Abstract
Relationships between aging, disease risks, and longevity are not yet well understood. For example, joint increases in cancer risk and total survival observed in many human populations and some experimental aging studies may be linked to a trade-off between cancer and aging as well as to the trade-off(s) between cancer and other diseases, and their relative impact is not clear. While the former trade-off (between cancer and aging) received broad attention in aging research, the latter one lacks respective studies, although its understanding is important for developing optimal strategies of increasing both longevity and healthy life span. In this paper, we explore the possibility of trade-offs between risks of cancer and selected major disorders. First, we review current literature suggesting that the trade-offs between cancer and other diseases may exist and be linked to the differential intensity of apoptosis. Then we select relevant disorders for the analysis (acute coronary heart disease [ACHD], stroke, asthma, and Alzheimer disease [AD]) and calculate the risk of cancer among individuals with each of these disorders, and vice versa, using the Framingham Study (5209 individuals) and the National Long Term Care Survey (NLTCS) (38,214 individuals) data. We found a reduction in cancer risk among old (80+) men with stroke and in risk of ACHD among men (50+) with cancer in the Framingham Study. We also found an increase in ACHD and stroke among individuals with cancer, and a reduction in cancer risk among women with AD in the NLTCS. The manifestation of trade-offs between risks of cancer and other diseases thus depended on sex, age, and study population. We discuss factors modulating the potential trade-offs between major disorders in populations, e.g., disease treatments. Further study is needed to clarify possible impact of such trade-offs on longevity.
Introduction
The relationships between aging, disease, and longevity are not yet well understood. For instance, joint increases in cancer risk and total survival observed in many human populations and some experimental aging studies could happen due to reasons related as well as not related to individual aging. They might result from a trade-off between cancer and aging, as well as from the trade-off between cancer and other diseases. The former trade-off (between cancer and aging) received significant attention in aging research and literature. It was hypothesized that cellular mechanisms of tumor suppression, such as senescence and apoptosis, may have a downside manifested in somatic aging. There is experimental evidence in support of this hypothesis1–3 (see also “Overview of Literature,” below). At the same time, the potential trade-off between cancer and other diseases lacks intensive research. This trade-off means that certain factors favoring cancer can be protective against other pathology and even improve overall survival, if the protective effect overweighs the detrimental one. In this case, the observed joint increases in cancer risk and longevity may not necessary be due to “a postponed aging at cost of an increased cancer risk” because they can also be due to “a lower risk or better survival from diseases other than cancer.” The relative contribution of these trade-offs to human longevity is not clear, while its understanding is important for developing optimal ways of increasing both maximal and healthy life span.
In this paper we explore the relationships between risks of cancer and selected major disorders, using the Framingham Heart Study (FHS) and the National Long Term Care Survey (NLTCS) data. First we review current literature allowing us to: (1) Support the outlined problem with evidence; (2) hypothesize a possibility of trade-offs between cancer and other disorders, in which the differential intensity of apoptosis plays significant role; and (3) select the appropriate diseases for the analysis.
Then we present own results of analyses of the relationships between risks of cancer and selected disorders (acute coronary heart disease [ACHD], stroke, asthma, and Alzheimer disease [AD]) and show that manifestation of trade-offs between the disease risks depends on age, sex and study population. Finally, we discuss the results in context of relevant literature, considering the role of factors modulating the potential trade-offs, such as disease treatments, among others.
Overview of Literature
Connection between cancer and aging: Is there a trade-off?
The hypothesis about a trade-off between cancer and aging implies that cellular mechanisms of tumor suppression, such as p53-dependent senescence and apoptosis, may not only reduce cancer risk but also cause or accelerate aging.1–3 For example, continually upregulated apoptosis, being cancer protective, may in the long term lead to a decline in tissue renewal capacity and its depletion, thus contributing to aging.
There is evidence in support of this hypothesis.1–15 Tyner et al.3 and Donehower4 showed that mice carrying the p53 mutation with a phenotypic effect analogous to the upregulation of this gene have a lower risk of cancer development, but their life span is reduced and accompanied by early tissue atrophy. Long-living mutant mice, p66Shc−/−, have shown an impaired p53 apoptotic response.5 Introducing the null p53 allele has protected Ku80−/− and mTR−/− mice from premature aging,6,7 indicating that the senescence phenotypes were p53 dependent.8 Bauer et al.9,10 found that reduction of p53 activity in flies leads to life span extension. It was suggested that mechanism by which p53 and possibly other tumor suppressors regulate life span depends on a balance between tumor suppression and tissue renewal, and that the trade-off between cancer and aging may reflect the opposite manifestation of apoptotic and growth signaling pathways in cancer and aging cells.1,2,11–14 There is also evidence of a positive correlation between cancer and survival in humans. Ørsted et al.15 found that the overall 12-year survival was increased in p53 Pro/Pro versus Arg/Arg homozygotes despite potential cancer-favoring properties of the former genotype. In humans, the replacement of arginine (Arg) by proline (Pro) decreases the p53 ability to initiate apoptosis.16
Despite this supporting evidence, there are many experimental studies in which an increase in survival is accompanied by a reduction in cancer risk, often together with signs of phenotypically postponed aging and normal or enhanced apoptosis17–25 (also reviewed in refs. 26 and 27), so that the traditional trade-off (when cancer suppression would result in acceleration of aging and/or reduced longevity) does not take place.21–24,25 Probably the most prominent example is the proven ‘‘antiaging’’ treatment, caloric restriction.21–23,25
This and other evidence suggests a possibility that apoptosis may have pro- or antiaging effects on body, depending on other factors, such as other aging-related pathways.28–30 It seems logical that interaction among the aging-related pathways, particularly among those that control the balance of cellular input–output in tissues (i.e., the balance of cell proliferation, damage, repair and apoptosis rates), would determine the final pro- or antiaging phenotype resulting from, e.g., chronically upregulated apoptosis.
Trade-offs between major disorders: Do they exist and impact longevity?
There is also a possibility that the relationships between apoptosis, cancer risk, and longevity, at least in some cases, do not involve an aging process at all. Indeed, even when reduced apoptosis is truly accompanied with increases in both cancer risk and longevity in a study, this situation does not necessary reflect a trade-off between cancer and aging per se, because it may also reflect another kind of trade-off—between vulnerabilities to cancer and other disease. For example, the high apoptotic activity that opposes tumor development may at the same time increase the risk of stroke or increase chances to die from it, because brain cells in ischemic state are more susceptible to apoptosis.31 If this is the case, then the reduced apoptosis could be associated with increased longevity because it lowers risk of/mortality from stroke rather than because it slows down the aging. Van Heemst et al.32 demonstrated that individuals with Pro/Pro genotype of p53 corresponding to reduced apoptosis in cells have a significantly increased both overall survival (by 41%) and mortality from cancer (2.54-fold) along with a decreased risk of death from cardiovascular disease (CVD) at the ages 85+. One may suppose that a trade-off between cancer and CVD contributed to the increase in overall survival in this case, not necessarily the trade-off between cancer and aging. Earlier we speculated that individuals with inherently reduced apoptosis could be more vulnerable to cancer but less vulnerable to stroke or myocardial infarction.33
Overall, available evidence suggests that: (1) The relationships between tumor suppression mechanisms and aging, and specifically between apoptosis and aging, appear complex and probably cannot be described in terms of simple trade-offs such as “better cancer protection–accelerated aging.” The pro- or antiaging effects of the upregulated apoptosis are likely conditional on other aging-related pathways. (2) The differential activity of apoptosis could be a factor that may contribute not only to the connection between cancer and aging but also to the connections (including trade-offs) between cancer and other disorders. (3) It is not clear yet which kind of the connections contribute more significantly to joint increases in longevity and cancer risk observed in experimental and human aging studies—the one between cancer and aging or the one between cancer and other diseases.
Thus, we hypothesize that there may exist trade-offs between cancer and other disorders, in which differential activity of apoptosis plays a role. To test this possibility, we next examine the relationships between risks of cancer and respective disorders in the FHS and the NLTCS data.
Data and Methods
Rationale for selecting particular diseases for the study
After reviewing relevant literature, we selected four major disorders (ACHD, stroke, asthma, and AD) in which the differential intensity of apoptosis plays a role and their risks/mortalities could be affected by it. Here we provide brief rationale for the choice of the particular diseases and suggest their prospective relationships with cancer.
Cancer is a group of diseases in which potentially immortal cells display uncontrolled growth and propensity to invasion and metastasis. The potential immortality of cancer cells results from their ability to avoid apoptosis.34 It is recognized that cancer is generally associated with downregulated apoptosis, and the lower susceptibility of cells to apoptosis may contribute to both increased cancer risk and tumor resistance to anticancer therapy, that is, to survival from cancer.34,35
Stroke is a rapidly developing loss of brain function due to ischemia (lack of blood supply) or due to a hemorrhage in brain. Cell death, via both necrosis and apoptosis, plays major role in developing this condition. Apoptosis has a dramatic impact on the loss of neurons in the region outside the stroke's core, where the oxygen supply is reduced but not eliminated.36 It was suggested that the suppressed apoptosis in brain may result in a lower incidence and/or better outcome for stroke because more neurons would survive damage following the brain ischemia.31 It becomes evident that inhibition of apoptosis in ischemic brain provides an important preventive and treatment strategy.36 Recent experimental studies of various compounds for treatment of acute stroke suggest that the medicated suppression of apoptosis may significantly improve survival and recovery after stroke.37–39 Preliminary results of a trial of the acute stroke treatment with DP-b99, a D-Pharm company compound (which protects primary cortical neurons from cell death induced by ischemia), demonstrated that such treatment may double the chances of the patients to recover from ischemic stroke.40,41 Overall, the available data imply that individuals with inherently reduced apoptotic activity might be less vulnerable to stroke and disability/death following it. These same individuals might be more susceptible to cancer or death from it. Thus, we hypothesize that the differential activity of apoptosis may be a ground for a trade-off between cancer and stroke and we are going to test the relationships between risks of these disorders in this study.
The above consideration about stroke can also be applied to ACHD, which includes myocardial infarction and angina pectoris, both associated with damage following the heart ischemia. Recent studies indicate that, in addition to necrosis, apoptosis plays a major role in the process of tissue damage after myocardial infarction, especially in the periinfarct viable myocardium.42–44 Van Heemst et al.32 found that individuals who have a genetically reduced apoptotic response have a higher mortality from cancer but lower from CVD. As in the case of cancer and stroke, these data suggest a possibility of trade-off between risks of cancer and ACHD that will be tested in this study.
AD is a complex neurodegenerative disorder linked to the accumulation of extracellular senile plaques and intracellular neurofibrillary tangles (NFT). Excessive apoptosis has shown association with AD in a number of studies. Kobayashi et al.45 found that senile plaques may be a cause of astrocytic apoptosis in the gray matter, and that the Bcl-2 pro-apoptotic protein is associated with NFT formation. Increasing evidence suggests that selective neuronal loss in AD involves activation of caspases, which initiate and execute apoptosis correlating with the disease.46 It was hypothesized that the excessive apoptosis in AD and the reduced apoptosis in cancer may reflect the opposite disturbances of the epigenetic homeostasis in these disorders,47,48 which could result in a trade-off between risks of cancer and AD.
Asthma is a chronic inflammatory disorder of the airways. It is now accepted that increased survival and decreased apoptosis of inflammatory cells, particularly T lymphocytes and eosinophils, may play a central role in the persistent inflammatory process characterizing this disease.49–51 The decreased apoptotic death favors the accumulation and the persistence of eosinophils in the asthmatic airways52–54 and prolongs the active life of T cells and their proinflammatory effects.55 The data suggest that cancer and asthma may share the proinflammatory background,51,56 in which the reduced apoptotic activity may promote both conditions. Thus, one might expect the increasing risk of asthma in the presence of cancer or vice versa rather than a trade-off between these two diseases in our study.
Thus, the current evidence suggests that the reduced apoptosis can be associated with increased risks of/death from cancer or asthma and with decreased risks of death from ACHD, stroke, or AD. This provides a ground for possibility of trade-offs between cancer and ACHD, stroke, or AD, as well as for cancer–asthma co-morbidity, manifested in reduced or increased risks of cancer appearance among people with other disease, and vice versa. To test the relationships between risks of cancer and the above disorders in population, we will calculate the risks of cancer in presence of each of the selected disorders, and vice versa, using the FHS and the NLTCS data.
Framingham Heart Study
To evaluate the risk of cancer in the presence of ACHD or stroke and vice versa, we used a sample of the FHS Original cohort data including 5209 individuals (55% females) aged 28–62 at the baseline and residing in Framingham, Massachusetts, between 1948 and 1951.57 Nearly all subjects were Caucasians. The FHS has continued with biennial examinations to the present, i.e., for more than 50 years. Examination included an interview, physical exam, and laboratory tests accessing numerous physiological indices and disease risk factors, with special emphasis on CVDs.58 The FHS cohort has been consistently followed for all incident cases of ACHD (including myocardial infarction and angina pectoris), stroke (acute cerebrovascular accident, CVA), and cancer, through surveillance of hospital admissions and other available sources. Table 1 shows numbers of people diagnosed with the diseases of interest in the FHS.
Table 1.
Numbers of People Diagnosed and Not Diagnosed with Different Diseases of Interest in the Framingham Heart Study (the Entire Sample) and National Long Term Care Survey (among Those Alive on January 1, 1991), by Sex
| Data | Disease | Sex | Diagnosed | Not diagnosed |
|---|---|---|---|---|
| FHS | ACHD | F | 878 | 1,995 |
| M | 1,101 | 1,235 | ||
| Cancer | F | 800 | 2,073 | |
| M | 743 | 1,593 | ||
| Stroke | F | 577 | 2,296 | |
| M | 440 | 1,896 | ||
| NLTCS | ACHD | F | 7,388 | 15,368 |
| M | 5,556 | 9,902 | ||
| AD | F | 3,354 | 19,402 | |
| M | 1,435 | 14,023 | ||
| Asthma | F | 3,617 | 19,139 | |
| M | 2,107 | 13,351 | ||
| Cancer | F | 8,504 | 14,252 | |
| M | 7,331 | 8,127 | ||
| Stroke | F | 5,761 | 16,995 | |
| M | 3,406 | 12,052 |
FHS, Framingham Heart Study; ACHD, acute coronary heart disease; M, male; F, female; NLTCS, National Long Term Care Survey; AD, Alzheimer disease.
We used the information on incident cases to evaluate the probability of staying cancer free in presence or absence of ACHD or stroke as background conditions and vice versa, among 5209 FHS participants, and drew respective “survival curves.” Note that we calculated incidence rates (and probabilities) for one condition for individuals with and without the second condition, taking into account whether the second condition occurred before or after the first one. That is, if the second condition occurred before the first one, then the individual contributes to the incidence rate of condition 1 for individuals with condition 2. Conversely, if an individual is free of condition 2 at the age of onset of condition 1 (i.e., condition 2 occurred after the onset of condition 1 or did not occur at all during the observation period), then the individual contributes to the incidence rate of condition 1 for individuals without condition 2.
Because there were no solid diagnoses of asthma or AD in the FHS available for the research use, we used a sample of the NLTCS data linked to Medicare records to assess the risk of cancer in presence of asthma or AD, and vice versa.
National Long Term Care Survey
The NLTCS contains longitudinal and cross-sectional data on a nationally representative sample of 49,240 U.S. elderly persons aged 65+ (from Medicare enrollees). Six surveys were conducted between 1982 and 2004/2005. Each NLTCS participant was linked to Medicare records containing information on the insurance claims, including respective International Classification of Diseases Coordination and Maintenance Committee (ICD-9-CM) diagnoses, from the beginning of the survey until 12/31/2005. Part A refers to the Medicare insurance program that covers inpatient hospital care, posthospital nursing health services, and hospice care. Part B pays for physicians' services, outpatient hospital care, durable medical equipment, and other medical services that are not covered by Part A (for more information see www.medicare.gov). Diagnostic information was not available on Part B until 1991, therefore we used Medicare data for the years 1991–2005, for which both Medicare Part A and Part B data were present. Therefore, we evaluated age-specific incidence rates of cancer in the presence or absence of ACHD, stroke, AD, or asthma, as background conditions, and vice versa, in the sample of the NLTCS participants linked to Medicare records, using pooled data for the years 1991–2005. The number of individuals included in calculations of the incidence rates for this period was 38,214 (22,756 females and 15,458 males). The date of disease onset was defined as the first date of a Medicare claim that contained respective International Classification of Diseases, 9th edition, Clinical Modification (ICD-9-CM) codes: for cancer (malignant neoplasm, all sites), 140-208; for ACHD, 410 (MI), 411 (other acute CHD), and 413 (angina pectoris); for stroke (both ischemic and hemorrhagic), 436, 431, 432.9, 433 (when the fifth digit is 1) and 434 (when the fifth digit is 1); for AD, 331.0, 290.1; for asthma, 493. One should note that some procedures aimed at distinguishing between incident and prevalent cases are important, especially for chronic diseases. In the analyses of NLTCS–Medicare data, we used a 6-month period after an individual entered the study (i.e., appeared in the Medicare files available for the study) to separate presumably prevalent cases from the incident ones. That is, if any Medicare claims with respective ICD-9-CM codes (either Part A or Part B) appeared within the 6-month period after the first date for which information on an individual is available in the NLTCS–Medicare files, then we assume that it is likely that the condition could have existed before that date. Such a case is considered prevalent and such an individual is not included in calculations of respective incidence rates. If no such claims appeared within that 6-month period, then we assume that the date of the first Medicare claim is the date of onset of that condition. Note also that we calculated incidence rates for one condition for individuals with and without the second condition, taking into account whether the second condition occurred before or after the first one, as described in the previous section. Table 1 shows numbers of people diagnosed with a particular disease in the NLTCS.
Results
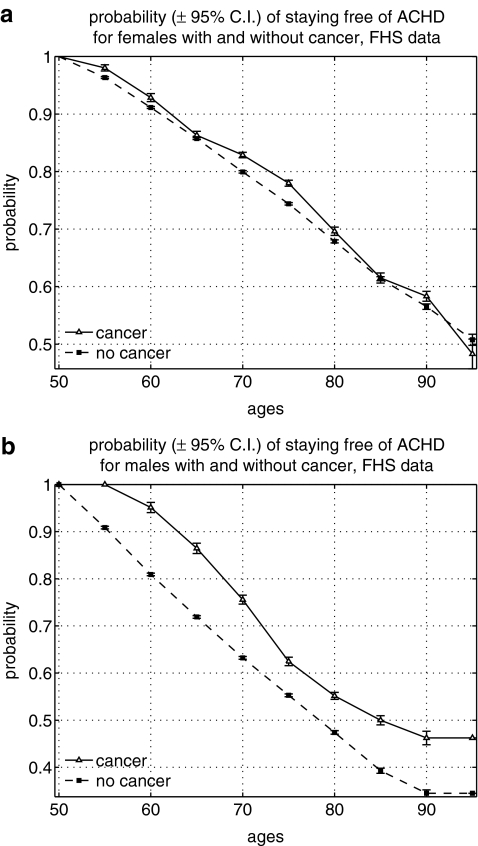
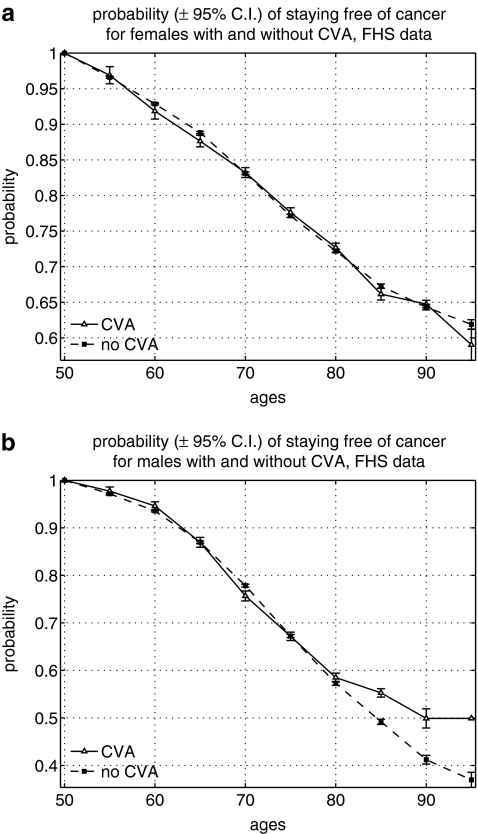
Figures 1–6 show the results of our calculations. We found the higher probability of staying cancer free among old (80+) men with stroke diagnosis and the higher probability of staying free of ACHD among men aged 50+ with cancer diagnosis, but not otherwise, in the FHS data (Figs. 1 and 2). That is, one disease appeared among individuals with other diseases less frequently than expected in these groups. The risk of stroke among individuals with cancer was not significantly different from that risk in individuals without cancer. The same was true for the risk of cancer among people with ACHD.
FIG. 1.
Probability of staying free of acute coronary heart disease (ACHD) among individuals with and without cancer in the Framingham Heart Study (FHS): (a) Females; (b) males. C.I., Confidence interval.
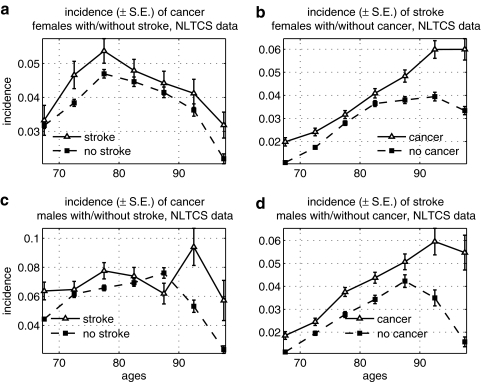
FIG. 6.
Age-specific incidence rates of cancer among males and females with stroke (cerebrovascular accident [CVA]), and vice versa, in the National Long Term Care Survey (NLTCS): (a) Incidence of cancer among females with and without stroke; (b) incidence of stroke among females with and without cancer; (c) incidence of cancer among males with and without stroke; (d) incidence of stroke among males with and without cancer. S.E., Standard error.
FIG. 2.
Probability of staying free of cancer among individuals with and without stroke (acute cerebrovascular accident [CVA]) in the Framingham Heart Study (FHS): (a) Females; (b) males. C.I., Confidence interval.
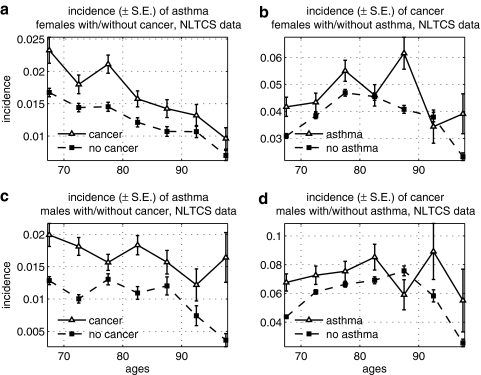
Cancer and asthma were positively correlated in the NLTCS data (Fig. 3) for both sexes. The effect was especially pronounced for the risk of asthma among individuals with cancer.
FIG. 3.
Age-specific incidence rates of cancer among males and females with asthma, and vice versa, in the National Long Term Care Survey (NLTCS): (a) Incidence of asthma among females with and without cancer; (b) incidence of cancer among females with and without asthma; (c) incidence of asthma among males with and without cancer; (d) incidence of cancer among males with and without asthma. S.E., Standard error.
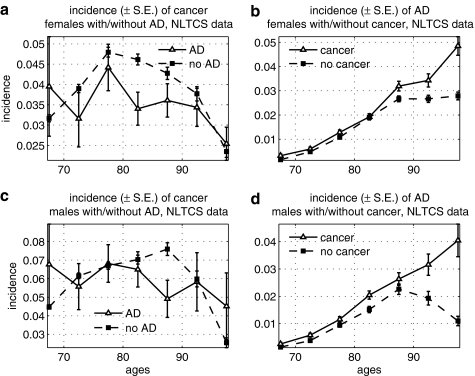
We found a significantly lower risk of cancer among elderly females with AD in the NLTCS (Fig. 4). The risk of AD was, however, higher among oldest old (85+) males and females with cancer, and patterns of the increase in the risk were similar in both sexes. We suggest an explanation for such behavior of the incidence rates in the Discussion section.
FIG. 4.
Age-specific incidence rates of cancer among males and females with Alzheimer disease (AD), and vice versa, in the National Long Term Care Survey (NLTCS): (a) Incidence of cancer among females with and without AD; (b) incidence of AD among females with and without cancer; (c) incidence of cancer among males with and without AD; (d) incidence of AD among males with and without cancer. S.E., Standard error.
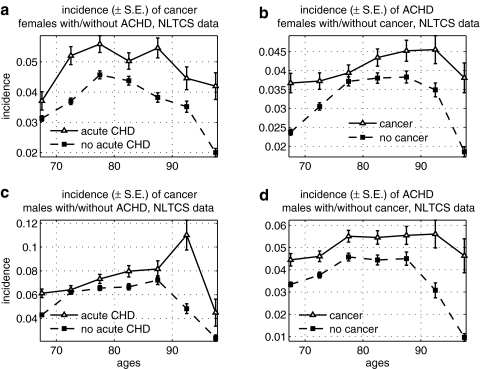
We also found an increase in risk of ACHD and stroke among individuals with cancer in the NLTCS (Figs. 5 and 6). For ACHD, the effect is more pronounced and is the opposite to what we observed for the risk of ACHD among cancer in the FHS data (see Fig. 1).
FIG. 5.
Age-specific incidence rates of cancer among males and females with acute coronary heart disease (ACHD), and vice versa, in the National Long Term Care Survey (NLTCS): (a) Incidence of cancer among females with and without ACHD; (b) incidence of ACHD among females with and without cancer; (c) incidence of cancer among males with and without ACHD; (d) incidence of ACHD among males with and without cancer. S.E., Standard error.
Discussion
As our results show, the trade-offs between risks of cancer and other diseases have been observed in our study only for some disease combinations and depended on age, sex, and study population. Although a positive correlation between cancer and asthma was expected on the ground of common risk factors for both pathologies (chronic inflammation and reduced apoptosis), the fact that we did not observe the trade-offs between disease risks for all combinations of cancer and ACHD, stroke, or AD, and, in a similar way, in both study populations, requires explanation. Two findings appear especially interesting and call for a deeper discussion: (1) While cancer risk was lower among individuals with AD, the risk of AD was higher among the oldest old with cancer (Fig. 4); and (2) while the risk of ACHD was lower among men with cancer in the FHS, the risk of ACHD was higher among individuals with cancer in the NLTCS (see Figs. 1 and 5).
To understand this, one should remember that the analyzed relationships are between disease incidence rates, not between the underlying risk factors. That is, the varying behavior of the incidence rates does not reject the fact that apoptosis intensity may oppositely influence chances of developing cancer and ACHD, stroke, or AD in body. The incidence rates reflect resulting impact of many risk factors, some of which can be opposite for the selected diseases and others can be common. Examples of the factors that can potentially interfere with the trade-offs between vulnerabilities to diseases include (but not limited to) the following. For cancer–AD: (1) Cancer treatment may increase the risk of consequent AD and other dementia.59,60 The effect can be related to a proapoptotic action of such treatment (chemotherapy or radiotherapy), as well as to other factors, such as surgery or anesthesia, that have been discussed as possible reasons for increased dementia risks, particularly among the elderly.61 (2) Common factors (e.g., chronic inflammation) in developing cancer and AD62,63 may favor both conditions.
For cancer–coronary heart disease (CHD) or stroke: (1) CHD treatment may potentially increase cancer risk. For example, low cholesterol levels achieved with some statin therapy in CVD patients are shown to be associated with increased risk of/mortality from cancer64,65 (meta-analysis of 23 statins studies). A pro-apoptotic cancer treatment (e.g., chemotherapy or radiation35) might favor the risk of/deaths from CHD or stroke. Although a reduced apoptosis is thought to favor cancer and protect against ACHD or stroke in the same individuals, the effect of proapoptotic cancer treatment may overcome this underlying trade-off and increase the risk of ACHD/stroke in cancer survivors, who have been exposed to such treatment. (2) Potential selection bias: Survivors of myocardial infarction may have lower apoptotic responses and so be more vulnerable to cancer compared to their peers who died from myocardial infarction and were eliminated from population.33 Similarly, cancer survivors may have more intense apoptosis compared with those who died from the disease and thus they may be more prone to CHD/stroke than the original group of individuals with cancer.66 (3) Impact of common risk factors for cancer and CHD/stroke (e.g., inflammation56,67) may overcome the impact of antagonistic factors.
Existence of these and other interfering factors may help to explain why we, for instance, observed a lower risk of cancer among individuals with AD, but the higher risk of AD among old cancer survivors. The latter could be because cancer therapy and surgery (with anesthesia) themselves may provoke dementia, especially among the oldest old.59–61 Importantly, similar to our study, several other researches reported inverse relationships between cancer and AD, such as the lower prevalence or history of cancer among individuals with AD,68,69 or the AD accompanying cancer deaths significantly less frequently than expected in case of independent diseases.70 The latter finding has been confirmed in our recent paper, where we also showed similar relationships for cancer and CHD/stroke as causes of death.27 Roe and colleagues47 reported significantly (p < 0.001) lower risk of developing cancer (all sites) among AD patients compared to nondemented participants in a prospective study, whereas the opposite association was nonsignificant. This is similar to what we observed in our study and could refer to the same explanation (cancer treatment provoking dementia).
Our results also show significant co-appearance of cancer and ACHD in the NLTCS, whereas a trade-off between these same diseases in the FHS. That is, the relationships between risks of cancer and ACHD were different in two data sets. To understand this, one should note that in the NLTCS, people aged 65+ belong to the period 1991–2005, whereas in the FHS the elderly started to accumulate in the data since early 1950s and became the majority of surviving participants by the end of 1970s. This means that the increased risk of ACHD among cancer in the NLTCS relates to more recent time period than the decreased risk of ACHD among cancer in the FHS. The manifestation of trade-off between cancer and ACHD may depend on factors specific for a time period, such as changes in cancer treatment. Treatment strategies substantially evolved over time so that the effect of cancer treatment on the potential trade-offs may substantially differ in recent years from that in the past,33 e.g., today it may affect risks of CHD or stroke more significantly than in 1950s. Considering this possibility, we plan to test the period effects on the relationships between disease risks in next larger study.
We are also going to study dependencies among a broader spectrum of elderly disorders, not only in the pairs “cancer–other disease.” It may be reasonable to focus on groups of diseases with similar types of interacting with aging-related metabolic/genetic pathway(s) instead of focusing on separate disorders. For instance, cancer, type 2 diabetes, and asthma are all disorders in which apoptosis is typically downregulated, whereas ACHD, stroke, and AD are all associated with enhanced apoptosis, and so they could be analyzed in groups.
In conclusion, we would like to emphasize the importance of continuing studying the trade-offs among disorders that are also major causes of death. Existence of such trade-offs (even limited) would mean that modern increases in human longevity may at least in part be causally related to the increased risks of/mortalities from some diseases that are overweighed by the reduced risks of/mortalities from some other disorders. This knowledge would not only help better understanding the relationships among aging, health, and longevity, but is also essential for practical development of optimal ways of increasing healthy longevity. Considering the trade-offs, such ways may involve not just increasing “free-of-all-diseases" life span but probably more delicate approaches targeting a balance between more and less fatal disorders, with a possibility of increasing the risks of some of them in favor of decreasing the risks of others, which have a higher impact to total mortality and/or disability.
Acknowledgments
The work was supported by National Institutes of Health/National Institute of Aging (NIH/NIA) research grants R01AG032319, R01 AG030612, and R01AG028259. The content is solely the responsibility of the authors and does not necessarily represent official views of the National Institute on Aging/the National Institutes of Health. The Framingham Heart Study (FHS) is conducted and supported by the National Heart, Lung and Blood Institute (NHLBI) in collaboration with the FHS Investigators. This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the FHS or the NHLBI. Authors are also grateful to Prof. Herbert K. Lyerly for valuable comments on the manuscript.
Author Disclosure Statement
Each author participating in this manuscript has no commercial associations that might create a conflict of interest in connection with submitted manuscript.
References
- 1.Campisi J. Cancer and Aging: Yin, Yang, and p53. Science of Aging Knowledge Environment. 2002;1:pe1. doi: 10.1126/sageke.2002.1.pe1. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J. Cancer and ageing: Rival demons? Nat Rev Cancer. 2003;3:339–349. doi: 10.1038/nrc1073. [DOI] [PubMed] [Google Scholar]
- 3.Tyner SD. Venkatachalam S. Choi J. Jones S. Ghebranious N. Igelmann H. Lu XB. Soron G. Cooper B. Brayton C. Park SH. Thompson T. Karsenty G. Bradley A. Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 4.Donehower LA. Does p53 affect organismal aging? J Cell Physiol. 2002;192:23–33. doi: 10.1002/jcp.10104. [DOI] [PubMed] [Google Scholar]
- 5.Migliaccio E. Giorgio M. Mele S. Pelicci G. Reboidl P. Pandolfi PP. Lanfrancone L. Pelicci PG. The p66(shc) adaptor protein controls oxidative stress response and life span in mammals. Nature. 1999;402:309–313. doi: 10.1038/46311. [DOI] [PubMed] [Google Scholar]
- 6.Vogel H. Lim DS. Karsenty G. Finegold M. Hasty P. Deletion of Ku86 causes early onset of senescence in mice. Proc Natl Acad Sci USA. 1999;96:10770–10775. doi: 10.1073/pnas.96.19.10770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chin L. Artandi SE. Shen Q. Tam A. Lee SL. Gottlieb GJ. Greider CW. DePinho RA. p53 deficiency rescues the adverse effects of telomere loss and cooperates with telomere dysfunction to accelerate carcinogenesis. Cell. 1999;97:527–538. doi: 10.1016/s0092-8674(00)80762-x. [DOI] [PubMed] [Google Scholar]
- 8.Lim DS. Vogel H. Willerford DM. Sands AT. Platt KA. Hasty P. Analysis of ku80-mutant mice and cells with deficient levels of p53. Mol Cell Biol. 2000;20:3772–3780. doi: 10.1128/mcb.20.11.3772-3780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bauer JH. Poon PC. Glatt-Deeley H. Abrams JM. Helfand SL. Neuronal expression of p53 dominant-negative proteins in adult Drosophila melanogaster extends life span. Curr Biol. 2005;15:2063–2068. doi: 10.1016/j.cub.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 10.Bauer JH. Helfand SL. New tricks of an old molecule: Lifespan regulation by p53. Aging Cell. 2006;5:437–440. doi: 10.1111/j.1474-9726.2006.00228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ukraintseva SV. Yashin AI. Individual aging and cancer risk: How are they related? Demogr Res. 2003;9:163–196. [Google Scholar]
- 12.Ukraintseva SV. Yashin AI. Opposite phenotypes of cancer and aging arise from alternative regulation of common signaling pathways. Ann NY Acad Sci. 2003;1010:489–492. doi: 10.1196/annals.1299.089. [DOI] [PubMed] [Google Scholar]
- 13.Ukraintseva SV. Yashin AI. Cancer as “Rejuvenescence.”. Ann NY Acad Sci. 2004;1019:200–205. doi: 10.1196/annals.1297.032. [DOI] [PubMed] [Google Scholar]
- 14.Golubovsky MD. Weisman NY. Arbeev KG. Ukraintseva SV. Yashin AI. Decrease in the lgl tumor suppressor dose in Drosophila increases survival and longevity in stress conditions. Exp Gerontol. 2006;41:819–827. doi: 10.1016/j.exger.2006.06.035. [DOI] [PubMed] [Google Scholar]
- 15.Orsted DD. Bojesen SE. Tybjaerg-Hansen A. Nordestgaard BG. Tumor suppressor p53 Arg72Pro polymorphism and longevity, cancer survival, and risk of cancer in the general population. J Exp Med. 2007;204:1295–1301. doi: 10.1084/jem.20062476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dumont P. Leu JIJ. Della Pietra AC. George DL. Murphy M. The codon 72 polymorphic variants of p53 have markedly different apoptotic potential. Nat Genet. 2003;33:357–365. doi: 10.1038/ng1093. [DOI] [PubMed] [Google Scholar]
- 17.Dilman VM. Anisimov VN. Effect of treatment with phenformin, diphenylhydantoin or L-DOPA on life-span and tumor-incidence in C3H/Sn mice. Gerontology. 1980;26:241–246. doi: 10.1159/000212423. [DOI] [PubMed] [Google Scholar]
- 18.Anisimov VN. Carcinogenesis and Aging, vol. II. CRC Press, Inc.; Boca Raton, Florida: 1987. [Google Scholar]
- 19.Anisimov VN. Khavinson VK. Mikhalski AI. Yashin AI. Effect of synthetic thymic and pineal peptides on biomarkers of ageing, survival and spontaneous tumour incidence in female CBA mice. Mech Ageing Dev. 2001;122:41–68. doi: 10.1016/s0047-6374(00)00184-6. [DOI] [PubMed] [Google Scholar]
- 20.Yashin AI. Ukraintseva SV. De Benedictis G. Anisimov VN. Butov AA. Arbeev K. Jdanov DA. Boiko SI. Begun AS. Bonafe M. Franceschi C. Have the oldest old adults ever been frail in the past? A hypothesis that explains modern trends in survival. J Gerontol A Biol Sci Med Sci. 2001;56:B432–B442. doi: 10.1093/gerona/56.10.b432. [DOI] [PubMed] [Google Scholar]
- 21.Weindruch R. Walford RL. Dietary restriction in mice beginning at 1 year of age: Effect on life-span and spontaneous cancer incidence. Science. 1982;215:1415–1418. doi: 10.1126/science.7063854. [DOI] [PubMed] [Google Scholar]
- 22.Blackwell BN. Bucci TJ. Hart RW. Turturro A. Longevity, body-weight, and neoplasia in ad libitum-fed and diet-restricted C57BL6 mice fed NIH-31 open formula diet. Toxicol Pathol. 1995;23:570–582. doi: 10.1177/019262339502300503. [DOI] [PubMed] [Google Scholar]
- 23.James SJ. Muskhelishvili L. Gaylor DW. Turturro A. Hart R. Upregulation of apoptosis with dietary restriction: Implications for carcinogenesis and aging. Environ Health Perspect. 1998;106:307–312. doi: 10.1289/ehp.98106s1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anisimov VN. Khavinson VK. Peptide bioregulation of aging: Results and prospects. Biogerontology. 2010;11:139–149. doi: 10.1007/s10522-009-9249-8. [DOI] [PubMed] [Google Scholar]
- 25.Colman RJ. Anderson RM. Johnson SC. Kastman EK. Kosmatka KJ. Beasley TM. Allison DB. Cruzen C. Simmons HA. Kemnitz JW. Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campisi J. Aging and cancer cell biology, 2008. Aging Cell. 2008;7:281–284. doi: 10.1111/j.1474-9726.2008.00383.x. [DOI] [PubMed] [Google Scholar]
- 27.Yashin AI. Ukraintseva SV. Akushevich IV. Arbeev KG. Kulminski A. Akushevich L. Trade-off between cancer and aging: What role do other diseases play? Evidence from experimental and human population studies. Mech Ageing Dev. 2009;130:98–104. doi: 10.1016/j.mad.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levine AJ. Feng ZH. Mak TW. You H. Jin SK. Coordination and communication between the p53 and IGF-1-AKT-TOR signal transduction pathways. Genes Dev. 2006;20:267–275. doi: 10.1101/gad.1363206. [DOI] [PubMed] [Google Scholar]
- 29.Matheu A. Maraver A. Serrano M. The Arf/p53 pathway in cancer and aging. Cancer Res. 2008;68:6031–6034. doi: 10.1158/0008-5472.CAN-07-6851. [DOI] [PubMed] [Google Scholar]
- 30.Gatza C. Hinkal G. Moore L. Dumble M. Donehower L. p53 and Mouse Aging Models. In: Masoro EJ, editor; Austad SN, editor. Handbook of Biology of Aging. 6th. Academic Press; 2006. [Google Scholar]
- 31.Barinaga M. Stroke-damaged neurons may commit cellular suicide. Science. 1998;281:1302–1303. doi: 10.1126/science.281.5381.1302. [DOI] [PubMed] [Google Scholar]
- 32.van Heemst D. Mooijaart SP. Beekman M. Schreuder J. de Craen AJM. Brandt BW. Slagboom PE. Westendorp RGJ. Long Life study g. Variation in the human TP53 gene affects old age survival and cancer mortality. Exp Gerontol. 2005;40:11–15. doi: 10.1016/j.exger.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Ukraintseva SV. Yashin AI. Economic Progress as Cancer Risk Factor: Part II. Why is Overall Cancer Risk Higher in More Developed Countries? Max Planck Institute for Demographic Research Working Paper series, WP-2005–022. 2005.
- 34.Hanahan D. Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 35.Haffty BG. Glazer PM. Molecular markers in clinical radiation oncology. Oncogene. 2003;22:5915–5925. doi: 10.1038/sj.onc.1206704. [DOI] [PubMed] [Google Scholar]
- 36.Yuan J. Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis. 2009;14:469–477. doi: 10.1007/s10495-008-0304-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrison C. Neurological disease—New avenues for stroke treatment. Nat Rev Drug Discov. 2007;6:520–520. [Google Scholar]
- 38.Kim HJ. Rowe M. Ren M. Hong JS. Chen PS. Chuang DM. Histone deacetylase inhibitors exhibit anti-inflammatory and neuroprotective effects in a rat permanent ischemic model of stroke: Multiple mechanisms of action. J Pharmacol Exp Ther. 2007;321:892–901. doi: 10.1124/jpet.107.120188. [DOI] [PubMed] [Google Scholar]
- 39.Rogalewski A. Schneider A. Ringelstein EB. Schabitz WR. Toward a multimodal neuroprotective treatment of stroke. Stroke. 2006;37:1129–1136. doi: 10.1161/01.STR.0000209330.73175.34. [DOI] [PubMed] [Google Scholar]
- 40.Rosenberg G. Angel I. Kozak A. Clinical pharmacology of DP-b99 in healthy volunteers: First administration to humans. Br J Clin Pharmacol. 2005;60:7–16. doi: 10.1111/j.1365-2125.2005.02378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D-Pharm. D-Pharm announcement, 2007 DP-b99 Phase IIb trial results presented at the European Stroke Conference 2007. www.dpharm.com/news.asp?year = 2007&type = 0&mode = 1&id = 912007 www.dpharm.com/news.asp?year = 2007&type = 0&mode = 1&id = 912007
- 42.Krijnen PAJ. Nijmeijer R. Meijer C. Visser CA. Hack CE. Niessen HWM. Apoptosis in myocardial ischaemia and infarction. J Clin Pathol. 2002;55:801–811. doi: 10.1136/jcp.55.11.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abbate A. Bussani R. Biondi-Zoccai GGL. Santini D. Petrolini A. De Giorgio F. Vasaturo F. Scarpa S. Severino A. Liuzzo G. Leone AM. Baldi F. Sinagra G. Silvestri F. Vetrovec GW. Crea F. Biasucci LM. Baldi A. Infarct-related artery occlusion, tissue markers of ischaemia, and increased apoptosis in the peri-infarct viable myocardium. Eur Heart J. 2005;26:2039–2045. doi: 10.1093/eurheartj/ehi419. [DOI] [PubMed] [Google Scholar]
- 44.Reeve JLV. Duffy AM. O'Brien T. Samali A. Don't lose heart—therapeutic value of apoptosis prevention in the treatment of cardiovascular disease. J Cell Mol Med. 2005;9:609–622. doi: 10.1111/j.1582-4934.2005.tb00492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobayashi K. Hayashi M. Nakano H. Shimazaki M. Sugimori K. Koshino Y. Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer's disease. J Alzheimers Dis. 2004;6:623–632. doi: 10.3233/jad-2004-6606. [DOI] [PubMed] [Google Scholar]
- 46.Dickson DW. Apoptotic mechanismsisms in Alzheimer neurofibrillary degeneration generation: cause or effect? J Clin Invest. 2004;114:23–27. doi: 10.1172/JCI22317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roe CM. Behrens MI. Xiong C. Miller JP. Morris JC. Alzheimer disease and cancer. Neurology. 2005;64:895–898. doi: 10.1212/01.WNL.0000152889.94785.51. [DOI] [PubMed] [Google Scholar]
- 48.Tremolizzo L. Rodriguez-Menendez V. Brighina L. Ferrarese C. Is the inverse association between Alzheimer's disease and cancer the result of a different propensity to methylate DNA? Med Hypotheses. 2006;66:1251–1252. doi: 10.1016/j.mehy.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 49.Kankaanranta H. Lindsay MA. Giembycz MA. Zhang XZ. Moilanen E. Barnes PJ. Delayed eosinophil apoptosis in asthma. J Allergy Clin Immunol. 2000;106:77–83. doi: 10.1067/mai.2000.107038. [DOI] [PubMed] [Google Scholar]
- 50.Trautmann A. Kruger K. Akdis M. Muller-Wening D. Akkaya A. Brocker EB. Blaser K. Akdis CA. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol. 2005;138:142–150. doi: 10.1159/000088436. [DOI] [PubMed] [Google Scholar]
- 51.Vignola AM. Chiappara G. Gagliardo R. Gjomarkaj M. Merendino A. Siena L. Bousquet J. Bonsignore G. Apoptosis and airway inflammation in asthma. Apoptosis. 2000;5:473–485. doi: 10.1023/a:1009661406440. [DOI] [PubMed] [Google Scholar]
- 52.Vignola AM. Chanez P. Chiappara G. Siena L. Merendino A. Reina C. Gagliardo R. Profita M. Bousquet J. Bonsignore G. Evaluation of apoptosis of eosinophils, macrophages, and T lymphocytes in mucosal biopsy specimens of patients with asthma and chronic bronchitis. J Allergy Clin Immunol. 1999;103:563–573. doi: 10.1016/s0091-6749(99)70225-3. [DOI] [PubMed] [Google Scholar]
- 53.Kodama T. Matsuyama T. Miyata S. Nishimura H. Nishioka Y. Kitada O. Sugita M. Kinetics of apoptosis in the lung of mice with allergic airway inflammation. Clin Exp Allergy. 1998;28:1435–1443. doi: 10.1046/j.1365-2222.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 54.Kankaanranta H. Moilanen E. Zhang X. Pharmacological regulation of human eosinophil apoptosis. Current Drug Targets—Inflammation and Allergy. 2005;4:433–445. doi: 10.2174/1568010054526395. [DOI] [PubMed] [Google Scholar]
- 55.Lamb JP. James A. Carroll N. Siena L. Elliot J. Vignola AM. Reduced apoptosis of memory T-cells in the inner airway wall of mild and severe asthma. Eur Respir J. 2005;6:265–270. doi: 10.1183/09031936.05.00144304. [DOI] [PubMed] [Google Scholar]
- 56.Coussens LM. Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dawber TR. Meadors GF. Moore FE. Epidemiological approaches to heart disease: The Framingham Study. Am J Public Health. 1951;41:279–286. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Govindaraju DR. Cupples LA. Kannel WB. O'Donnell CJ. Atwood LD. D'Agostino RB. Fox CS. Larson M. Levy D. Murabito J. Vasan RS. Splansky GL. Wolf PA. Benjamin EJ. Jeffrey CH. Genetics of the Framingham Heart Study population. Adv Genet. 2008;62:33–65. doi: 10.1016/S0065-2660(08)00602-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Backon J. Dementia in cancer patients undergoing chemotherapy: Implication of free radical injury and relevance to Alzheimer disease. Med Hypotheses. 1991;35:146–147. doi: 10.1016/0306-9877(91)90038-z. [DOI] [PubMed] [Google Scholar]
- 60.Monje ML. Palmer T. Radiation injury and neurogenesis. Curr Opin Neurol. 2003;16:129–134. doi: 10.1097/01.wco.0000063772.81810.b7. [DOI] [PubMed] [Google Scholar]
- 61.Lewis MC. Nevo I. Paniagua MA. Ben-Ari A. Pretto E. Eisdorfer S. Davidson E. Matot I. Eisdorfer C. Uncomplicated general anesthesia in the elderly results in cognitive decline: Does cognitive decline predict morbidity and mortality? Med Hypotheses. 2007;68:484–492. doi: 10.1016/j.mehy.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 62.Herrup K. Yang Y. Pictures in molecular medicine—Contemplating Alzheimer's disease as cancer: a loss of cell-cycle control. Trends Mol Med. 2001;7:527–527. doi: 10.1016/s1471-4914(01)02158-x. [DOI] [PubMed] [Google Scholar]
- 63.Finch CE. Morgan TE. Systemic inflammation, infection, apoE alleles, and Alzheimer disease: A position paper. Curr Alzheimer Res. 2007;4:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- 64.Sherwin RW. Wentworth DN. Cutler JA. Hulley SB. Kuller LH. Stamler J. Serum cholesterol levels and cancer mortality in 361662 men screened for the multiple risk factor intervention trial. JAMA. 1987;257:943–948. [PubMed] [Google Scholar]
- 65.Alsheikh-Ali AA. Maddukuri PV. Han H. Karas RH. Effect of the magnitude of lipid lowering on risk of elevated liver enzymes, rhabdomyolysis, and cancer— Insights from large randomized statin trials. J Am Coll Cardiol. 2007;50:409–418. doi: 10.1016/j.jacc.2007.02.073. [DOI] [PubMed] [Google Scholar]
- 66.Perik PJ. Van der Graaf WTA. De Vries EGE. Boomsma F. Messerschmidt J. Van Veldhuisen DJ. Sleijfer DT. Gietema JA. Circulating apoptotic proteins are increased in long-term disease-free breast cancer survivors. Acta Oncol. 2006;45:175–183. doi: 10.1080/02841860500482225. [DOI] [PubMed] [Google Scholar]
- 67.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease—Reply. New Engl J Med. 2005;353:429–430. doi: 10.1056/NEJM200507283530425. [DOI] [PubMed] [Google Scholar]
- 68.Yamada M. Sasaki H. Mimori Y. Kasagi F. Sudoh S. Ikeda J. Hosoda Y. Nakamura S. Kodama K. Prevalence and risks of dementia in the Japanese population: RERF's Adult Health Study Hiroshima subjects. J Am Geriatr Soc. 1999;47:189–195. doi: 10.1111/j.1532-5415.1999.tb04577.x. [DOI] [PubMed] [Google Scholar]
- 69.Tirumalasetti F. Han L. Birkett DP. The relationship between cancer and Alzheimer's disease. J Am Geriatr Soc. 1991;39:840–840. doi: 10.1111/j.1532-5415.1991.tb02713.x. [DOI] [PubMed] [Google Scholar]
- 70.Stallard E. Underlying and multiple cause mortality at advanced ages: United States 1980–1998. N Amer Actuarial J. 2002;6:64–87. [Google Scholar]