Abstract
To determine whether decreased renal responsiveness to atrial natriuretic peptide (ANP) in diabetes is mediated by alterations in the renal ANP receptor, ANP receptor density and affinity were measured 17-20 d after streptozotocin injection and compared with values in vehicle-treated controls and streptozotocin-treated rats made euglycemic with insulin. Plasma ANP concentration was significantly greater in hyperglycemic diabetic rats than in control or euglycemic diabetic rats. Both in glomeruli and inner medulla, ANP receptor dissociation constant did not differ among the three study groups, whereas the maximum binding capacity was decreased significantly in hyperglycemic diabetics in comparison with controls and euglycemic diabetics. Glomerular clearance receptors were also decreased significantly in hyperglycemic diabetic rats in comparison with control and euglycemic diabetic rats. To determine whether the decreased number of renal ANP receptors in diabetic rats was associated with a decreased biological response, we measured ANP-dependent cyclic GMP (cGMP) accumulation by isolated glomeruli and inner medullary collecting duct cells in vitro. cGMP accumulation was significantly less in hyperglycemic diabetic rats than in controls or euglycemic diabetic rats both in the presence or absence of the phosphodiesterase inhibitor zaprinast. cGMP phosphodiesterase activity in inner medullary collecting duct cells obtained from control and hyperglycemic diabetic rats did not differ. Thus, the decreased number of biologically active ANP receptors in the kidneys of diabetic rats is accompanied by decreased biological responsiveness in vitro and provides a potential explanation for the reduction in renal sensitivity to ANP in this condition.
Full text
PDF

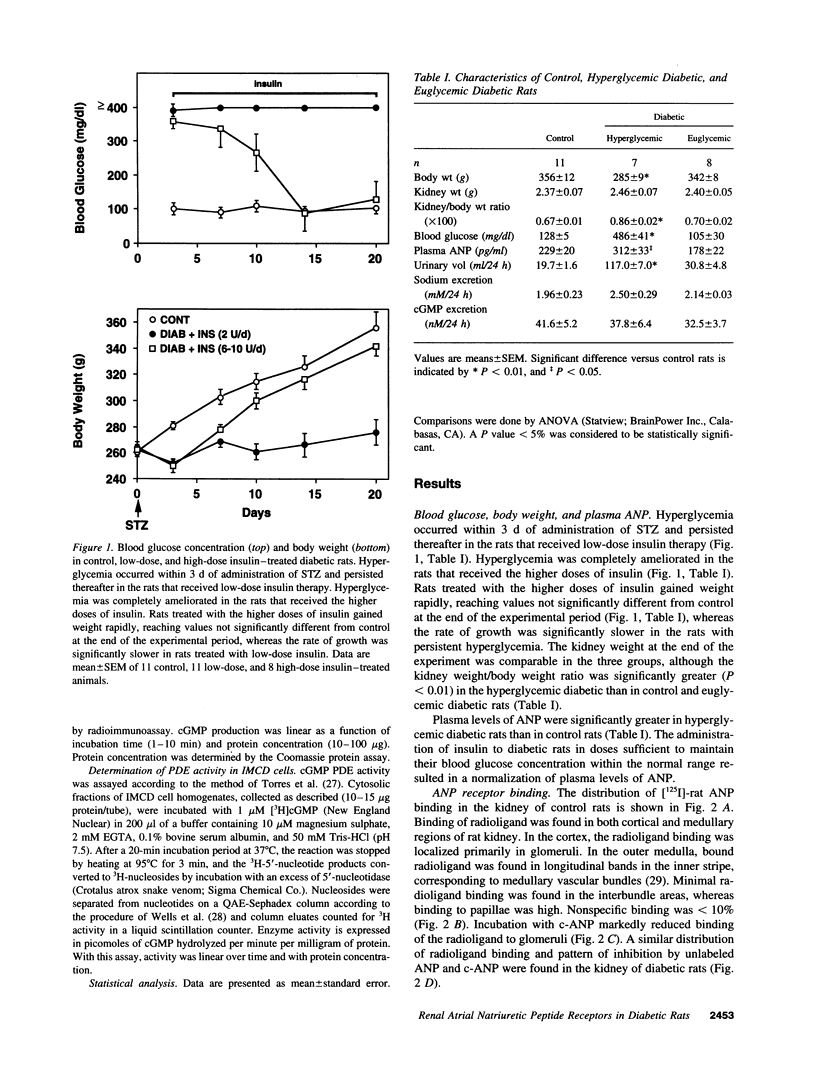
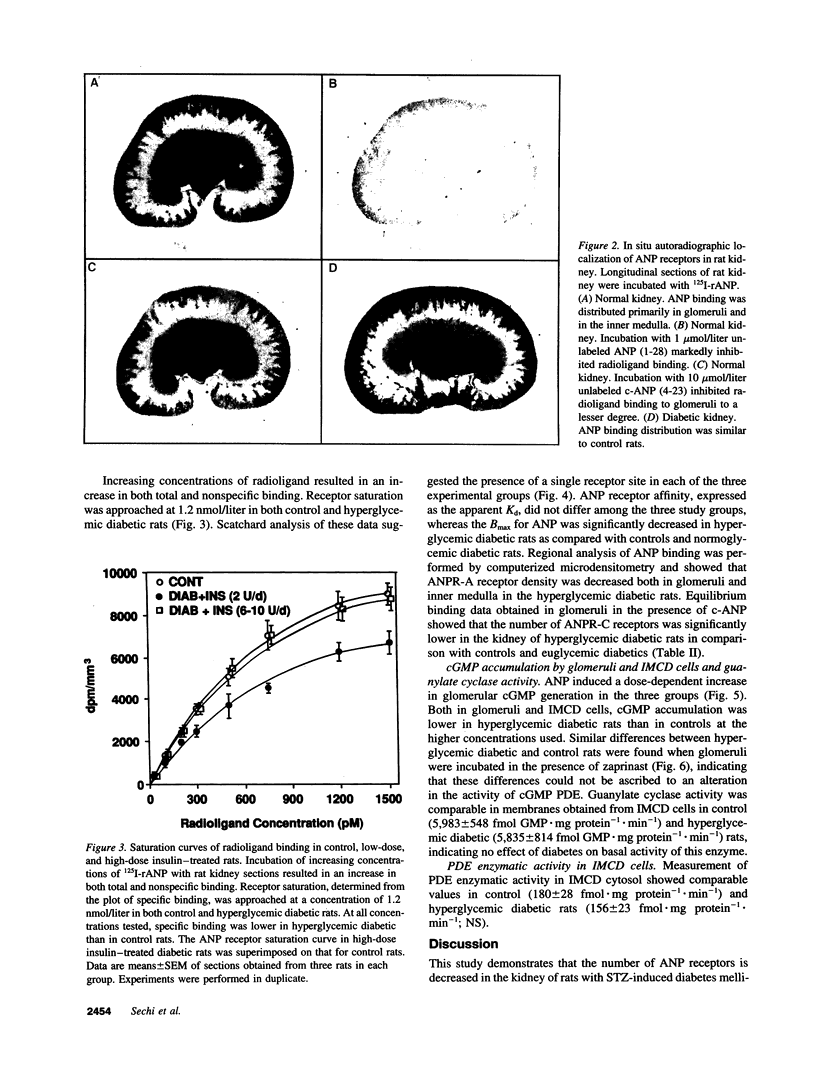
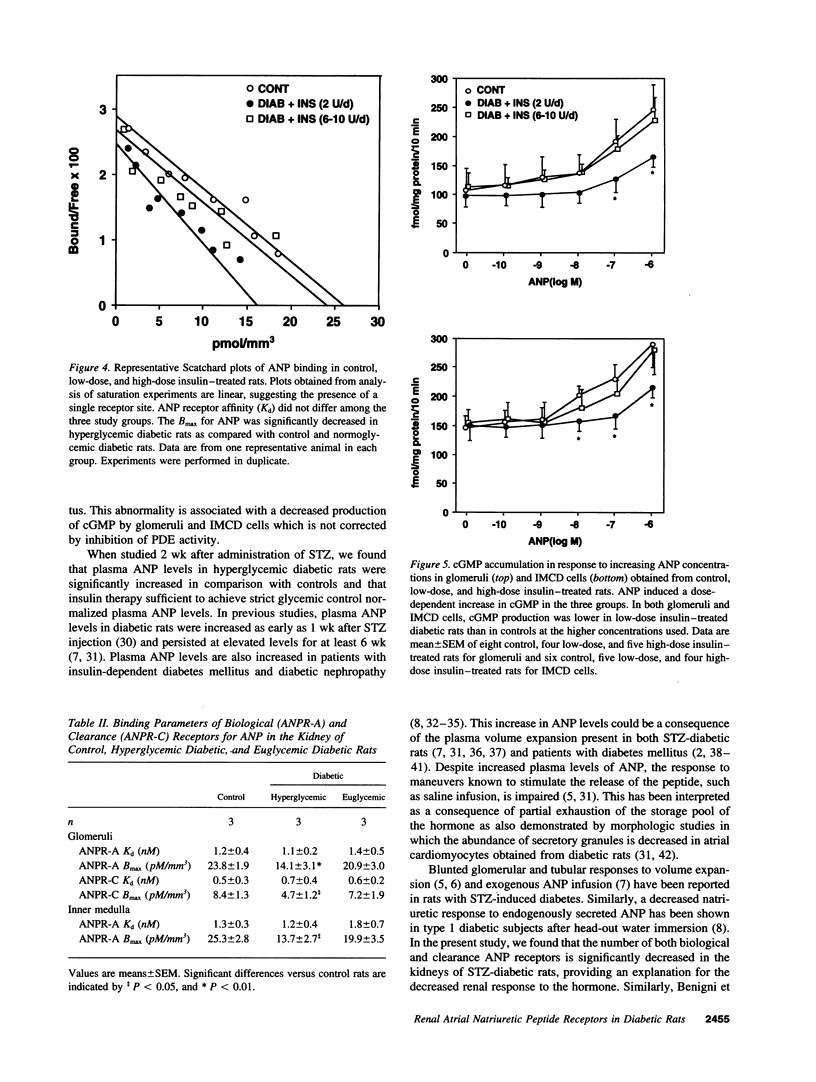

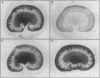
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. J., Cooper M. E., O'Brien R. C., Bach L. A., Jackson B., Jerums G. Glomerular filtration rate in streptozocin-induced diabetic rats. Role of exchangeable sodium, vasoactive hormones, and insulin therapy. Diabetes. 1990 Oct;39(10):1182–1190. doi: 10.2337/diab.39.10.1182. [DOI] [PubMed] [Google Scholar]
- Almeida F. A., Suzuki M., Scarborough R. M., Lewicki J. A., Maack T. Clearance function of type C receptors of atrial natriuretic factor in rats. Am J Physiol. 1989 Feb;256(2 Pt 2):R469–R475. doi: 10.1152/ajpregu.1989.256.2.R469. [DOI] [PubMed] [Google Scholar]
- Anand-Srivastava M. B., Sairam M. R., Cantin M. Ring-deleted analogs of atrial natriuretic factor inhibit adenylate cyclase/cAMP system. Possible coupling of clearance atrial natriuretic factor receptors to adenylate cyclase/cAMP signal transduction system. J Biol Chem. 1990 May 25;265(15):8566–8572. [PubMed] [Google Scholar]
- Ballermann B. J., Bloch K. D., Seidman J. G., Brenner B. M. Atrial natriuretic peptide transcription, secretion, and glomerular receptor activity during mineralocorticoid escape in the rat. J Clin Invest. 1986 Sep;78(3):840–843. doi: 10.1172/JCI112650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. M., Bernstein R. K., Laragh J. H., Atlas S. A., James G. D., Pecker M. S., Sealey J. E. Increased plasma atrial natriuretic factor and reduced plasma renin in patients with poorly controlled diabetes mellitus. Clin Sci (Lond) 1989 Aug;77(2):177–182. doi: 10.1042/cs0770177. [DOI] [PubMed] [Google Scholar]
- Benigni A., Perico N., Dadan J., Gabanelli M., Galbusera M., Boccardo P., Mennini T., Remuzzi G. Functional implications of decreased renal cortical atrial natriuretic peptide binding in experimental diabetes. Circ Res. 1990 Jun;66(6):1453–1460. doi: 10.1161/01.res.66.6.1453. [DOI] [PubMed] [Google Scholar]
- Brown J., Salas S. P., Singleton A., Polak J. M., Dollery C. T. Autoradiographic localization of atrial natriuretic peptide receptor subtypes in rat kidney. Am J Physiol. 1990 Jul;259(1 Pt 2):F26–F39. doi: 10.1152/ajprenal.1990.259.1.F26. [DOI] [PubMed] [Google Scholar]
- Bröchner-Mortensen J. Glomerular filtration rate and extracellular fluid volumes during normoglycemia and moderate hyperglycemia in diabetics. Scand J Clin Lab Invest. 1973 Dec;32(4):311–316. doi: 10.3109/00365517309084353. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Chai S. Y., Sexton P. M., Allen A. M., Figdor R., Mendelsohn F. A. In vitro autoradiographic localization of ANP receptors in rat kidney and adrenal gland. Am J Physiol. 1986 Apr;250(4 Pt 2):F753–F757. doi: 10.1152/ajprenal.1986.250.4.F753. [DOI] [PubMed] [Google Scholar]
- Christlieb A. R. Renin, angiotensin, and norepinephrine in alloxan diabetes. Diabetes. 1974 Dec;23(12):962–970. doi: 10.2337/diab.23.12.962. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Protein kinase C is activated in glomeruli from streptozotocin diabetic rats. Possible mediation by glucose. J Clin Invest. 1989 May;83(5):1667–1675. doi: 10.1172/JCI114066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldt-Rasmussen B., Mathiesen E. R., Deckert T., Giese J., Christensen N. J., Bent-Hansen L., Nielsen M. D. Central role for sodium in the pathogenesis of blood pressure changes independent of angiotensin, aldosterone and catecholamines in type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1987 Aug;30(8):610–617. doi: 10.1007/BF00277316. [DOI] [PubMed] [Google Scholar]
- Fuller F., Porter J. G., Arfsten A. E., Miller J., Schilling J. W., Scarborough R. M., Lewicki J. A., Schenk D. B. Atrial natriuretic peptide clearance receptor. Complete sequence and functional expression of cDNA clones. J Biol Chem. 1988 Jul 5;263(19):9395–9401. [PubMed] [Google Scholar]
- Gauquelin G., Schiffrin E. L., Cantin M., Garcia R. Specific binding of atrial natriuretic factor to renal glomeruli in Doca- and Doca-salt-treated rats correlation with atrial and plasma levels. Biochem Biophys Res Commun. 1987 May 29;145(1):522–531. doi: 10.1016/0006-291x(87)91352-0. [DOI] [PubMed] [Google Scholar]
- Gunning M., Silva P., Brenner B. M., Zeidel M. L. Characteristics of ANP-sensitive guanylate cyclase in inner medullary collecting duct cells. Am J Physiol. 1989 May;256(5 Pt 2):F766–F775. doi: 10.1152/ajprenal.1989.256.5.F766. [DOI] [PubMed] [Google Scholar]
- Hebden R. A., Todd M. E., McNeill J. H. Relationship between atrial granularity and release of atrial natriuretic factor in rats with diabetes mellitus. Am J Physiol. 1989 Oct;257(4 Pt 2):R932–R938. doi: 10.1152/ajpregu.1989.257.4.R932. [DOI] [PubMed] [Google Scholar]
- Hostetter T. H., Troy J. L., Brenner B. M. Glomerular hemodynamics in experimental diabetes mellitus. Kidney Int. 1981 Mar;19(3):410–415. doi: 10.1038/ki.1981.33. [DOI] [PubMed] [Google Scholar]
- Ilstrup K. M., Keane W. F., Michels L. D. Intravascular and extracellular volumes in the diabetic rat. Life Sci. 1981 Aug 17;29(7):717–724. doi: 10.1016/0024-3205(81)90025-4. [DOI] [PubMed] [Google Scholar]
- Koller K. J., Lowe D. G., Bennett G. L., Minamino N., Kangawa K., Matsuo H., Goeddel D. V. Selective activation of the B natriuretic peptide receptor by C-type natriuretic peptide (CNP). Science. 1991 Apr 5;252(5002):120–123. doi: 10.1126/science.1672777. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee T. S., Saltsman K. A., Ohashi H., King G. L. Activation of protein kinase C by elevation of glucose concentration: proposal for a mechanism in the development of diabetic vascular complications. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5141–5145. doi: 10.1073/pnas.86.13.5141. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Lieberman J. S., Parra L., Newton L., Scandling J. D., Loon N., Myers B. D. Atrial natriuretic peptide and response to changing plasma volume in diabetic nephropathy. Diabetes. 1991 Jul;40(7):893–901. doi: 10.2337/diab.40.7.893. [DOI] [PubMed] [Google Scholar]
- Maack T., Suzuki M., Almeida F. A., Nussenzveig D., Scarborough R. M., McEnroe G. A., Lewicki J. A. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987 Oct 30;238(4827):675–678. doi: 10.1126/science.2823385. [DOI] [PubMed] [Google Scholar]
- Munson P. J., Rodbard D. Ligand: a versatile computerized approach for characterization of ligand-binding systems. Anal Biochem. 1980 Sep 1;107(1):220–239. doi: 10.1016/0003-2697(80)90515-1. [DOI] [PubMed] [Google Scholar]
- Patel K. P., Zhang P. L. Attenuated renal responses to atrial natriuretic factor in streptozotocin-induced diabetic rats. Can J Physiol Pharmacol. 1990 Mar;68(3):425–430. doi: 10.1139/y90-060. [DOI] [PubMed] [Google Scholar]
- Patel K. P., Zhang P. L. Reduced renal responses to volume expansion in streptozotocin-induced diabetic rats. Am J Physiol. 1989 Sep;257(3 Pt 2):R672–R679. doi: 10.1152/ajpregu.1989.257.3.R672. [DOI] [PubMed] [Google Scholar]
- Roubert P., Gillard V., Plas P., Guillon J. M., Chabrier P. E., Braquet P. Angiotensin II and phorbol-esters potently down-regulate endothelin (ET-1) binding sites in vascular smooth muscle cells. Biochem Biophys Res Commun. 1989 Oct 31;164(2):809–815. doi: 10.1016/0006-291x(89)91531-3. [DOI] [PubMed] [Google Scholar]
- Sahai A., Ganguly P. K. Congestive heart failure in diabetes with hypertension may be due to uncoupling of the atrial natriuretic peptide receptor-effector system in the kidney basolateral membrane. Am Heart J. 1991 Jul;122(1 Pt 1):164–170. doi: 10.1016/0002-8703(91)90774-c. [DOI] [PubMed] [Google Scholar]
- Sawicki P. T., Heinemann L., Rave K., Hohmann A., Berger M. Atrial natriuretic factor in various stages of diabetic nephropathy. J Diabet Complications. 1988 Oct-Dec;2(4):207–209. doi: 10.1016/s0891-6632(88)80010-2. [DOI] [PubMed] [Google Scholar]
- Scarborough R. M., Schenk D. B., McEnroe G. A., Arfsten A., Kang L. L., Schwartz K., Lewicki J. A. Truncated atrial natriuretic peptide analogs. Comparison between receptor binding and stimulation of cyclic GMP accumulation in cultured vascular smooth muscle cells. J Biol Chem. 1986 Oct 5;261(28):12960–12964. [PubMed] [Google Scholar]
- Schambelan M., Blake S., Sraer J., Bens M., Nivez M. P., Wahbe F. Increased prostaglandin production by glomeruli isolated from rats with streptozotocin-induced diabetes mellitus. J Clin Invest. 1985 Feb;75(2):404–412. doi: 10.1172/JCI111714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda T., Ishihara M., Kurimoto F., Aizawa T., Hiramatsu K., Shirota T., Takasu N., Yamada T. Elevated plasma atrial natriuretic peptide level in the early phase of microalbuminuria in patients with non-insulin-dependent diabetes mellitus. Clin Nephrol. 1990 Nov;34(5):202–207. [PubMed] [Google Scholar]
- Song D. L., Kohse K. P., Murad F. Brain natriuretic factor. Augmentation of cellular cyclic GMP, activation of particulate guanylate cyclase and receptor binding. FEBS Lett. 1988 May 9;232(1):125–129. doi: 10.1016/0014-5793(88)80400-9. [DOI] [PubMed] [Google Scholar]
- Suzuki Y., Suzuki H., Ohtake R., Tsuchiya T., Muramatsu H., Hashigami Y., Kobori H., Shimoda S. Plasma and urine concentrations of atrial natriuretic peptide in patients with diabetes mellitus. Pancreas. 1988;3(4):404–408. doi: 10.1097/00006676-198808000-00006. [DOI] [PubMed] [Google Scholar]
- Takayanagi R., Inagami T., Snajdar R. M., Imada T., Tamura M., Misono K. S. Two distinct forms of receptors for atrial natriuretic factor in bovine adrenocortical cells. Purification, ligand binding, and peptide mapping. J Biol Chem. 1987 Sep 5;262(25):12104–12113. [PubMed] [Google Scholar]
- Torres V. E., Hui Y. S., Shah S. V., Northrup T. E., Dousa T. P. Cyclic nucleotide phosphodiesterases in glomeruli of rat renal cortex. Kidney Int. 1978 Nov;14(5):444–451. doi: 10.1038/ki.1978.149. [DOI] [PubMed] [Google Scholar]
- Tsunoda K., Mendelsohn F. A., Sexton P. M., Chai S. Y., Hodsman G. P., Johnston C. I. Decreased atrial natriuretic peptide binding in renal medulla in rats with chronic heart failure. Circ Res. 1988 Jan;62(1):155–161. doi: 10.1161/01.res.62.1.155. [DOI] [PubMed] [Google Scholar]
- Valentin J. P., Qiu C., Muldowney W. P., Ying W. Z., Gardner D. G., Humphreys M. H. Cellular basis for blunted volume expansion natriuresis in experimental nephrotic syndrome. J Clin Invest. 1992 Oct;90(4):1302–1312. doi: 10.1172/JCI115995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin J. P., Sechi L. A., Qui C., Schambelan M., Humphreys M. H. Urodilatin binds to and activates renal receptors for atrial natriuretic peptide. Hypertension. 1993 Apr;21(4):432–438. doi: 10.1161/01.hyp.21.4.432. [DOI] [PubMed] [Google Scholar]
- Weidmann P., Beretta-Piccoli C., Keusch G., Glück Z., Mujagic M., Grimm M., Meier A., Ziegler W. H. Sodium-volume factor, cardiovascular reactivity and hypotensive mechanism of diuretic therapy in mild hypertension associated with diabetes mellitus. Am J Med. 1979 Nov;67(5):779–784. doi: 10.1016/0002-9343(79)90734-4. [DOI] [PubMed] [Google Scholar]
- Wells J. N., Baird C. E., Hardman Y. J., Wu J. G. Cyclic nucleotide phosphodiesterase activities of pig coronary arteries. Biochim Biophys Acta. 1975 Apr 19;384(2):430–442. doi: 10.1016/0005-2744(75)90044-3. [DOI] [PubMed] [Google Scholar]
- Wilcox J. N., Augustine A., Goeddel D. V., Lowe D. G. Differential regional expression of three natriuretic peptide receptor genes within primate tissues. Mol Cell Biol. 1991 Jul;11(7):3454–3462. doi: 10.1128/mcb.11.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkes B. M., Kaplan R., Mento P. F., Aynedjian H. S., Macica C. M., Schlondorff D., Bank N. Reduced glomerular thromboxane receptor sites and vasoconstrictor responses in diabetic rats. Kidney Int. 1992 Apr;41(4):992–999. doi: 10.1038/ki.1992.151. [DOI] [PubMed] [Google Scholar]
- Wilkes B. M. Reduced glomerular angiotensin II receptor density in diabetes mellitus in the rat: time course and mechanism. Endocrinology. 1987 Apr;120(4):1291–1298. doi: 10.1210/endo-120-4-1291. [DOI] [PubMed] [Google Scholar]
- Wong K. R., Xie M. H., Shi L. B., Liu F. Y., Huang C. L., Gardner D. G., Cogan M. G. Urinary cGMP as biological marker of the renal activity of atrial natriuretic factor. Am J Physiol. 1988 Dec;255(6 Pt 2):F1220–F1224. doi: 10.1152/ajprenal.1988.255.6.F1220. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Brenner B. M., Seifter J. L. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol. 1987 Mar;252(3 Pt 2):F551–F559. doi: 10.1152/ajprenal.1987.252.3.F551. [DOI] [PubMed] [Google Scholar]