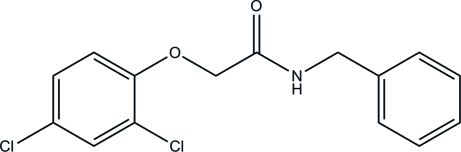
Abstract
In the title compound, C15H13Cl2NO2, the dihedral angle between the aromatic rings is 27.17 (11)°. In the crystal the molecules are linked by N—H⋯O hydrogen bonds.
Related literature
Experimental
Crystal data
C15H13Cl2NO2
M r = 310.16
Monoclinic,
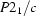
a = 4.7447 (6) Å
b = 26.821 (3) Å
c = 11.3962 (15) Å
β = 90.402 (2)°
V = 1450.2 (3) Å3
Z = 4
Mo Kα radiation
μ = 0.45 mm−1
T = 298 (2) K
0.30 × 0.10 × 0.05 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶)T min = 0.877, T max = 0.978
8418 measured reflections
3254 independent reflections
2233 reflections with I > 2σ(I)
R int = 0.027
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.103
S = 1.03
3254 reflections
185 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.19 e Å−3
Δρmin = −0.24 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: SAINT (Bruker, 2005 ▶); data reduction: SAINT; program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: WinGX (Farrugia, 1999 ▶).
Supplementary Material
Crystal structure: contains datablocks I, New_Global_Publ_Block. DOI: 10.1107/S1600536808029371/cs2090sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808029371/cs2090Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N—H0A⋯O2i | 0.83 (2) | 2.06 (2) | 2.883 (2) | 169 (2) |
Symmetry code: (i) 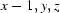 .
.
Acknowledgments
This study was supported by the Science and Technology Key Project of Chongqing Science and Technology Commission, China (grant No. CSTC, 2008 A A1001)
supplementary crystallographic information
Comment
The structure determination was performed as a part of a project on the interactions of small molecules with proteins. The single-crystal characterization should be valuable to understand such interactions. In our previous papers we reported single crystal structures of N-benzyl-2-(2-chloro-4-methylphenoxy)acetamide (Li et al., 2008a) and N-benzyl-2-(2,6-dichlorophenoxy)acetamide (Li et al., 2008b). In the title compound, C15H13Cl2NO2, all bond lengths and angles are normal. The dihedral angle between the two aryl rings is 27.17 (11)o. The molecules are connected via N-H···O hydrogen bonding into chains.
Experimental
The solution of 2,4-dichlorophenol (1.0 mmol), N-benzyl-2-chloroacetamide (1.1 mmol), K2CO3 (1.1 mmol) and CH3CN (20 ml) was refluxed for 3 h. After completion of the reaction, the solution was cooled; solvent was evaporated under reduced pressure. The residue was poured into water and adjusted the pH to 6–7 with dilute hydrochloric acid (10%) and extracted with ethyl acetate, washed with brine and dried over anhydrous MgSO4 to obtain the corresponding crude product. The product was purified by column chromatography on silica gel using ethyl acetate as eluent (yield 87%). Crystals suitable for X-ray diffraction were obtained by slow evaporation of a solution of the solid dissolved in ethyl acetate/hexane at room temperatures for 4 days.
Refinement
All H atoms were placed in geometrically calculated positions and refined using a riding model with C—H = 0.97 Å (for CH2 groups) and 0.96 Å (for CH3 groups), their isotropic displacement parameters were set to 1.2 times (1.5 times for CH3 groups) the equivalent displacement parameter of their parent atoms, except the N-H one which was freely refined.
Figures
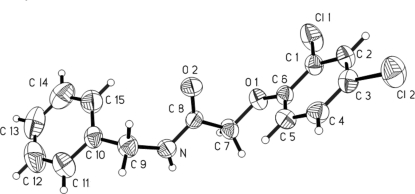
Fig. 1.
The molecular structure of the title compound showing displacement ellipsoids drawn at 50% probability level.
Crystal data
| C15H13Cl2NO2 | F(000) = 640 |
| Mr = 310.16 | Dx = 1.421 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 2023 reflections |
| a = 4.7447 (6) Å | θ = 2.4–24.3° |
| b = 26.821 (3) Å | µ = 0.45 mm−1 |
| c = 11.3962 (15) Å | T = 298 K |
| β = 90.402 (2)° | Prism, colourless |
| V = 1450.2 (3) Å3 | 0.30 × 0.10 × 0.05 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 3254 independent reflections |
| Radiation source: fine-focus sealed tube | 2233 reflections with I > 2σ(I) |
| graphite | Rint = 0.027 |
| phi and ω scans | θmax = 27.5°, θmin = 1.5° |
| Absorption correction: multi-scan (PROGRAM? REFERENCE?) | h = −6→6 |
| Tmin = 0.878, Tmax = 0.978 | k = −26→34 |
| 8418 measured reflections | l = −14→14 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.103 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0422P)2 + 0.2337P] where P = (Fo2 + 2Fc2)/3 |
| 3254 reflections | (Δ/σ)max = 0.001 |
| 185 parameters | Δρmax = 0.19 e Å−3 |
| 0 restraints | Δρmin = −0.24 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N | −0.5859 (3) | 0.13972 (6) | 1.13239 (14) | 0.0432 (4) | |
| H0A | −0.757 (4) | 0.1456 (7) | 1.1390 (16) | 0.045 (6)* | |
| Cl1 | −0.01187 (15) | 0.32457 (2) | 1.38966 (5) | 0.0718 (2) | |
| Cl2 | 0.22803 (14) | 0.39046 (2) | 0.95534 (5) | 0.0711 (2) | |
| O1 | −0.3722 (3) | 0.25520 (5) | 1.27258 (11) | 0.0467 (3) | |
| O2 | −0.1562 (3) | 0.17031 (5) | 1.18000 (13) | 0.0533 (4) | |
| C1 | −0.0568 (4) | 0.32072 (6) | 1.23900 (15) | 0.0419 (4) | |
| C2 | 0.0880 (4) | 0.35291 (7) | 1.16733 (16) | 0.0470 (5) | |
| H2A | 0.2087 | 0.3767 | 1.1991 | 0.056* | |
| C3 | 0.0511 (4) | 0.34928 (7) | 1.04765 (16) | 0.0462 (5) | |
| C4 | −0.1215 (4) | 0.31367 (7) | 0.99970 (17) | 0.0496 (5) | |
| H4A | −0.1415 | 0.3112 | 0.9187 | 0.059* | |
| C5 | −0.2659 (4) | 0.28142 (7) | 1.07243 (16) | 0.0468 (5) | |
| H5A | −0.3834 | 0.2573 | 1.0398 | 0.056* | |
| C6 | −0.2379 (4) | 0.28461 (6) | 1.19306 (15) | 0.0386 (4) | |
| C7 | −0.5631 (4) | 0.21854 (7) | 1.23010 (18) | 0.0468 (5) | |
| H7A | −0.6842 | 0.2334 | 1.1707 | 0.056* | |
| H7B | −0.6816 | 0.2075 | 1.2941 | 0.056* | |
| C8 | −0.4137 (4) | 0.17379 (6) | 1.17797 (15) | 0.0370 (4) | |
| C9 | −0.4894 (4) | 0.09348 (7) | 1.08006 (17) | 0.0507 (5) | |
| H9A | −0.2910 | 0.0966 | 1.0615 | 0.061* | |
| H9B | −0.5912 | 0.0880 | 1.0071 | 0.061* | |
| C10 | −0.5293 (4) | 0.04881 (7) | 1.15846 (17) | 0.0459 (5) | |
| C11 | −0.7149 (5) | 0.01174 (9) | 1.1278 (2) | 0.0726 (7) | |
| H11A | −0.8146 | 0.0140 | 1.0574 | 0.087* | |
| C12 | −0.7554 (7) | −0.02898 (10) | 1.2005 (3) | 0.0908 (9) | |
| H12A | −0.8836 | −0.0536 | 1.1788 | 0.109* | |
| C13 | −0.6107 (7) | −0.03343 (10) | 1.3027 (3) | 0.0872 (9) | |
| H13A | −0.6395 | −0.0608 | 1.3514 | 0.105* | |
| C14 | −0.4209 (6) | 0.00298 (11) | 1.3337 (2) | 0.0822 (8) | |
| H14A | −0.3190 | 0.0001 | 1.4034 | 0.099* | |
| C15 | −0.3801 (5) | 0.04402 (9) | 1.2619 (2) | 0.0640 (6) | |
| H15A | −0.2512 | 0.0685 | 1.2838 | 0.077* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N | 0.0327 (9) | 0.0426 (9) | 0.0543 (10) | 0.0032 (7) | 0.0025 (7) | −0.0059 (7) |
| Cl1 | 0.1088 (5) | 0.0673 (4) | 0.0393 (3) | −0.0261 (3) | −0.0006 (3) | −0.0106 (2) |
| Cl2 | 0.0869 (5) | 0.0663 (4) | 0.0604 (3) | −0.0168 (3) | 0.0117 (3) | 0.0131 (3) |
| O1 | 0.0517 (8) | 0.0405 (7) | 0.0479 (7) | −0.0087 (6) | 0.0045 (6) | −0.0019 (6) |
| O2 | 0.0307 (7) | 0.0527 (8) | 0.0764 (10) | 0.0030 (6) | 0.0010 (6) | −0.0051 (7) |
| C1 | 0.0511 (11) | 0.0370 (10) | 0.0376 (9) | −0.0001 (8) | 0.0002 (8) | −0.0071 (8) |
| C2 | 0.0539 (12) | 0.0377 (10) | 0.0492 (11) | −0.0072 (9) | 0.0011 (9) | −0.0065 (8) |
| C3 | 0.0506 (11) | 0.0407 (10) | 0.0475 (11) | 0.0004 (9) | 0.0053 (9) | 0.0029 (9) |
| C4 | 0.0584 (13) | 0.0515 (12) | 0.0388 (10) | 0.0030 (10) | −0.0048 (9) | 0.0002 (9) |
| C5 | 0.0488 (11) | 0.0439 (11) | 0.0478 (11) | −0.0042 (9) | −0.0083 (9) | −0.0046 (9) |
| C6 | 0.0394 (10) | 0.0320 (9) | 0.0445 (10) | 0.0029 (8) | 0.0014 (8) | −0.0020 (8) |
| C7 | 0.0377 (10) | 0.0403 (10) | 0.0626 (12) | −0.0041 (8) | 0.0075 (9) | −0.0021 (9) |
| C8 | 0.0329 (10) | 0.0354 (9) | 0.0428 (10) | 0.0010 (7) | 0.0025 (7) | 0.0057 (7) |
| C9 | 0.0542 (13) | 0.0478 (12) | 0.0501 (11) | 0.0018 (9) | 0.0049 (9) | −0.0093 (9) |
| C10 | 0.0456 (11) | 0.0404 (10) | 0.0519 (11) | 0.0043 (9) | 0.0101 (9) | −0.0087 (9) |
| C11 | 0.0747 (17) | 0.0613 (15) | 0.0816 (17) | −0.0152 (13) | −0.0013 (13) | −0.0055 (13) |
| C12 | 0.098 (2) | 0.0560 (16) | 0.119 (3) | −0.0247 (15) | 0.020 (2) | −0.0025 (16) |
| C13 | 0.103 (2) | 0.0540 (16) | 0.105 (2) | 0.0148 (16) | 0.0368 (19) | 0.0151 (15) |
| C14 | 0.096 (2) | 0.0809 (19) | 0.0693 (17) | 0.0239 (17) | 0.0031 (14) | 0.0134 (15) |
| C15 | 0.0697 (15) | 0.0590 (14) | 0.0632 (14) | 0.0012 (11) | −0.0021 (12) | −0.0024 (11) |
Geometric parameters (Å, °)
| N—C8 | 1.329 (2) | C7—C8 | 1.517 (2) |
| N—C9 | 1.451 (2) | C7—H7A | 0.9700 |
| N—H0A | 0.83 (2) | C7—H7B | 0.9700 |
| Cl1—C1 | 1.7318 (18) | C9—C10 | 1.507 (3) |
| Cl2—C3 | 1.7448 (19) | C9—H9A | 0.9700 |
| O1—C6 | 1.363 (2) | C9—H9B | 0.9700 |
| O1—C7 | 1.420 (2) | C10—C11 | 1.372 (3) |
| O2—C8 | 1.225 (2) | C10—C15 | 1.377 (3) |
| C1—C2 | 1.376 (3) | C11—C12 | 1.385 (4) |
| C1—C6 | 1.394 (2) | C11—H11A | 0.9300 |
| C2—C3 | 1.377 (3) | C12—C13 | 1.353 (4) |
| C2—H2A | 0.9300 | C12—H12A | 0.9300 |
| C3—C4 | 1.369 (3) | C13—C14 | 1.373 (4) |
| C4—C5 | 1.383 (3) | C13—H13A | 0.9300 |
| C4—H4A | 0.9300 | C14—C15 | 1.386 (3) |
| C5—C6 | 1.383 (2) | C14—H14A | 0.9300 |
| C5—H5A | 0.9300 | C15—H15A | 0.9300 |
| C8—N—C9 | 123.59 (16) | O2—C8—N | 124.41 (16) |
| C8—N—H0A | 115.6 (14) | O2—C8—C7 | 121.43 (16) |
| C9—N—H0A | 120.7 (14) | N—C8—C7 | 114.16 (15) |
| C6—O1—C7 | 118.32 (14) | N—C9—C10 | 113.22 (16) |
| C2—C1—C6 | 121.47 (17) | N—C9—H9A | 108.9 |
| C2—C1—Cl1 | 119.52 (14) | C10—C9—H9A | 108.9 |
| C6—C1—Cl1 | 119.00 (14) | N—C9—H9B | 108.9 |
| C1—C2—C3 | 118.88 (17) | C10—C9—H9B | 108.9 |
| C1—C2—H2A | 120.6 | H9A—C9—H9B | 107.7 |
| C3—C2—H2A | 120.6 | C11—C10—C15 | 118.4 (2) |
| C4—C3—C2 | 121.07 (18) | C11—C10—C9 | 120.5 (2) |
| C4—C3—Cl2 | 119.33 (15) | C15—C10—C9 | 121.05 (19) |
| C2—C3—Cl2 | 119.60 (15) | C10—C11—C12 | 120.7 (3) |
| C3—C4—C5 | 119.63 (18) | C10—C11—H11A | 119.7 |
| C3—C4—H4A | 120.2 | C12—C11—H11A | 119.7 |
| C5—C4—H4A | 120.2 | C13—C12—C11 | 120.9 (3) |
| C6—C5—C4 | 120.84 (18) | C13—C12—H12A | 119.6 |
| C6—C5—H5A | 119.6 | C11—C12—H12A | 119.6 |
| C4—C5—H5A | 119.6 | C12—C13—C14 | 119.1 (3) |
| O1—C6—C5 | 125.67 (16) | C12—C13—H13A | 120.5 |
| O1—C6—C1 | 116.25 (15) | C14—C13—H13A | 120.5 |
| C5—C6—C1 | 118.09 (17) | C13—C14—C15 | 120.5 (3) |
| O1—C7—C8 | 112.50 (14) | C13—C14—H14A | 119.8 |
| O1—C7—H7A | 109.1 | C15—C14—H14A | 119.8 |
| C8—C7—H7A | 109.1 | C10—C15—C14 | 120.4 (2) |
| O1—C7—H7B | 109.1 | C10—C15—H15A | 119.8 |
| C8—C7—H7B | 109.1 | C14—C15—H15A | 119.8 |
| H7A—C7—H7B | 107.8 | ||
| C6—C1—C2—C3 | 0.3 (3) | C9—N—C8—O2 | 0.7 (3) |
| Cl1—C1—C2—C3 | 179.74 (15) | C9—N—C8—C7 | −178.70 (16) |
| C1—C2—C3—C4 | −1.4 (3) | O1—C7—C8—O2 | 3.5 (3) |
| C1—C2—C3—Cl2 | 178.90 (14) | O1—C7—C8—N | −177.05 (15) |
| C2—C3—C4—C5 | 1.3 (3) | C8—N—C9—C10 | 103.3 (2) |
| Cl2—C3—C4—C5 | −178.97 (15) | N—C9—C10—C11 | 113.8 (2) |
| C3—C4—C5—C6 | −0.1 (3) | N—C9—C10—C15 | −66.5 (3) |
| C7—O1—C6—C5 | −1.4 (2) | C15—C10—C11—C12 | 1.4 (3) |
| C7—O1—C6—C1 | 178.78 (15) | C9—C10—C11—C12 | −179.0 (2) |
| C4—C5—C6—O1 | 179.23 (17) | C10—C11—C12—C13 | −0.8 (4) |
| C4—C5—C6—C1 | −1.0 (3) | C11—C12—C13—C14 | −0.3 (4) |
| C2—C1—C6—O1 | −179.29 (16) | C12—C13—C14—C15 | 0.7 (4) |
| Cl1—C1—C6—O1 | 1.3 (2) | C11—C10—C15—C14 | −0.9 (3) |
| C2—C1—C6—C5 | 0.9 (3) | C9—C10—C15—C14 | 179.4 (2) |
| Cl1—C1—C6—C5 | −178.57 (14) | C13—C14—C15—C10 | −0.1 (4) |
| C6—O1—C7—C8 | 75.3 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N—H0A···O2i | 0.83 (2) | 2.06 (2) | 2.883 (2) | 169 (2) |
Symmetry codes: (i) x−1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CS2090).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Bruker (2005). SADABS and APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
- Li, Z.-B., Luo, Y.-H., Dong, W.-L., Li, J. & Zuo, H. (2008a). Acta Cryst. E64, o1610. [DOI] [PMC free article] [PubMed]
- Li, Z.-B., Zuo, H., Dong, W.-L., He, X.-Y. & Chen, Z.-B. (2008b). Acta Cryst. E64, o1609. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, New_Global_Publ_Block. DOI: 10.1107/S1600536808029371/cs2090sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808029371/cs2090Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report