Abstract
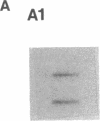
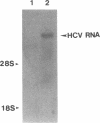
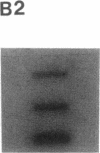
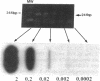
Diagnostic testing for hepatitis C virus (HCV) infection currently is based on the presence of anti-HCV antibodies or a positive HCV RNA polymerase chain reaction (PCR) test. Although HCV RNA PCR is a sensitive and specific technique, widespread application is limited. Moreover, HCV RNA PCR is subject to false-positive reactions through contamination and is inherently difficult to standardize and quantitate. To overcome limitations of HCV RNA PCR, we produced both cDNA and riboprobes from a 241 nucleotide sequence of the 5' untranslated region of the HCV genome for slot hybridization. Hybridization was absent using normal human serum, horse serum, or hepatic cellular RNA from noninfected liver. Hybridization occurred predominantly with positive-stranded HCV RNA and was abolished by pretreatment with RNase A. Slot hybridization was performed on serum samples from 60 patients with chronic HCV infection and a positive HCV RNA PCR and 20 patients with liver diseases unrelated to HCV who had a negative HCV RNA PCR. Slot hybridization with cDNA and riboprobes showed concordance with HCV RNA PCR of 95 and 98.3%, respectively. There were no false-positive reactions in controls. The sensitivity of riboprobe hybridization was comparable to that of one stage HCV RNA PCR using 5' untranslated region primers. Riboprobe hybridization with the HCV H strain standard was positive in the dilution corresponding to 10(-6) chimpanzee infectious doses50/ml. The density of the hybridization signals correlated significantly with the mass of an RNA standard extracted from the liver of a patient with HCV infection. The relative quantities of HCV RNA in the sera of selected patients varied and were not correlated with the duration of disease or the histopathological stage. The highest relative quantities were associated with concurrent immunosuppression. We conclude that slot hybridization is a sensitive, specific alternative to HCV RNA PCR that can be directly quantitated using appropriate HCV RNA standards.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aach R. D., Stevens C. E., Hollinger F. B., Mosley J. W., Peterson D. A., Taylor P. E., Johnson R. G., Barbosa L. H., Nemo G. J. Hepatitis C virus infection in post-transfusion hepatitis. An analysis with first- and second-generation assays. N Engl J Med. 1991 Nov 7;325(19):1325–1329. doi: 10.1056/NEJM199111073251901. [DOI] [PubMed] [Google Scholar]
- Alberti A. Diagnosis of hepatitis C. Facts and perspectives. J Hepatol. 1991 May;12(3):279–282. doi: 10.1016/0168-8278(91)90827-x. [DOI] [PubMed] [Google Scholar]
- Alter H. J., Purcell R. H., Shih J. W., Melpolder J. C., Houghton M., Choo Q. L., Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989 Nov 30;321(22):1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- Alter M. J., Sampliner R. E. Hepatitis C: and miles to go before we sleep. N Engl J Med. 1989 Nov 30;321(22):1538–1540. doi: 10.1056/NEJM198911303212208. [DOI] [PubMed] [Google Scholar]
- Brillanti S., Garson J. A., Tuke P. W., Ring C., Briggs M., Masci C., Miglioli M., Barbara L., Tedder R. S. Effect of alpha-interferon therapy on hepatitis C viraemia in community-acquired chronic non-A, non-B hepatitis: a quantitative polymerase chain reaction study. J Med Virol. 1991 Jun;34(2):136–141. doi: 10.1002/jmv.1890340213. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Kuo G., Weiner A. J., Overby L. R., Bradley D. W., Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989 Apr 21;244(4902):359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- Choo Q. L., Richman K. H., Han J. H., Berger K., Lee C., Dong C., Gallegos C., Coit D., Medina-Selby R., Barr P. J. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiano K., Di Bisceglie A. M., Hoofnagle J. H., Feinstone S. M. Hepatitis C viral RNA in serum of patients with chronic non-A, non-B hepatitis: detection by the polymerase chain reaction using multiple primer sets. Hepatology. 1991 Jul;14(1):51–55. doi: 10.1002/hep.1840140109. [DOI] [PubMed] [Google Scholar]
- Farci P., Alter H. J., Wong D., Miller R. H., Shih J. W., Jett B., Purcell R. H. A long-term study of hepatitis C virus replication in non-A, non-B hepatitis. N Engl J Med. 1991 Jul 11;325(2):98–104. doi: 10.1056/NEJM199107113250205. [DOI] [PubMed] [Google Scholar]
- Feinstone S. M., Alter H. J., Dienes H. P., Shimizu Y., Popper H., Blackmore D., Sly D., London W. T., Purcell R. H. Non-A, non-B hepatitis in chimpanzees and marmosets. J Infect Dis. 1981 Dec;144(6):588–598. doi: 10.1093/infdis/144.6.588. [DOI] [PubMed] [Google Scholar]
- Fong T. L., Shindo M., Feinstone S. M., Hoofnagle J. H., Di Bisceglie A. M. Detection of replicative intermediates of hepatitis C viral RNA in liver and serum of patients with chronic hepatitis C. J Clin Invest. 1991 Sep;88(3):1058–1060. doi: 10.1172/JCI115368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garson J. A., Tedder R. S., Briggs M., Tuke P., Glazebrook J. A., Trute A., Parker D., Barbara J. A., Contreras M., Aloysius S. Detection of hepatitis C viral sequences in blood donations by "nested" polymerase chain reaction and prediction of infectivity. Lancet. 1990 Jun 16;335(8703):1419–1422. doi: 10.1016/0140-6736(90)91446-h. [DOI] [PubMed] [Google Scholar]
- Gray J. J., Wreghitt T. G., Friend P. J., Wight D. G., Sundaresan V., Calne R. Y. Differentiation between specific and non-specific hepatitis C antibodies in chronic liver disease. Lancet. 1990 Mar 10;335(8689):609–610. doi: 10.1016/0140-6736(90)90398-o. [DOI] [PubMed] [Google Scholar]
- Han J. H., Shyamala V., Richman K. H., Brauer M. J., Irvine B., Urdea M. S., Tekamp-Olson P., Kuo G., Choo Q. L., Houghton M. Characterization of the terminal regions of hepatitis C viral RNA: identification of conserved sequences in the 5' untranslated region and poly(A) tails at the 3' end. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1711–1715. doi: 10.1073/pnas.88.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Weiner A., Han J., Kuo G., Choo Q. L. Molecular biology of the hepatitis C viruses: implications for diagnosis, development and control of viral disease. Hepatology. 1991 Aug;14(2):381–388. [PubMed] [Google Scholar]
- Inchauspe G., Abe K., Zebedee S., Nasoff M., Prince A. M. Use of conserved sequences from hepatitis C virus for the detection of viral RNA in infected sera by polymerase chain reaction. Hepatology. 1991 Oct;14(4 Pt 1):595–600. doi: 10.1016/0270-9139(91)90044-v. [DOI] [PubMed] [Google Scholar]
- Katkov W. N., Dienstag J. L., Cody H., Evans A. A., Choo Q. L., Houghton M., Kuo G. Role of hepatitis C virus in non-B chronic liver disease. Arch Intern Med. 1991 Aug;151(8):1548–1552. [PubMed] [Google Scholar]
- Kato N., Hijikata M., Ootsuyama Y., Nakagawa M., Ohkoshi S., Sugimura T., Shimotohno K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9524–9528. doi: 10.1073/pnas.87.24.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo G., Choo Q. L., Alter H. J., Gitnick G. L., Redeker A. G., Purcell R. H., Miyamura T., Dienstag J. L., Alter M. J., Stevens C. E. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989 Apr 21;244(4902):362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. H., Purcell R. H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto H., Okada S., Sugiyama Y., Yotsumoto S., Tanaka T., Yoshizawa H., Tsuda F., Miyakawa Y., Mayumi M. The 5'-terminal sequence of the hepatitis C virus genome. Jpn J Exp Med. 1990 Jun;60(3):167–177. [PubMed] [Google Scholar]
- Saito I., Miyamura T., Ohbayashi A., Harada H., Katayama T., Kikuchi S., Watanabe Y., Koi S., Onji M., Ohta Y. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Zhang L. Q., Watson H. G., Rebus S., Ferguson E. D., Balfe P., Leadbetter G. H., Yap P. L., Peutherer J. F., Ludlam C. A. Hepatitis C quantification and sequencing in blood products, haemophiliacs, and drug users. Lancet. 1990 Dec 15;336(8729):1469–1472. doi: 10.1016/0140-6736(90)93179-s. [DOI] [PubMed] [Google Scholar]
- Smedile A., Bergmann K. F., Baroudy B. M., Wells F. V., Purcell R. H., Bonino F., Rizzetto M., Gerin J. L. Riboprobe assay for HDV RNA: a sensitive method for the detection of the HDV genome in clinical serum samples. J Med Virol. 1990 Jan;30(1):20–24. doi: 10.1002/jmv.1890300105. [DOI] [PubMed] [Google Scholar]
- Tabor E., Gerety R. J., Drucker J. A., Seeff L. B., Hoofnagle J. H., Jackson D. R., April M., Barker L. F., Pineda-Tamondong G. Transmission of non-A, non-B hepatitis from man to chimpanzee. Lancet. 1978 Mar 4;1(8062):463–466. doi: 10.1016/s0140-6736(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Takamizawa A., Mori C., Fuke I., Manabe S., Murakami S., Fujita J., Onishi E., Andoh T., Yoshida I., Okayama H. Structure and organization of the hepatitis C virus genome isolated from human carriers. J Virol. 1991 Mar;65(3):1105–1113. doi: 10.1128/jvi.65.3.1105-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K., Kubo Y., Boonmar S., Watanabe Y., Katayama T., Choo Q. L., Kuo G., Houghton M., Saito I., Miyamura T. The putative nucleocapsid and envelope protein genes of hepatitis C virus determined by comparison of the nucleotide sequences of two isolates derived from an experimentally infected chimpanzee and healthy human carriers. J Gen Virol. 1990 Dec;71(Pt 12):3027–3033. doi: 10.1099/0022-1317-71-12-3027. [DOI] [PubMed] [Google Scholar]
- Ulrich P. P., Romeo J. M., Lane P. K., Kelly I., Daniel L. J., Vyas G. N. Detection, semiquantitation, and genetic variation in hepatitis C virus sequences amplified from the plasma of blood donors with elevated alanine aminotransferase. J Clin Invest. 1990 Nov;86(5):1609–1614. doi: 10.1172/JCI114882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]