chang CY. Jin DY. Lin PJ. Khvorova A. Stafford DW. Identification of the gene for vitamin K epoxide reductase. Nature. 2004;427:541–544. doi: 10.1038/nature02254. [DOI] [PubMed] [Google Scholar]
- 69.Liu GP. Topping TB. Cover WH. Randall LL. Retardation of folding as a possible means of suppression of a mutation in the leader sequence of an exported protein. J Biol Chem. 1988;263:14790–14793. [PubMed] [Google Scholar]
- 70.Liu X. Wang CC. Disulfide-dependent folding and export of Escherichia coli DsbC. J Biol Chem. 2001;276:1146–1151. doi: 10.1074/jbc.M004929200. [DOI] [PubMed] [Google Scholar]
- 71.Magnet S. Bellais S. Dubost L. Fourgeaud M. Mainardi JL. Petit-Frère S. Marie A. Mengin-Lecreulx D. Arthur M. Gutmann L. Identification of the L,D-transpeptidases responsible for attachment of the Braun lipoprotein to Escherichia coli peptidoglycan. J Bacteriol. 2007;189:3927–3931. doi: 10.1128/JB.00084-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mallick P. Boutz DR. Eisenberg D. Yeates TO. Genomic evidence that the intracellular proteins of archaeal microbes contain disulfide bonds. Proc Natl Acad Sci U S A. 2002;99:9679–9684. doi: 10.1073/pnas.142310499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Malojčić G. Owen RL. Grimshaw JP. Glockshuber R. Preparation and structure of the charge-transfer intermediate of the transmembrane redox catalyst DsbB. FEBS Lett. 2008;582:3301–3307. doi: 10.1016/j.febslet.2008.07.063. [DOI] [PubMed] [Google Scholar]
- 74.Martin JL. Bardwell JC. Kuriyan J. Crystal structure of the DsbA protein required for disulphide bond formation in vivo. Nature. 1993;365:464–468. doi: 10.1038/365464a0. [DOI] [PubMed] [Google Scholar]
- 75.Martin JL. Thioredoxin: a fold for all reasons. Structure. 1995;3:245–250. doi: 10.1016/s0969-2126(01)00154-x. [DOI] [PubMed] [Google Scholar]
- 76.Masip L. Pan JL. Haldar S. Penner-Hahn JE. DeLisa MP. Georgiou G. Bardwell JC. Collet JF. An engineered pathway for the formation of protein disulfide bonds. Science. 2004;303:1185–1189. doi: 10.1126/science.1092612. [DOI] [PubMed] [Google Scholar]
- 77.McCarthy AA. Haebel PW. Törronen A. Rybin V. Baker EN. Metcalf P. Crystal structure of the protein disulfide bond isomerase, DsbC, from Escherichia coli. Nat Struct Biol. 2000;7:196–199. doi: 10.1038/73295. [DOI] [PubMed] [Google Scholar]
- 78.Messens J. Collet JF. Van Belle K. Brosens E. Loris R. Wyns L. The oxidase DsbA folds a protein with a nonconsecutive disulfide. J Biol Chem. 2007;282:31302–31307. doi: 10.1074/jbc.M705236200. [DOI] [PubMed] [Google Scholar]
- 79.Missiakas D. Schwager F. Raina S. Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 1995;14:3415–3424. doi: 10.1002/j.1460-2075.1995.tb07347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Narzi D. Siu SW. Stirnimann CU. Grimshaw JP. Glockshuber R. Capitani G. Böckmann RA. Evidence for proton shuffling in a thioredoxin-like protein during catalysis. J Mol Biol. 2008;382:978–986. doi: 10.1016/j.jmb.2008.07.061. [DOI] [PubMed] [Google Scholar]
- 81.Nelson JW. Creighton TE. Reactivity and ionization of the active site cysteine residues of DsbA, a protein required for disulfide bond formation in vivo. Biochemistry. 1994;33:5974–5983. doi: 10.1021/bi00185a039. [DOI] [PubMed] [Google Scholar]
- 82.Osborne AR. Rapoport TA. Protein translocation is mediated by oligomers of the SecY complex with one SecY copy forming the channel. Cell. 2007;129:97–110. doi: 10.1016/j.cell.2007.02.036. [DOI] [PubMed] [Google Scholar]
- 83.Paxman JJ. Borg NA. Horne J. Thompson PE. Chin Y. Sharma P. Simpson JS. Wielens J. Piek S. Kahler CM. Sakellaris H. Pearce M. Bottomley SP. Rossjohn J. Scanlon MJ. The structure of the bacterial oxidoreductase enzyme DsbA in complex with a peptide reveals a basis for substrate specificity in the catalytic cycle of DsbA enzymes. J Biol Chem. 2009;284:17835–17845. doi: 10.1074/jbc.M109.011502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perez-Jimenez R. Li J. Kosuri P. Sanchez-Romero I. Wiita AP. Rodriguez-Larrea D. Chueca A. Holmgren A. Miranda-Vizuete A. Becker K. Cho SH. Beckwith J. Gelhaye E. Jacquot JP. Gaucher EA. Sanchez-Ruiz JM. Berne BJ. Fernandez JM. Diversity of chemical mechanisms in thioredoxin catalysis revealed by single-molecule force spectroscopy. Nat Struct Mol Biol. 2009;16:1331. doi: 10.1038/nsmb.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pettersen EF. Goddard TD. Huang CC. Couch GS. Greenblatt DM. Meng EC. Ferrin TE. UCSF Chimera: a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 86.Pugsley AP. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quan S. Schneider I. Pan J. Von Hacht A. Bardwell JC. The CXXC motif is more than a redox rheostat. J Biol Chem. 2007;282:28823–28833. doi: 10.1074/jbc.M705291200. [DOI] [PubMed] [Google Scholar]
- 88.Regeimbal J. Bardwell JC. DsbB catalyzes disulfide bond formation de novo. J Biol Chem. 2002;277:32706–32713. doi: 10.1074/jbc.M205433200. [DOI] [PubMed] [Google Scholar]
- 89.Ren G. Stephan D. Xu Z. Zheng Y. Tang D. Harrison RS. Kurz M. Jarrott R. Shouldice SR. Hiniker A. Martin JL. Heras B. Bardwell JC. Properties of the thioredoxin fold superfamily are modulated by a single amino acid residue. J Biol Chem. 2009;284:10150–10159. doi: 10.1074/jbc.M809509200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rietsch A. Belin D. Martin N. Beckwith J. An in vivo pathway for disulfide bond isomerization in Escherichia coli. Proc Natl Acad Sci U S A. 1996;93:13048–13053. doi: 10.1073/pnas.93.23.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rietsch A. Bessette P. Georgiou G. Beckwith J. Reduction of the periplasmic disulfide bond isomerase, DsbC, occurs by passage of electrons from cytoplasmic thioredoxin. J Bacteriol. 1997;179:6602–6608. doi: 10.1128/jb.179.21.6602-6608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rost S. Fregin A. Ivaskevicius V. Conzelmann E. Hörtnagel K. Pelz HJ. Lappegard K. Seifried E. Scharrer I. Tuddenham EG. Muller CR. Strom TM. Oldenburg J. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 93.Rozhkova A. Glockshuber R. Thermodynamic aspects of DsbD-mediated electron transport. J Mol Biol. 2008;380:783–788. doi: 10.1016/j.jmb.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 94.Rozhkova A. Stirnimann CU. Frei P. Grauschopf U. Brunisholz R. Grütter MG. Capitani G. Glockshuber R. Structural basis and kinetics of inter- and intramolecular disulfide exchange in the redox catalyst DsbD. EMBO J. 2004;23:1709–1719. doi: 10.1038/sj.emboj.7600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Segatori L. Murphy L. Arredondo S. Kadokura H. Gilbert H. Beckwith J. Georgiou G. Conserved role of the linker α-helix of the bacterial disulfide isomerase DsbC in the avoidance of misoxidation by DsbB. J Biol Chem. 2006;281:4911–4919. doi: 10.1074/jbc.M505453200. [DOI] [PubMed] [Google Scholar]
- 96.Segatori L. Paukstelis PJ. Gilbert HF. Georgiou G. Engineered DsbC chimeras catalyze both protein oxidation and disulfide-bond isomerization in Escherichia coli: reconciling two competing pathways. Proc Natl Acad Sci USA. 2004;101:10018–10023. doi: 10.1073/pnas.0403003101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sevier CS. Kaiser CA. Conservation and diversity of the cellular disulfide bond formation pathways. Antioxid Redox Signal. 2006;8:797–811. doi: 10.1089/ars.2006.8.797. [DOI] [PubMed] [Google Scholar]
- 98.Shao F. Bader MW. Jakob U. Bardwell JC. DsbG, a protein disulfide isomerase with chaperone activity. J Biol Chem. 2000;275:13349–13352. doi: 10.1074/jbc.275.18.13349. [DOI] [PubMed] [Google Scholar]
- 99.Shevchik VE. Condemine G. Robert-Baudouy J. Characterization of DsbC, a periplasmic protein of Erwinia chrysanthemi and Escherichia coli with disulfide isomerase activity. EMBO J. 1994;13:2007–2012. doi: 10.1002/j.1460-2075.1994.tb06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shouldice SR. Cho SH. Boyd D. Heras B. Eser M. Beckwith J. Riggs P. Martin JL. Berkmen M. In vivo oxidative protein folding is facilitated by oxidation-reduction cycling. Mol Microbiol. 2010;75:13–28. doi: 10.1111/j.1365-2958.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- 101.Singh AK. Bhattacharyya-Pakrasi M. Pakrasi HB. Identification of an atypical membrane protein involved in the formation of protein disulfide bonds in oxygenic photosynthetic organisms. J Biol Chem. 2008;283:15762–15770. doi: 10.1074/jbc.M800982200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tapley TL. Eichner T. Gleiter S. Ballou DP. Bardwell JC. Kinetic characterization of the disulfide bond-forming enzyme DsbB. J Biol Chem. 2007;282:10263–10271. doi: 10.1074/jbc.M611541200. [DOI] [PubMed] [Google Scholar]
- 103.Vertommen D. Depuydt M. Pan J. Leverrier P. Knoops L. Szikora JP. Messens J. Bardwell JC. Collet JF. The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner. Mol Microbiol. 2008;67:336–349. doi: 10.1111/j.1365-2958.2007.06030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vinci F. Couprie J. Pucci P. Quéméneur E. Moutiez M. Description of the topographical changes associated to the different stages of the DsbA catalytic cycle. Protein Sci. 2002;11:1600–1612. doi: 10.1110/ps.4960102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vivian JP. Scoullar J. Rimmer K. Bushell SR. Beddoe T. Wilce MC. Byres E. Boyle TP. Doak B. Simpson JS. Graham B. Heras B. Kahler CM. Rossjohn J. Scanlon MJ. Structure and function of the oxidoreductase DsbA1 from Neisseria meningitidis. J Mol Biol. 2009;394:931–943. doi: 10.1016/j.jmb.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 106.Vlamis-Gardikas A. The multiple functions of the thiol-based electron flow pathways of Escherichia coli: eternal concepts revisited. Biochim Biophys Acta. 2008;1780:1170–1200. doi: 10.1016/j.bbagen.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 107.Wiita AP. Perez-Jimenez R. Walther KA. Gräter F. Berne BJ. Holmgren A. Sanchez-Ruiz JM. Fernandez JM. Probing the chemistry of thioredoxin catalysis with force. Nature. 2007;450:124–127. doi: 10.1038/nature06231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wunderlich M. Otto A. Seckler R. Glockshuber R. Bacterial protein disulfide isomerase: efficient catalysis of oxidative protein folding at acidic pH. Biochemistry. 1993;32:12251–12256. doi: 10.1021/bi00096a039. [DOI] [PubMed] [Google Scholar]
- 109.Zapun A. Bardwell JC. Creighton TE. The reactive and destabilizing disulfide bond of DsbA, a protein required for protein disulfide bond formation in vivo. Biochemistry. 1993;32:5083–5092. doi: 10.1021/bi00070a016. [DOI] [PubMed] [Google Scholar]
- 110.Zapun A. Missiakas D. Raina S. Creighton TE. Structural and functional characterization of DsbC, a protein involved in disulfide bond formation in Escherichia coli. Biochemistry. 1995;34:5075–5089. doi: 10.1021/bi00015a019. [DOI] [PubMed] [Google Scholar]
- 111.Zhao Z. Peng Y. Hao SF. Zeng ZH. Wang CC. Dimerization by domain hybridization bestows chaperone and isomerase activities. J Biol Chem. 2003;278:43292–43298. doi: 10.1074/jbc.M306945200. [DOI] [PubMed] [Google Scholar]
- 112.Zhou Y. Cierpicki T. Jimenez RH. Ellena JF. Cafiso DS. Kadokura H. Beckwith J. Bushweller JH. NMR solution structure of the integral membrane enzyme DsbB: functional insights into DsbB-catalyzed disulfide bond formation. Mol Cell. 2008;31:896–908. doi: 10.1016/j.molcel.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]