Abstract
Peroxisome proliferator-activated receptor-α (PPARα) agonists have been shown to have a therapeutic benefit in experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS). In this study, we investigated the mechanism by which the PPARα agonist gemfibrozil induces immune deviation and protects mice from EAE. We demonstrated that treatment with gemfibrozil increases expression of the Th2 transcription factor GATA-3 and decreases expression of the Th1 transcription factor T-bet in vitro and directly ex vivo. These changes correlated with an increase in nuclear PPARα expression. Moreover, the protective effects of PPARα agonists in EAE were shown to be partially dependent on IL-4 and to occur in a receptor-dependent manner. PPARα was demonstrated, for the first time, to regulate the IL-4 and IL-5 genes and to bind the IL-4 promoter in the presence of steroid receptor coactivator-1, indicating that PPARα can directly transactivate the IL-4 gene. Finally, therapeutic administration of PPARα agonists ameliorated clinically established EAE, suggesting that PPARα agonists may provide a treatment option for immune-mediated inflammatory diseases.
Experimental autoimmune encephalomyelitis (EAE)3 is an immune-mediated inflammatory disease that serves as a model for the human disease multiple sclerosis (MS). EAE can be induced in genetically susceptible strains of rodents by immunization with myelin or myelin peptides emulsified in CFA or by adoptive transfer of myelin-specific CD4+ Th1 lymphocytes into naive recipient animals (1, 2). It has also been demonstrated recently that EAE can be induced by the adoptive transfer of IL-23-stimulated, myelin-specific T cells that produce IL-17, and these cells are believed to play a major role in EAE pathogenesis (3). It has been shown by our laboratory and others that shifting the phenotype of autoreactive CD4+ T cells from proinflammatory Th1 cells to IL-4 producing Th2 cells can be beneficial in EAE (1, 4, 5). Several different reagents such as altered peptide ligands, retinoids, and peroxisome proliferator-activated receptor (PPAR) agonists have been shown to ameliorate disease in this manner (4, 6–8). However, the mechanism by which these agents induce a Th2-like phenotype is not clearly defined.
PPARs are ligand-activated transcription factors belonging to the nuclear hormone receptor superfamily that includes steroid, retinoic acid, and thyroid hormone receptors (9). Three different isoforms of PPARs have been identified to date, PPARα, PPARγ, and PPARβ/δ. These isoforms are encoded by separate genes and have varied tissue distribution and ligand specificity (10, 11). Upon heterodimerizing with retinoid X receptors (RXR), PPARs can positively or negatively regulate gene expression by binding to PPAR response elements (PPREs) in the regulatory regions of target genes (12–14). PPARα, the first PPAR to be cloned, is known to regulate lipid homeostasis and is a target of the class of drugs known as fibrates (15–18). Fibrates, such as gemfibrozil and fenofibrate, have been used clinically for the treatment of hypertri-glyceridemia for a number of years and are safe and well tolerated by patients. Recently, PPARα has been shown to be expressed in immune cells, including macrophages, dendritic cells, and B and T lymphocytes, and PPARα agonists are believed to play a role in regulating the inflammatory response (14, 19–22). More specifically, PPARα ligands have been shown to inhibit IL-2, TNF-α, and IFN-γ production by activated CD4+ T cells and to induce IL-4 production in splenocytes, suggesting the ability of these agonists to induce a Th2-like phenotype (8, 23, 24). In addition, PPARα agonists may partially regulate inflammation by sustaining expression of the negative regulator IκBα, thereby preventing nuclear translocation and activation of NF-κB, a major transcription factor involved in initiating proinflammatory immune responses (14, 25). Furthermore, the PPARα agonist WY14,643 was shown to induce apoptosis of lymphocytes and to inhibit IgG responses in myelin oligodendrocyte glycoprotein peptide (MOG35–55)-immunized mice (20). More recently, we have demonstrated that oral administration of PPARα agonists can prevent the development of EAE. We also found that PPARα agonists can increase the production of the Th2 cytokine IL-4, reduce the production of the Th1 cytokine IFN-γ, suppress Ag-specific T cell proliferation, and reduce NO production by microglia (8).
In this study, we characterized the mechanism by which the PPARα agonist gemfibrozil can induce a Th2-like response and protect mice from EAE. More specifically, we investigated the ability of gemfibrozil to activate its receptor, PPARα, and modulate the transcription of genes involved in the immune response that occurs during EAE. We examined the effects of gemfibrozil on the expression of two key transcription factors involved in Th cell differentiation, T-bet and GATA3, and determined whether this agonist mediates its effects in EAE in an IL-4- and receptor-dependent manner using IL-4−/− mice and a small interfering RNA (siRNA) specific for PPARα. This study also demonstrated that PPARα can positively regulate the Th2 cytokine genes IL-4 and IL-5 and illustrated specifically that PPARα binds the IL-4 promoter in the presence of the steroid receptor coactivator (SRC)-1, indicating its ability to transactivate this gene. Finally, we show that the PPARα agonists gemfibrozil and fenofibrate can effectively ameliorate established EAE, suggesting that these drugs could be used therapeutically.
Materials and Methods
Mice
Vβ8.2 TCR transgenic mice were provided by Dr. J. Goverman (University of Washington, Seattle, WA) (26). These mice were bred and maintained in a federally approved animal facility at the University of Texas Southwestern Medical Center (Dallas, TX) or the Ohio State University Medical Center in accordance with the Institutional Animal Care and Use Committee. B10.PL and C57BL/6 mice were purchased from The Jackson Laboratory and bred in our animal facility. IL-4−/− mice were purchased from The Jackson Laboratory and backcrossed onto the B10.PL background in our animal facility. All mice were between 7 and 10 wk of age when the experiments were performed.
Induction of EAE and in vivo administration of siRNA
EAE was induced in IL-4−/− B10.PL mice, wild-type (WT) B10.PL mice, and C57BL/6 mice by s.c. injection over four sites in the flank with 50 μg of myelin basic protein (MBP) N-terminally acetylated peptide (Ac) 1–11 or 200 μg of the myelin oligodendrocyte glycoprotein peptide MOG35–55 in an emulsion with CFA (Difco). Pertussis toxin (200 ng/mouse) was injected i.p. at the time of immunization and 48 h later. The mice were evaluated daily for clinical signs of EAE as previously described (8, 27). Mean clinical scores from two EAE experiments were combined for Figs. 2 and 3 and SEM is indicated on the graphs. Mice that died from EAE were removed from the analysis following death.
FIGURE 2.
Gemfibrozil partially mediates its protective effects in EAE in an IL-4 dependent manner. A, B10.PL mice deficient in IL-4 or WT for the IL-4 gene were given a diet supplemented with 0.25% (w/w) gemfibrozil (Gem) or vehicle control (EtOH) beginning 1 day before immunization with MBP Ac1–11 to induce EAE. Mice were monitored for clinical signs of disease. Disease incidence is indicated in parentheses. Mann-Whitney nonparametric analysis was performed; gemfibrozil WT vs gemfibrozil IL4−/−, p < 0.0001; EtOH WT vs EtOH IL4−/−, p = 0.0557. B, Thirty days postimmunization feeding splenocytes were isolated from representative mice in A and nuclear extracts were made. PPARα expression was measured directly ex vivo by Western blotting. Densitometry was performed and relative PPARα expression was determined by normalizing to actin. Results shown are representative of at least three experiments.
FIGURE 3.
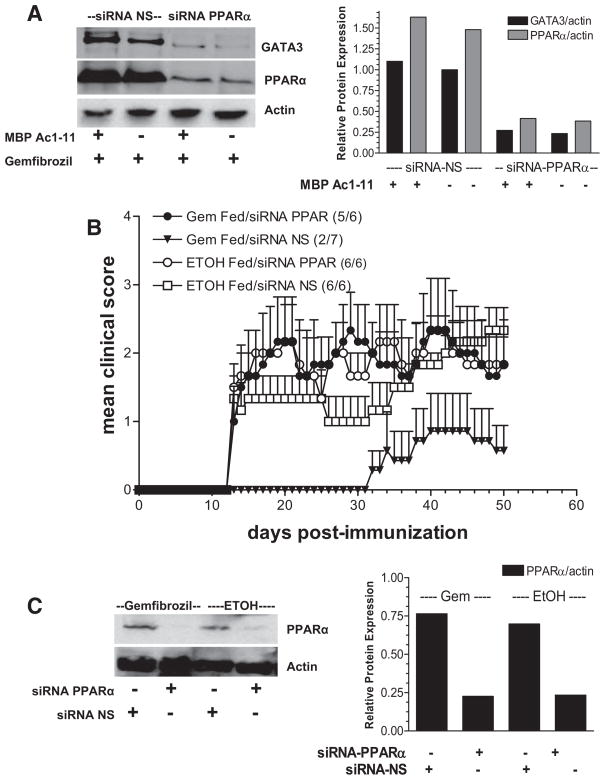
Gemfibrozil modulates the immune response in a receptor-dependent manner. A, Splenocytes isolated from a Vβ8.2 TCR transgenic B10.PL mouse were transfected with an siRNA specific for PPARα or an siRNA NS control. Cells were cultured in the presence of 100 μM gemfibrozil or vehicle control (EtOH) with or without the Ag MBP Ac1–11 for 48 h. Whole cell lysates were made and PPARα and GATA3 expression were measured by Western blotting. B, siRNA PPARα or siRNA NS were administered to B10.PL mice in vivo via tail vein and diet was supplemented with 0.25% (w/w) gemfibrozil (Gem) or vehicle control (EtOH) 1 day before immunization with MBP Ac1–11. Mice were monitored for clinical signs of EAE. Disease incidence is indicated in parentheses. Mann-Whitney nonparametric analysis was performed; gemfibrozil plus siRNA NS vs gemfibrozil plus siRNA PPARα, p < 0.0001; EtOH plus siRNA NS vs EtOH plus siRNA PPARα, p <0.0169; gemfibrozil plus siRNA PPARα vs EtOH plus siRNA PPARα, p = 0.8668; gemfibrozil plus siRNA NS vs EtOH + siRNA NS, p < 0.0001. C, Splenocytes were isolated from mice receiving siRNA PPARα or siRNA NS 15 days postimmunization/feeding and PPARα expression was measured directly ex vivo by Western blotting to verify gene silencing in vivo. Densitometry was performed and relative GATA3 and PPARα expression was determined by normalizing to actin.
For adoptive transfer studies, splenocytes were removed from B10.PL mice induced to develop EAE that had been fed gemfibrozil or ethanol (EtOH) diets. Splenocytes were activated in vitro with MBP Ac1–11 (2 μg/ml) for 3 days and subsequently transferred into naive recipients as previously described (27).
Synthetic siRNA specific for PPARα and a nonsense (NS) control siRNA were purchased from Dharmacon and stocks were prepared in the manufacturer’s buffer at 160 μM stocks and diluted to 50 μg per 100 μl of PBS (2 mg/kg/mouse) for i.v. administration via the tail vein. The sequence of siRNA PPARα is as follows: 5′-UCACGGAGCUCACAGAAUUUU-3′ and 3′-AAUUCUGUGAGCUCCGUGAUU5-′.
Administration of PPARα agonists
For administration of gemfibrozil and fenofibrate, stock solutions were made by dissolving these agents in EtOH (gemfibrozil) or DMSO (fenofibrate) (50 mg/ml). When mice were fed by gavage (supplementary Fig. 1),4 stock solution was diluted in PBS so that the indicated dose of agonist (500 μg) was administered in a total of 200 μl. For all other EAE experiments, mouse chow was supplemented with gemfibrozil or fenofibrate by adding the stock solution to the chow (0.25% (w/w) in EtOH), allowing the EtOH to evaporate, and then using this chow as the source of food. Control mice were given chow treated with an equivalent amount of EtOH without the PPARα agonist.
Transfection with siRNA, preparation of lysates, and Western blotting
Splenocytes from a Vβ8.2 TCR transgenic mouse were transfected in vitro with siRNA PPARα or siRNA NS in the presence of 100 μM gemfibrozil with or without MBP Ac1–11 for 48 h and cell lysates were prepared as previously described (27). Similarly, splenocytes from a Vβ8.2 TCR transgenic mouse were cultured in the presence or absence of 100 μM gemfibrozil with or without MBP Ac1–11 for 48 h and nuclear extracts were prepared using the NE-PER nuclear and cytoplasmic extraction reagents (Pierce) as previously described (27). For ex vivo experiments, splenocytes were isolated from mice treated with or without siRNA that were fed gemfibrozil or vehicle control and cell lysates were prepared. Briefly, cells were collected, spun down, and re-suspended in SDS-lysis buffer. Cells were lysed on ice for 30 min and spun down to remove cell debris. Protease inhibitors (aprotinin, leupeptin, and pep-statin) were added to all lysates at the time of preparation. The protein concentration of all lysates was determined by using the BioRad protein assay. The lysates were diluted in 5× SDS loading buffer and boiled for 3 min. Lysates were electrophoretically separated on 4–20% SDS-PAGE gels and transferred to polyvinylidene difluoride membranes. Western blotting and densitometry were performed as previously described (27) using the following Abs: T-bet, GATA3, actin, goat anti-mouse IgG-HRP, rabbit anti-goat IgG-HRP, and goat anti-rabbit IgG-HRP purchased from Santa Cruz Biotechnology; IL-23R purchased from R&D systems; and PPARα purchased from Affinity Bioreagents.
ELISA
Cytokine expression was determined in the supernatants of splenocytes taken from siRNA PPARα- or siRNA NS-treated mice that were fed gemfibrozil or EtOH and were restimulated at 5 × 106 splenocytes/ml in 24-well plates by activation with MBP Ac1–11 (2 μg/ml). IL-4 and IL-5 ELISA were performed as previously described (8). IL-17 ELISA was performed using the mouse IL-17 DuoSet (R&D Systems).
Chromatin immunoprecipitation (ChIP) and ChIP re-ChIP assays
The ChIP assay was performed as described previously using a PPARα Ab from Affinity Bioreagents (27). For ChIP re-ChIP assay, crosslinking, lysis, sonication, and immunoprecipitation with a PPARα-specific Ab were performed per normal ChIP assay. Following initial immunoprecipitation, eluted immune complexes were diluted 10× with a re-ChIP buffer and then immunoprecipitated again using an Ab specific for the coactivator SRC-1 (Affinity Bioreagents). Following this step, the assay was continued as a routine ChIP assay. Input samples were diluted 100-fold, and for re-ChIP, the initial PPARα immunoprecipitation sample was diluted 10-fold. Five μl of DNA was used for each PCR. PCR amplification of the IL-4, IL-5, GATA3, and cMaf promoters was performed. The primers used were as follows: IL-4, 5′-CGGACACCTGTGACCTCTTCCTTC-3′ and 5′-CCAATCAGCACCTCTCTTCCAGGAG-3′; IL-5, 5′-CCTGAGTTTCAGGACTCGCC-3′ and 5′-CCAGACACAGCTGAGAGTCA-3′; GATA3, 5′-GGAAAGCTGGTTCGGAGGCA-3′ and 5′-GCCGATTCATTCGGGCTCAG-3′; and cMaf, 5′-CGAGGGATCCGGAGAGAGAA-3′ and 5′-GCGCTTTGCATAAGGAGGGC-3′. PCR conditions were 55°C for 1 min, 72°C for 1 min, and 94°C for 30 cycles using previously described PCR mixes (27).
Statistics
Student’s two-tailed t test was used to determine statistical significance of cytokine production between different experimental groups. The Mann-Whitney U test was used for analysis of mean clinical scores. All statistics were performed using GraphPad Prism software.
Results
Gemfibrozil increases GATA3 and decreases T-bet expression in vitro and ex vivo in mouse splenocytes
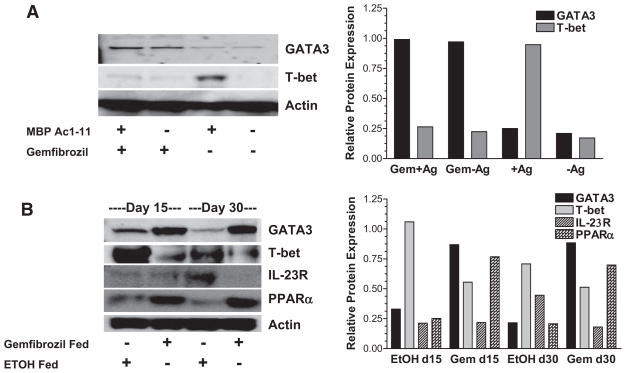
It has been shown previously that PPARα agonists can increase IL-4 production and decrease IFN-γ production in immune cells (8, 20, 24, 28). We wanted to determine whether the agonists were mediating these effects by modulating the transcription factors GATA3 and T-bet, which are known to regulate IL-4 and IFN-γ production, respectively (29, 30). TCR transgenic splenocytes that express Vβ8.2, which recognizes MBP Ac1–11 when paired with Vα2.3, were stimulated with MBP Ac1–11 in the presence or absence of gemfibrozil. Vβ8.2 transgenic mice have a frequency of MBP Ac1–11-specific T cells of 1 in 103 to 104, providing a model for studying Ag-specific T cells in the context of a diverse T cell repertoire. Western blot analysis indicated that GATA3 expression increased and T-bet expression decreased when splenocytes were cultured with gemfibrozil, suggesting that this PPARα agonist cannot only alter IL-4 and IFN-γ production, as shown previously (8), but can also modulate the transcription factors that regulate expression of these cytokines (Fig. 1A).
FIGURE 1.
Gemfibrozil increases GATA3 and decreases T-bet expression in vitro and ex vivo in mouse splenocytes. A, The PPARα agonist gemfibrozil (Gem) (100 μM) or vehicle control (EtOH) was added to Vβ8.2 TCR transgenic splenocytes from a B10.PL mouse, and cells were stimulated with MBP Ac1–11 for 48 h. Nuclear extracts were made and GATA3 and T-bet expression were measured by Western blotting. B, B10.PL mice were given a diet supplemented with 0.25% (w/w) gemfibrozil or vehicle control (EtOH) beginning 1 day before immunization with MBP Ac1–11. On days (d) 15 and 30 postimmunization, splenocytes were isolated and GATA3, T-bet, IL-23R, and PPARα expression were measured ex vivo by Western blotting. Densitometry was performed on Western blots and relative GATA3, T-bet, IL-23R, and PPARα expression was determined by normalizing to actin. All results shown are representative of at least three experiments.
Directly ex vivo, similar to what was observed in vitro, GATA3 expression was increased and T-bet expression was decreased in the splenocytes of mice immunized with MBP Ac1–11 and fed gemfibrozil compared with vehicle-fed controls. These changes correlated with an increase in nuclear PPARα expression in the gemfibrozil-fed mice (Fig. 1B). We also found that IL-23R expression was decreased in the splenocytes of EAE mice that were given gemfibrozil 30 days postimmunization per feeding compared with vehicle-fed controls (Fig. 1B). This is in agreement with our recent observation that T-bet regulates IL-23R expression (31). Furthermore, activation of splenocytes with MBP Ac1–11 from vehicle-fed controls resulted in successful adoptive transfer in 9/9 mice, whereas 4/17 mice receiving MBP Ac1–11-activated splenocytes from gemfibrozil fed mice developed EAE (supplemental Table I).4
Gemfibrozil partially mediates its protective effects in EAE in an IL-4-dependent manner
To determine whether the efficacy of gemfibrozil treatment in EAE was dependent on increased production of IL-4, IL-4 deficient or WT B10.PL mice were given a diet continuously supplemented with gemfibrozil before and after the induction of EAE. Gemfibrozil-fed mice that were deficient in IL-4 were not protected from disease to the same extent as the WT gemfibrozil-fed controls (Fig. 2A). Incidence and mean disease severity of the mice in this experiment are shown in Table I. In addition, IL-4-deficient mice were found to have decreased nuclear PPARα expression ex vivo compared with WT controls even in the presence of gemfibrozil (Fig. 2B). This decreased expression correlated with increased disease severity.
Table I.
Incidence and severity of EAE in IL-4−/− vs WT mice treated with gemfibrozil
| Incidence | Mean Severitya | |
|---|---|---|
| EtOH IL4−/− | 10/11 | 3.1 |
| EtOH WT | 6/7 | 3.0 |
| Gemfibrozil IL4−/− | 6/11 | 2.3 |
| Gemfibrozil WT | 3/7 | 1.7 |
Mean of the maximum clinical disease score of individual animals in each treatment group.
The PPARα agonist gemfibrozil modulates the immune response in a receptor-dependent manner
It has been suggested previously that PPARα agonists mediate their anti-inflammatory effects in a PPARα-independent manner (24, 32). To determine whether this was true in our system, splenocytes were transfected with a siRNA specific for PPARα and stimulated in the presence of gemfibrozil in vitro. Following administration of siRNA, PPARα expression was decreased in vitro (Fig. 3A). Furthermore, when PPARα expression was suppressed, there was no longer an increase in GATA3 expression in the presence of gemfibrozil (Fig. 3A). This data suggests that the increase in GATA3 expression that occurs in the presence of gemfibrozil (Fig. 1, A and B) occurs in a receptor-dependent manner.
To investigate whether the PPARα agonist gemfibrozil was mediating its protective effects in EAE in a receptor-dependent manner in vivo, siRNA specific for PPARα or a siRNA NS control was administered to gemfibrozilfed mice before the induction of EAE. Silencing PPARα in vivo decreased the efficacy of gemfibrozil treatment in EAE (Fig. 3B). Overall, disease was most severe in mice that received siRNA PPARα (gemfibrozil and EtOH fed), suggesting an important role for this receptor and possibly its endogenous ligands in protection from EAE. The incidence and mean severity of disease for the mice in this experiment are shown in Table II. To confirm that PPARα expression was successfully silenced in vivo, splenocytes were isolated from mice on day 15 and PPARα expression was measured ex vivo by Western blotting. PPARα expression was decreased in mice given siRNA specific for PPARα compared with siRNA NS controls, confirming that knockdown of PPARα was successful in vivo (Fig. 3C).
Table II.
Incidence and severity of EAE in siRNA PPAR-α vs siRNA NS-treated mice fed gemfibrozil
| Incidence | Mean Severitya | |
|---|---|---|
| Gemfibrozil fed/siRNA PPAR | 5/6 | 3.0 |
| Gemfibrozil fed/siRNA NS | 2/7 | 1.0 |
| EtOH fed/siRNA PPAR | 6/6 | 2.5 |
| EtOH fed/siRNA NS | 6/6 | 2.3 |
Mean of the maximum clinical disease score of individual animals in each treatment group.
To verify that gemfibrozil induced a shift to a Th2-like phenotype in siRNA PPARα-treated mice, splenocytes were removed from the mice in the previous experiment, activated with MBP Ac1–11, and cytokine secretion was measured by ELISA. Interestingly, splenocytes taken from siRNA PPARα-treated mice were unable to secrete significant amounts of IL-4 and IL-5 even if the mice were fed gemfibrozil. Mice given siRNA NS and fed gemfibrozil were able to produce increased IL-4 and IL-5 compared with EtOH-fed controls (Fig. 4, A and B). These data suggest that the ability of the PPARα agonist gemfibrozil to induce a Th2-like phenotype, as determined by increased Th2 cytokine secretion, is receptor dependent. Furthermore, we found that splenocytes taken from siRNA PPARα-treated mice produced increased amounts of IL-17 even if the mice were fed gemfibrozil in vivo compared with EtOH-fed controls (Fig. 4C). Contrary to what was found for IL-4 and IL-5, siRNA NS-treated mice that were fed gemfibrozil were impaired in their ability to secrete IL-17 compared with EtOH-fed controls. Therefore, this suggests that gemfibrozil, via interaction with its receptor PPARα, ameliorates EAE by increasing Th2 cytokine production and decreasing IL-17 production.
FIGURE 4.
Gemfibrozil induces Th2 cytokine secretion in a receptor-dependent manner. Splenocytes were isolated from representative EAE mice in B and restimulated in vitro with MBP Ac1–11. At 72 h supernatants were collected and IL-4 (A), IL-5 (B), and IL-17 (C) secretion were measured by ELISA. siRNA PPARα plus gemfibrozil (Gem) vs siRNA NS plus gemfibrozil, p < 0.001 for IL-4 and IL-5. siRNA PPARα plus gemfibrozil vs siRNA NS plus Gem, p < 0.01 for IL-17. All results shown are representative of two or more independent experiments.
PPARα regulates IL-4 and IL-5
Because we no longer observed an increase in the Th2 cytokines IL-4 and IL-5 in the presence of gemfibrozil when PPARα is silenced, we wanted to ascertain whether PPARα could directly regulate these genes. To determine whether PPARα binds directly to the IL-4 and/or IL-5 promoter regions, ChIP assays were performed. Prior work had identified a PPRE in the IL-4 promoter (24). In the presence of gemfibrozil, following stimulation with Ag, PPARα bound the IL-4 and IL-5 promoters (Fig. 5A). This binding was not observed in the absence of ligand or when we immunoprecipitated with an isotype control Ab. In addition, the IL-4 and IL-5 promoters contain a PPRE located ~317 and 1738 bp upstream from the transcription start site, respectively, and this is the region of each promoter that was amplified. To determine whether PPARα could also regulate IL-4 and IL-5 production indirectly via regulation of the transcription factors GATA3 and c-Maf, which are known to regulate Th2 cytokine genes, the DNA immunoprecipitated with a PPAR-α specific Ab was also amplified using primer sets specific for the GATA3 and c-Maf promoters. We were unable to detect binding to either of these regulatory regions by PPARα (Fig. 5A), suggesting that regulation of IL-4 and IL-5 production is not occurring indirectly through regulation of GATA3 or c-Maf. Rather, it is possible that PPARα-dependent Th2 cytokine production, specifically IL-4 production, can lead to the increased GATA3 expression that is seen in the presence of gemfibrozil (Fig. 1, A and B).
FIGURE 5.
PPARα regulates transcription of Th2 cytokine genes in a ligand-dependent manner. A, Splenocytes were isolated from a Vβ8.2 TCR transgenic B10.PL mouse and cultured in the presence of 100 μM gemfibrozil or vehicle control (EtOH). Cells were stimulated with or without MBP Ac1–11 for 48 h and crosslinked for ChIP. ChIP assays were performed using an Ab specific for PPARα and immunoprecipitated DNA was amplified using primers specific for the IL-4, IL-5, GATA3, and cMaf, promoter regions. B, Splenocytes were isolated as described in A and transfected with an siRNA specific for PPARα or an siRNA NS control. Cells were cultured in the presence of 100 μM gemfibrozil or vehicle control (EtOH) with or without MBP-Ac1–11 for 48 h and crosslinked for ChIP assays. ChIP assays were performed with a PPARα-specific Ab and immunoprecipitated DNA was amplified with primers specific for the IL-4 and IL-5 promoter regions that contained PPREs. C, Splenocytes were isolated and cultured as described in A. Cells were crosslinked for ChIP re-ChIP assay. ChIP re-ChIP was performed by immunoprecipitating first with a PPARα-specific Ab. Eluted immune complexes were then immunoprecipitated again using an Ab specific for SRC-1. Immunoprecipitated DNA was amplified using primers specific for the IL-4 promoter containing a PPRE. Results shown are representative of multiple experiments.
To verify the specificity of this binding, another ChIP assay was performed in which the Vβ8.2 transgenic splenocytes were transfected with siRNA PPARα or siRNA NS before activation. When PPARα expression is inhibited using RNA interference (RNAi), we no longer observe binding to the IL-4 or IL-5 promoters in the presence of gemfibrozil compared with the siRNA NS-transfected controls (Fig. 5B).
To provide further evidence that PPARα is transactivating the IL-4 gene, which would therefore indicate functional binding, we examined whether PPARα is bound to the coactivator SRC-1 when it is bound to the IL-4 promoter. To accomplish this, a ChIP re-ChIP assay was performed. Splenocytes were stimulated in the presence or absence of gemfibrozil and ChIP was performed using an Ab specific for PPARα. Following immunoprecipitation with PPARα, another immunoprecipitation was performed using an Ab specific for the coactivator SRC-1. DNA that was specifically bound to PPARα when SRC-1 was bound was then used as a template in a PCR. We found that a primer set that spans a PPRE amplified a sequence within the IL-4 promoter when the cells were cultured in the presence of gemfibrozil (Fig. 5C). These data, in combination with the inability of gemfibrozil to increase IL-4 production when PPARα is silenced (Fig. 4), suggest that PPARα can transactivate the IL-4 promoter and that the binding is functional.
Treatment of EAE mice with the PPARα agonists gemfibrozil and fenofibrate ameliorates disease course
To determine whether treatment with the PPARα agonists gemfibrozil and fenofibrate could ameliorate EAE once the disease has already been established, mice with clinically definite EAE were fed gemfibrozil, fenofibrate, or vehicle controls by gavage for 5 days to ensure that the mice were getting an equivalent dose. After 5 days, the mice were given a diet supplemented with the PPARα agonists for the duration of the experiment. Treating mice with gemfibrozil and fenofibrate after disease was established ameliorated their disease course (supplemental Fig. 1). The incidence and mean severity of EAE for the animals in this experiment are depicted in Table III.
Table III.
Incidence and severity of EAE in PPAR-α agonist-treated mice
| Incidence | MCS Day 32a | Mean Severityb | |
|---|---|---|---|
| Gemfibrozil fed | 5/5 | 0.8 | 2.4 |
| Fenofibrate fed | 6/7 | 0.9 | 2.0 |
| EtOH fed | 4/4 | 2.0 | 2.8 |
| DMSO fed | 6/6 | 2.5 | 3.5 |
Mean clinical disease score of individual animals in each treatment group at day 32 postimmunization (day 18 posttreatment).
Mean of the maximum clinical disease score of individual animals in each treatment group.
Discussion
The current study investigates the mechanism by which PPARα agonists induce an anti-inflammatory phenotype and protect mice from EAE. Gemfibrozil has been shown to affect cytokine production but was also found in this study to alter the expression of the transcription factors T-bet and GATA3, which are essential for the differentiation of CD4+ T cells. Changes in the expression of these transcription factors were observed following culture in vitro, as well as directly ex vivo when taken from mice that had been given a diet supplemented with gemfibrozil. In addition, it has been shown by us and others that decreased T-bet expression correlates with protection from EAE (27, 31, 33). T-bet knockout mice and mice that have had T-bet silenced using RNAi are protected from disease and have been shown to have a correlative increase in GATA3 expression (27, 31, 33). Furthermore, we have demonstrated that T-bet, via direct regulation of the IL-23R, can influence the fate of pathogenic IL-17-producing cells (31). Although Th17 cells have been shown to differentiate independently of T-bet, they appear to rely on T-bet for optimal IL-23 responsiveness and, therefore, survival. We have found that T-bet can directly regulate the IL-23R and when T-bet is silenced using RNAi, IL-23R expression and IL-17 expression are decreased, resulting in protection from EAE (31). The current study suggests that at least one mechanism by which PPARα agonists exert their protective effects in EAE is through down-regulation of T-bet and up-regulation of GATA3. This altered transcription factor expression results in a Th2-like phenotype and may lead to decreased proliferation/expansion of encephalitogenic Th1 as previously demonstrated (8) or Th17 cells, as evidenced by a decrease in IL-23R expression and decreased IL-17 production in gemfibrozil-fed mice. Furthermore, it has been shown that T-bet and GATA3 can regulate one another and, in doing so, may affect downstream signaling (34, 35). Therefore, if PPARα, through interaction with its ligand, can regulate either of these transcription factors directly or indirectly, this could result in the regulation of the other transcription factor.
This study also demonstrates that the protective effects of gemfibrozil treatment in EAE are partially dependent on IL-4. The data indicate that there may be more than one mechanism by which gemfibrozil exerts it protective effects in EAE, but suggest that IL-4 does play an important role in this protection. An additional mechanism for protection may be mediated via APCs such as microglia, which have been implicated in the pathology of EAE and MS (36). PPARα and RXR agonists have been shown to inhibit microglial and astrocyte production of NO, IL-1β, TNF-α, IL-6, and MCP-1, all of which contribute to pathogenesis in EAE (37, 38), and the PPARα agonist fenofibrate has been demonstrated to suppress LPS induction of IL-12, IL-23, and IL-27p28 by microglia (39). In addition, PPARα agonists may mediate protection from EAE in part by repression of transcription factors such as NF-κB and T-bet, which regulate Th1 or Th17 inflammatory genes, or by negatively regulating the production of proinflammatory cytokines, such as IFN-γ, as has been demonstrated for PPARs (8, 40–42). Increased nuclear expression of PPARα in WT gemfibrozil-fed mice was also observed (Fig. 1). However, this expression was decreased in IL-4−/− gemfibrozil-fed mice to levels seen in vehicle controls, suggesting an important role for IL-4 in the regulation of PPARα. There is evidence of cross-talk between PPAR signaling pathways and STAT and GATA transcription factors (43). It is possible that PPARα agonists induce increased IL-4 expression that then drives Th2 differentiation via STAT6 and GATA3. Additional studies are required to further investigate the role of IL-4 in induction of PPARα expression and to elucidate other mechanisms of protection by gemfibrozil in IL-4-deficient mice.
It has previously been suggested that fibrates increase IL-4 production via a PPARα-independent mechanism. The PPARα agonist WY14,643 was found to induce modest IL-4 production in splenocytes from PPARα knockout mice after stimulation with a T cell mitogen (24). In addition, PPARα knockout mice were found to benefit from gemfibrozil treatment (32). Contrary to these results, we found that gemfibrozil increases GATA3 expression and ex vivo IL-4 and IL-5 production by Ag-specific T cells in a PPARα-dependent manner. Furthermore, gemfibrozil was shown to exert its protective effects in EAE in a receptor-dependent manner when PPARα was silenced in vivo using siRNA. Our data suggest that IL-4 at least partially mediates the therapeutic effect of gemfibrozil in a receptor-dependent manner but do not exclude the possibility that receptor-independent mechanisms may contribute to the therapeutic effect. There may be a number of reasons for the discrepancy between our data and that generated in PPARα knockout mice. Rather than using PPARα knockout mice, we silenced PPARα expression using RNAi, thereby avoiding the redundancy or compensation that may occur when a gene is not expressed during development. For example, PPARγ and PPARδ expression are increased in T cells from PPARα-deficient mice (44). In addition, the genetic background of PPARα-deficient mice is distinct from that of the B10.PL mice used this study (45). Moreover, there may be additional mechanisms by which gemfibrozil mediates protection in EAE other than increasing IL-4 production, such as inhibiting production of the proinflammatory cytokines IL-17 or IL-23, which may be dependent on receptor-ligand interactions (39). Importantly, this study demonstrates that PPARα directly regulates IL-4 and IL-5 by binding to PPREs in the regulatory regions of these genes as shown by ChIP assay. These data, in combination with the inability of siRNA PPARα-treated mice to produce increased IL-4/IL-5 and decreased IL-17, suggest that regulation of these cytokine genes is one way in which gemfibrozil induces immune deviation to a Th2-like phenotype and protects mice from EAE. Furthermore, the finding that PPARα binds the IL-4 promoter when the coactivator SRC-1 is bound is further support that PPARα, following ligand binding, can directly transactivate the IL-4 gene. In an unliganded state, PPARα is bound to a corepressor complex. In the presence of ligand, this corepressor complex dissociates and is targeted for degradation. The PPARα/RXR heterodimer can then associate with a coactivator complex and bind to PPREs in promoters and enhancers of target genes. It is this coactivator complex that induces chromosomal modifications such as chromatin acetylation and remodeling and allows transcriptional machinery to gain access to the regulatory regions of genes that are regulated by PPARα (46). Association of PPARα with the coactivator complex on the IL-4 promoter is strong evidence of transactivation and receptor-dependent regulation of IL-4 in the presence of gemfibrozil.
Finally, in this study established EAE was ameliorated with the PPARα agonists gemfibrozil and fenofibrate. This is important because it suggests that PPARα agonists could be used clinically for the treatment of immune-mediated inflammatory diseases. Fibrates are taken orally; this differs from the method of administration of current MS therapies, which are given by s.c. or i.m. injections. Therefore, the use of these drugs could improve the quality of life of the MS patient if proven to be effective in reducing disease severity.
Overall, this study provides new insight into the anti-inflammatory mechanism of the PPARα agonist gemfibrozil and further delineates how this drug ameliorates EAE. It suggests for the first time that PPARα agonists mediate their protective effects in a receptor-dependent manner via transcriptional activation of the Th2 cytokine genes IL-4 and IL-5. This regulation of IL-4 and IL-5 results in immune deviation to a Th2-like phenotype via altered expression of the transcription factors T-bet and GATA3. The model we propose for the protective mechanism of gemfibrozil in EAE is as follows (Fig. 6). In the presence of PPARα agonists, PPARα heterodimerizes with RXR, dissociates from its nuclear corepressor complex, associates with a coactivator complex, and binds to PPREs in the promoter region of IL-4 and/or IL-5. The transactivation of IL-4/IL-5 leads to increased expression of GATA3, which in turn results in decreased T-bet expression and down-regulation of the Th1/Th17 inflammatory response. This study suggests that fibrates could provide an effective therapy for MS, and insight gained from dissecting the mechanism of action of fibrates in EAE could be used for the development of new drugs with fewer side effects to be used for the treatment of immune-mediated inflammatory diseases.
FIGURE 6.
A model for PPARα-mediated protection in EAE. In the presence of PPARα agonists, PPARα heterodimerizes with RXR, dissociates from its nuclear corepressor complex, associates with a coactivator complex, and binds to PPREs in the promoter region of IL-4 and/or IL-5. The trans-activation of IL-4/IL-5 leads to increased expression of GATA3, which in turn results in decreased T-bet expression and down-regulation of the Th1/Th17 inflammatory response. This shift in the immune response to a Th2-like phenotype results in amelioration of EAE.
Supplementary Material
Footnotes
This work was supported National Multiple Sclerosis Society Grants RG3427-A-8 and RG3812-A-3 and the National Institutes of Health Grants NS44250 and NS37513. A.E.L.-R. is a National Multiple Sclerosis Society Harry Weaver Neuroscience Scholar.
Abbreviations used in this paper: EAE, experimental autoimmune encephalomyelitis; Ac, N-terminally acetylated peptide; ChIP, chromatin immunoprecipitation; EtOH, ethanol; MBP, myelin basic protein; MS, multiple sclerosis; NS, nonsense; PPAR, peroxisome proliferator-activated receptor; PPRE, PPAR response element; RNAi, RNA interference; RXR, retinoid X receptor; siRNA, small interfering RNA; SRC, steroid receptor coactivator; WT, wild type.
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflict of interest.
References
- 1.Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induce immune deviation as a therapy for inflammatory autoimmune disease. J Exp Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ando DG, Clayton J, Kono D, Urban L, Sercarz EE. Encephalitogenic T cells in the B10.PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 3.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Racke MK, Burnett D, Pak SH, Albert PS, Cannella B, Raine CS, McFarlin DE, Scott DE. Retinoid treatment of experimental allergic encephalomyelitis: IL-4 production correlates with improved disease course. J Immunol. 1995;154:450–458. [PubMed] [Google Scholar]
- 5.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 6.Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci USA. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholson LB, Greer JM, Sobel RA, Lees MB, Kuchroo VK. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 8.Lovett-Racke AE, Hussain RZ, Northrop S, Choy J, Rocchini A, Matthes L, Chavis JA, Diab A, Drew PD, Racke MK. Peroxisome proliferators-activated receptor α agonists as therapy for autoimmune disease. J Immunol. 2004;172:5790–5798. doi: 10.4049/jimmunol.172.9.5790. [DOI] [PubMed] [Google Scholar]
- 9.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–688. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 10.Braissant O, Foufelle F, Scotto C, Dauca M, Wahli W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- 11.Kliewer SA, Forman BM, Blumberg B, Ong ES, Borgmeyer U, Mangelsdorf DJ, Umesono K, Evans RM. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359. doi: 10.1073/pnas.91.15.7355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tugwood JD, Issemann I, Anderson RG, Bundell KR, McPheat WL, Green S. The mouse peroxisome proliferator activated receptor recognizes a response element in the 5′ flanking sequence of the rat acyl CoA oxidase gene. EMBO J. 1992;11:433–439. doi: 10.1002/j.1460-2075.1992.tb05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyata KS, McCaw SE, Marcus SL, Rachubinski RA, Capone JP. The peroxisome proliferator-activated receptor interacts with the retinoid X receptor in vivo. Gene. 1994;148:327–330. doi: 10.1016/0378-1119(94)90707-2. [DOI] [PubMed] [Google Scholar]
- 14.Jones DC, Ding X, Daynes RA. Nuclear receptor PPARα is expressed in resting murine lymphocytes: the PPARα in T and B lymphocytes is both transactivation and transrepression competent. J Biol Chem. 2002;277:6838–6845. doi: 10.1074/jbc.M106908200. [DOI] [PubMed] [Google Scholar]
- 15.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature. 1990;347:645–649. doi: 10.1038/347645a0. [DOI] [PubMed] [Google Scholar]
- 16.Issemann I, Prince RA, Tugwood JD, Green S. The peroxisome proliferator-activated receptor: retinoid X receptor heterodimer is activated by fatty acids and fibrate hypolipidaemic drugs. J Mol Endocrinol. 1993;11:37–47. doi: 10.1677/jme.0.0110037. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann JM, Lenhard JM, Oliver BB, Ringold GM, Kliewer SA. Peroxisome proliferator-activated receptors α and γ are activated by indo-methacin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1997;272:3406–3410. doi: 10.1074/jbc.272.6.3406. [DOI] [PubMed] [Google Scholar]
- 18.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyun-saturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devchand PR, Keller H, Peters JM, Vazquez M, Gonzalez FJ, Wahli W. The PPARα-leukotriene B4 pathway to inflammation control. Nature. 1996;384:39–43. doi: 10.1038/384039a0. [DOI] [PubMed] [Google Scholar]
- 20.Cunard R, DiCampli D, Archer DC, Stevenson JL, Ricote M, Glass CK, Kelly CJ. WY14,643, a PPARα ligand, has profound effects on immune responses in vivo. J Immunol. 2002;169:6806–6812. doi: 10.4049/jimmunol.169.12.6806. [DOI] [PubMed] [Google Scholar]
- 21.Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, Fruchart JC, Tedgui A, Haegeman G, Staels B. Peroxisome proliferator-activated receptor α negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-κB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- 22.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, Torra IP, Teissier E, Minnich A, Jaye M, Duverger N, et al. PPAR-α and PPAR-γ activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. doi: 10.1038/83348. [DOI] [PubMed] [Google Scholar]
- 23.Marx N, Kehrle B, Kohlhammer K, Grub M, Koenig W, Hombach V, Libby P, Plutzky J. PPAR activators as anti-inflammatory mediators in human T lymphocytes: implications for atherosclerosis and transplantation-associated arteriosclerosis. Circ Res. 2002;90:703–710. doi: 10.1161/01.res.0000014225.20727.8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunard R, Ricote M, DiCampli D, Archer DC, Kahn DA, Glass CK, Kelly CJ. Regulation of cytokine expression by ligands of peroxisome proliferator activated receptors. J Immunol. 2002;168:2795–2802. doi: 10.4049/jimmunol.168.6.2795. [DOI] [PubMed] [Google Scholar]
- 25.Delerive P, Gervois P, Fruchart JC, Staels B. Induction of IκBα expression as a mechanism contributing to the anti-inflammatory activities of peroxisome proliferator-activated receptor-α activators. J Biol Chem. 2000;275:36703–36707. doi: 10.1074/jbc.M004045200. [DOI] [PubMed] [Google Scholar]
- 26.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM. Transgenic mice that express myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell. 1993;72:551–560. doi: 10.1016/0092-8674(93)90074-z. [DOI] [PubMed] [Google Scholar]
- 27.Lovett-Racke AE, Rocchini AE, Choy J, Northrop SC, Hussain RZ, Ratts RB, Sikder D, Racke MK. Silencing T-bet defines critical role in the differentiation of autoreactive T lymphocytes. Immunity. 2004;21:719–731. doi: 10.1016/j.immuni.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Jones DC, Ding X, Zhang TY, Daynes RA. Peroxisome proliferator-activated receptor α negatively regulates T-bet transcription through suppression of p38 mitogen-activated protein kinase activation. J Immunol. 2003;171:196–203. doi: 10.4049/jimmunol.171.1.196. [DOI] [PubMed] [Google Scholar]
- 29.Zheng WP, Flavell RA. The transcription factor GATA-3 is necessary and sufficient for Th2 cytokine gene expression in CD4+ T cells. Cell. 1997;89:587–596. doi: 10.1016/s0092-8674(00)80240-8. [DOI] [PubMed] [Google Scholar]
- 30.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100:655–669. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 31.Gocke AR, Cravens PD, Ben LH, Hussain RZ, Northrop SC, Racke MK, Lovett-Racke AE. T-bet regulates the fate of Th1 and Th17 lymphocytes in autoimmunity. J Immunol. 2007;178:1341–1348. doi: 10.4049/jimmunol.178.3.1341. [DOI] [PubMed] [Google Scholar]
- 32.Dasgupta S, Roy A, Jana M, Hartley DM, Pahan K. Gemfibrozil ameliorates relapsing-remitting experimental autoimmune encephalomyelitis independent of peroxisome proliferators-activated receptor-α. Mol Pharmacol. 2007;72:935–946. doi: 10.1124/mol.106.033787. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Sullivan B, Szabo SJ, Sobel RA, Glimcher LH, Kuchroo VK. Loss of T-bet, but not STAT1, prevents the development of experimental autoimmune encephalomyelitis. J Exp Med. 2004;200:79–87. doi: 10.1084/jem.20031819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang ES, Szabo SJ, Schwartzberg PL, Glimcher LH. T helper cell fate specified by kinase-mediated interaction of T-bet with GATA-3. Science. 2005;307:430–433. doi: 10.1126/science.1103336. [DOI] [PubMed] [Google Scholar]
- 35.Usui T, Preiss JC, Kanno Y, Yao ZJ, Bream JH, O’Shea JJ, Strober W. T-bet regulates Th1 responses through essential effects on GATA-3 function rather than on IFNG gene acetylation and transcription. J Exp Med. 2006;203:755–766. doi: 10.1084/jem.20052165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sriram S, Rodriguez M. Indictment of microglia as the villain in multiple sclerosis. Neurology. 1997;48:464–470. doi: 10.1212/wnl.48.2.464. [DOI] [PubMed] [Google Scholar]
- 37.Xu J, Storer PD, Chavis JA, Racke MK, Drew PD. Agonists for the peroxisome proliferator-activated receptor-α and the retinoid X receptor inhibit inflammatory responses of microglia. J Neurosci Res. 2005;81:403–411. doi: 10.1002/jnr.20518. [DOI] [PubMed] [Google Scholar]
- 38.Xu J, Chavis JA, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-α and retinoid X receptor agonists inhibit inflammatory responses of astrocytes. J Neuroimmunol. 2006;176:95–105. doi: 10.1016/j.jneuroim.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Racke MK, Drew PD. Peroxisome proliferator-activated receptor-α agonist fenofibrate regulates IL-12 family cytokine expression in the CNS: relevance to multiple sclerosis. J Neurochem. 2007;103:1801–1810. doi: 10.1111/j.1471-4159.2007.04875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cunard R, Eto Y, Muljadi JT, Glass CK, Kelly CJ, Ricote M. Repression of IFN-γ expression by peroxisome proliferator-activated receptor-γ. J Immunol. 2004;172:7530–7536. doi: 10.4049/jimmunol.172.12.7530. [DOI] [PubMed] [Google Scholar]
- 41.Pascual G, Fong AL, Ogawa S, Gamliel A, Li AC, Perissi V, Rose DW, Willson TM, Rosenfeld MG, Glass CK. A SUMOylation-dependent pathway mediates transrepression of inflammatory response genes by PPAR-γ. Nature. 2005;437:759–763. doi: 10.1038/nature03988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, Hoffmann A, Subramaniam S, David M, Rosenfeld MG, Glass CK. Molecular determinants of cross-talk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang XY, Wang LH, Chen T, Hodge DR, Resau JH, DaSilva L, Farrar WL. Activation of human T lymphocytes is inhibited by peroxisome proliferator-activated receptor-γ (PPARγ) agonists. PPARγ co-association with transcription factor NFAT. J Biol Chem. 2000;275:4541–4544. doi: 10.1074/jbc.275.7.4541. [DOI] [PubMed] [Google Scholar]
- 44.Dunn SE, Ousman SS, Sobel RA, Zuniga L, Baranzini SE, Youssef S, Crowell A, Loh J, Oksenberg J, Steinman L. Peroxisome proliferator-activated receptor (PPAR)α expression in T cells mediates gender differences in development of T cell-mediated autoimmunity. J Exp Med. 2007;204:321–330. doi: 10.1084/jem.20061839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee SS, Pineau T, Drago J, Lee EJ, Owens JW, Kroetz DL, Fernandez-Salguero PM, Westphal H, Gonzalez FJ. Targeted disruption of the α isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol Cell Biol. 1995;15:3012–3022. doi: 10.1128/mcb.15.6.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jones DC, Manning BM, Daynes RA. A role for the peroxisome proliferator-activated receptor α in T cell physiology and ageing immunobiology. Proc Nutr Soc. 2002;61:363–369. doi: 10.1079/pns2002173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.