Abstract
The title compound, C25H28O7, was prepared by the base-catalysed reaction of 3,4,5-trimethoxybenzaldehyde with cyclopentanone. The molecule has crystallographic twofold rotation symmetry and adopts an E-configuration about the central olefinic bonds. The two benzene rings and the central cyclopentanone ring are almost coplanar [dihedral angle = 4.7 (2)°].
Related literature
For background literature, see: Guilford et al. (1999 ▶); Xue et al. (2008 ▶); Wu et al. (2008 ▶); Das et al. (2008 ▶). For related crystal structures, see: Sun & Cui (2007 ▶); Du et al. (2007 ▶); Wei et al. (2008 ▶).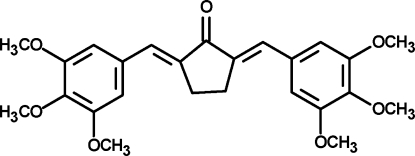
Experimental
Crystal data
C25H28O7
M r = 440.47
Monoclinic,

a = 18.573 (4) Å
b = 15.231 (3) Å
c = 8.8460 (18) Å
β = 113.99 (3)°
V = 2286.2 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 293 (2) K
0.30 × 0.20 × 0.20 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: ψ scan (North et al., 1968 ▶) T min = 0.973, T max = 0.982
2123 measured reflections
2058 independent reflections
1422 reflections with I > 2σ(I)
R int = 0.048
3 standard reflections every 200 reflections intensity decay: none
Refinement
R[F 2 > 2σ(F 2)] = 0.055
wR(F 2) = 0.164
S = 1.00
2058 reflections
146 parameters
H-atom parameters constrained
Δρmax = 0.21 e Å−3
Δρmin = −0.27 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius, 1989 ▶); cell refinement: CAD-4 Software; data reduction: XCAD4 (Harms & Wocadlo, 1995 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808029474/fj2151sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808029474/fj2151Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected torsion angles (°).
| O1—C1—C2—C4 | −2.7 (3) |
| C1—C2—C4—C5 | 178.0 (2) |
| C2—C4—C5—C6 | 179.6 (3) |
| C13—O4—C7—C6 | 1.8 (4) |
| C12—O3—C8—C7 | 86.9 (3) |
| C11—O2—C9—C8 | 174.8 (3) |
Acknowledgments
This project was supported by the Jiangsu Planned Projects for Postdoctoral Research Funds (No.0701001B) and the Foundation of Taishan University.
supplementary crystallographic information
Comment
Bis(arylmethylidene)cycloalkanones are widely used as building blocks for the synthesis of biologically active heterocycles (Guilford et al., 1999), and reported to exhibit promising two-photon absorption (TPA) property (Xue et al., 2008; Wu et al., 2008). Moreover, it has been reported that some compounds containing the 3-(3,4,5-trimethoxyphenyl)-2-propenoyl group displayed potent multidrug resistance (MDR) reversal properties in cancer chemotherapy. In particular, 2,5-bis(3,4,5-trimethoxybenzylidene)cyclopentanone was 31 times more potent than verapamil as a MDR revertant (Das et al., 2008). In this contribution, we report the crystal structure of the title compound, 2,5-bis(3,4,5-trimethoxybenzylidene) cyclopentanone, Fig.1.
The molecule possesses normal geometric parameters and adopts an E configuration about the central olefinic bonds (Fig. 1). The cyclopentanone ring and the two benzene rings are almost coplanar which allows conjugation. Among the six methoxy groups, only O3/C12 and O3A/C12A deviate from the molecule mean plane on the opposite side, the others are nearly coplanar with their attached benzene ring (Table 1).
Similar structures have been observed in the related substituted cyclohexanone and cyclopentanone analogues reported by Sun & Cui (2007), Du et al. (2007) and Wei et al. (2008).
Experimental
The title compound was synthesized from cyclopenthexanone and 3,4,5-trimethoxybenzaldehyde as reported (Sun et al., 2007).Yellow block crystals suitable for an X-ray structural analysis were obtained by slowly evaporating an ethanol solution at room temperature.
Refinement
All H atoms were initially located in a difference Fourier map. The methyl H atoms were then constrained to an ideal geometry with C—H distances of 0.96 Å and Uiso(H) = 1.5Ueq(C). All other H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with C—H distances 0.93–0.97 Å and Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
View of the molecule of (I) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms are shown as small spheres of arbitrary radii. Suffix A corresponds to symmetry code (-x+1, y, -z+1/2).
Crystal data
| C25H28O7 | F(000) = 936 |
| Mr = 440.47 | Dx = 1.280 Mg m−3 |
| Monoclinic, C2/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -C 2yc | Cell parameters from 25 reflections |
| a = 18.573 (4) Å | θ = 10–13° |
| b = 15.231 (3) Å | µ = 0.09 mm−1 |
| c = 8.8460 (18) Å | T = 293 K |
| β = 113.99 (3)° | Block, yellow |
| V = 2286.2 (10) Å3 | 0.30 × 0.20 × 0.20 mm |
| Z = 4 |
Data collection
| Enraf–Nonius CAD-4 diffractometer | 1422 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.048 |
| graphite | θmax = 25.2°, θmin = 1.8° |
| ω/2θ scans | h = −22→20 |
| Absorption correction: ψ scan (North et al., 1968) | k = 0→18 |
| Tmin = 0.973, Tmax = 0.982 | l = 0→10 |
| 2123 measured reflections | 3 standard reflections every 200 reflections |
| 2058 independent reflections | intensity decay: none |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.055 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.164 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.080P)2 + 2.P] where P = (Fo2 + 2Fc2)/3 |
| 2058 reflections | (Δ/σ)max < 0.001 |
| 146 parameters | Δρmax = 0.21 e Å−3 |
| 0 restraints | Δρmin = −0.27 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.5000 | 0.43398 (16) | 0.2500 | 0.0707 (8) | |
| C1 | 0.5000 | 0.3537 (2) | 0.2500 | 0.0510 (8) | |
| C2 | 0.47174 (14) | 0.29645 (15) | 0.3510 (3) | 0.0480 (6) | |
| O2 | 0.36310 (14) | 0.07554 (11) | 0.6941 (3) | 0.0806 (7) | |
| O3 | 0.32676 (10) | 0.18198 (11) | 0.89316 (19) | 0.0592 (5) | |
| C3 | 0.47864 (15) | 0.20225 (14) | 0.3086 (3) | 0.0512 (6) | |
| H3A | 0.4268 | 0.1760 | 0.2546 | 0.061* | |
| H3B | 0.5085 | 0.1690 | 0.4080 | 0.061* | |
| O4 | 0.33734 (12) | 0.35568 (11) | 0.8758 (2) | 0.0683 (6) | |
| C4 | 0.44589 (14) | 0.33133 (16) | 0.4587 (3) | 0.0517 (6) | |
| H4A | 0.4489 | 0.3922 | 0.4665 | 0.062* | |
| C5 | 0.41371 (14) | 0.28904 (15) | 0.5662 (3) | 0.0473 (6) | |
| C6 | 0.39179 (15) | 0.34335 (15) | 0.6677 (3) | 0.0526 (6) | |
| H6A | 0.3979 | 0.4038 | 0.6648 | 0.063* | |
| C7 | 0.36086 (14) | 0.30734 (16) | 0.7728 (3) | 0.0504 (6) | |
| C8 | 0.35313 (13) | 0.21799 (15) | 0.7816 (3) | 0.0478 (6) | |
| C9 | 0.37410 (15) | 0.16327 (15) | 0.6797 (3) | 0.0542 (6) | |
| C10 | 0.40400 (15) | 0.19887 (16) | 0.5729 (3) | 0.0555 (6) | |
| H10A | 0.4177 | 0.1620 | 0.5050 | 0.067* | |
| C11 | 0.3772 (3) | 0.01797 (19) | 0.5835 (5) | 0.1075 (13) | |
| H11A | 0.3679 | −0.0414 | 0.6071 | 0.161* | |
| H11B | 0.4309 | 0.0240 | 0.5965 | 0.161* | |
| H11C | 0.3425 | 0.0323 | 0.4718 | 0.161* | |
| C12 | 0.24333 (17) | 0.1766 (2) | 0.8306 (4) | 0.0739 (8) | |
| H12A | 0.2284 | 0.1507 | 0.9126 | 0.111* | |
| H12B | 0.2239 | 0.1410 | 0.7326 | 0.111* | |
| H12C | 0.2212 | 0.2345 | 0.8044 | 0.111* | |
| C13 | 0.3430 (2) | 0.44758 (18) | 0.8706 (4) | 0.0886 (11) | |
| H13A | 0.3249 | 0.4735 | 0.9478 | 0.133* | |
| H13B | 0.3111 | 0.4681 | 0.7611 | 0.133* | |
| H13C | 0.3969 | 0.4639 | 0.8995 | 0.133* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.118 (2) | 0.0480 (15) | 0.0791 (18) | 0.000 | 0.0745 (17) | 0.000 |
| C1 | 0.074 (2) | 0.048 (2) | 0.0498 (18) | 0.000 | 0.0438 (17) | 0.000 |
| C2 | 0.0656 (14) | 0.0492 (13) | 0.0459 (12) | −0.0033 (10) | 0.0397 (11) | −0.0017 (10) |
| O2 | 0.1371 (18) | 0.0460 (11) | 0.0952 (15) | −0.0030 (10) | 0.0846 (14) | 0.0043 (9) |
| O3 | 0.0752 (12) | 0.0681 (11) | 0.0527 (10) | −0.0055 (9) | 0.0450 (9) | 0.0105 (8) |
| C3 | 0.0719 (16) | 0.0494 (13) | 0.0494 (13) | −0.0013 (11) | 0.0422 (12) | 0.0001 (10) |
| O4 | 0.1063 (14) | 0.0569 (11) | 0.0782 (12) | −0.0088 (9) | 0.0749 (12) | −0.0123 (9) |
| C4 | 0.0734 (16) | 0.0497 (14) | 0.0515 (13) | 0.0006 (11) | 0.0452 (12) | 0.0011 (10) |
| C5 | 0.0631 (14) | 0.0482 (13) | 0.0463 (12) | −0.0029 (10) | 0.0384 (11) | −0.0005 (10) |
| C6 | 0.0770 (16) | 0.0447 (13) | 0.0575 (14) | −0.0010 (11) | 0.0493 (13) | 0.0004 (10) |
| C7 | 0.0666 (15) | 0.0550 (14) | 0.0474 (12) | −0.0045 (11) | 0.0414 (12) | −0.0058 (10) |
| C8 | 0.0585 (14) | 0.0542 (14) | 0.0433 (12) | −0.0037 (11) | 0.0336 (11) | 0.0049 (10) |
| C9 | 0.0779 (17) | 0.0449 (13) | 0.0564 (14) | −0.0038 (11) | 0.0443 (13) | 0.0040 (11) |
| C10 | 0.0807 (17) | 0.0506 (14) | 0.0556 (14) | 0.0013 (12) | 0.0487 (13) | −0.0018 (11) |
| C11 | 0.188 (4) | 0.0482 (18) | 0.130 (3) | −0.005 (2) | 0.109 (3) | −0.0127 (18) |
| C12 | 0.0775 (19) | 0.085 (2) | 0.0795 (19) | −0.0150 (15) | 0.0526 (16) | 0.0035 (16) |
| C13 | 0.149 (3) | 0.0538 (17) | 0.108 (2) | −0.0021 (18) | 0.098 (2) | −0.0120 (16) |
Geometric parameters (Å, °)
| O1—C1 | 1.222 (4) | C5—C6 | 1.398 (3) |
| C1—C2 | 1.489 (3) | C6—C7 | 1.389 (3) |
| C1—C2i | 1.489 (3) | C6—H6A | 0.9300 |
| C2—C4 | 1.339 (3) | C7—C8 | 1.374 (3) |
| C2—C3 | 1.501 (3) | C8—C9 | 1.395 (3) |
| O2—C9 | 1.365 (3) | C9—C10 | 1.386 (3) |
| O2—C11 | 1.415 (3) | C10—H10A | 0.9300 |
| O3—C8 | 1.381 (2) | C11—H11A | 0.9600 |
| O3—C12 | 1.420 (3) | C11—H11B | 0.9600 |
| C3—C3i | 1.541 (4) | C11—H11C | 0.9600 |
| C3—H3A | 0.9700 | C12—H12A | 0.9600 |
| C3—H3B | 0.9700 | C12—H12B | 0.9600 |
| O4—C7 | 1.373 (3) | C12—H12C | 0.9600 |
| O4—C13 | 1.406 (3) | C13—H13A | 0.9600 |
| C4—C5 | 1.462 (3) | C13—H13B | 0.9600 |
| C4—H4A | 0.9300 | C13—H13C | 0.9600 |
| C5—C10 | 1.390 (3) | ||
| O1—C1—C2 | 125.87 (13) | C7—C8—O3 | 120.62 (19) |
| O1—C1—C2i | 125.87 (13) | C7—C8—C9 | 119.49 (19) |
| C2—C1—C2i | 108.3 (3) | O3—C8—C9 | 119.9 (2) |
| C4—C2—C1 | 120.7 (2) | O2—C9—C10 | 124.2 (2) |
| C4—C2—C3 | 130.4 (2) | O2—C9—C8 | 115.7 (2) |
| C1—C2—C3 | 108.86 (18) | C10—C9—C8 | 120.1 (2) |
| C9—O2—C11 | 117.7 (2) | C9—C10—C5 | 120.7 (2) |
| C8—O3—C12 | 113.10 (19) | C9—C10—H10A | 119.7 |
| C2—C3—C3i | 106.72 (11) | C5—C10—H10A | 119.7 |
| C2—C3—H3A | 110.4 | O2—C11—H11A | 109.5 |
| C3i—C3—H3A | 110.4 | O2—C11—H11B | 109.5 |
| C2—C3—H3B | 110.4 | H11A—C11—H11B | 109.5 |
| C3i—C3—H3B | 110.4 | O2—C11—H11C | 109.5 |
| H3A—C3—H3B | 108.6 | H11A—C11—H11C | 109.5 |
| C7—O4—C13 | 117.66 (19) | H11B—C11—H11C | 109.5 |
| C2—C4—C5 | 130.4 (2) | O3—C12—H12A | 109.5 |
| C2—C4—H4A | 114.8 | O3—C12—H12B | 109.5 |
| C5—C4—H4A | 114.8 | H12A—C12—H12B | 109.5 |
| C10—C5—C6 | 118.77 (19) | O3—C12—H12C | 109.5 |
| C10—C5—C4 | 123.87 (19) | H12A—C12—H12C | 109.5 |
| C6—C5—C4 | 117.4 (2) | H12B—C12—H12C | 109.5 |
| C7—C6—C5 | 120.3 (2) | O4—C13—H13A | 109.5 |
| C7—C6—H6A | 119.9 | O4—C13—H13B | 109.5 |
| C5—C6—H6A | 119.9 | H13A—C13—H13B | 109.5 |
| O4—C7—C8 | 115.13 (18) | O4—C13—H13C | 109.5 |
| O4—C7—C6 | 124.2 (2) | H13A—C13—H13C | 109.5 |
| C8—C7—C6 | 120.7 (2) | H13B—C13—H13C | 109.5 |
| O1—C1—C2—C4 | −2.7 (3) | O4—C7—C8—O3 | −2.8 (3) |
| C2i—C1—C2—C4 | 177.3 (3) | C6—C7—C8—O3 | 176.1 (2) |
| O1—C1—C2—C3 | 177.49 (12) | O4—C7—C8—C9 | 178.8 (2) |
| C2i—C1—C2—C3 | −2.51 (12) | C6—C7—C8—C9 | −2.2 (4) |
| C4—C2—C3—C3i | −173.4 (3) | C12—O3—C8—C7 | 86.9 (3) |
| C1—C2—C3—C3i | 6.4 (3) | C12—O3—C8—C9 | −94.7 (3) |
| C1—C2—C4—C5 | 178.0 (2) | C11—O2—C9—C10 | −5.3 (4) |
| C3—C2—C4—C5 | −2.2 (5) | C11—O2—C9—C8 | 174.8 (3) |
| C2—C4—C5—C10 | −0.3 (4) | C7—C8—C9—O2 | −178.8 (2) |
| C2—C4—C5—C6 | 179.6 (3) | O3—C8—C9—O2 | 2.9 (4) |
| C10—C5—C6—C7 | 0.0 (4) | C7—C8—C9—C10 | 1.3 (4) |
| C4—C5—C6—C7 | −180.0 (2) | O3—C8—C9—C10 | −177.0 (2) |
| C13—O4—C7—C8 | −179.2 (3) | O2—C9—C10—C5 | −179.7 (2) |
| C13—O4—C7—C6 | 1.8 (4) | C8—C9—C10—C5 | 0.2 (4) |
| C5—C6—C7—O4 | −179.6 (2) | C6—C5—C10—C9 | −0.8 (4) |
| C5—C6—C7—C8 | 1.6 (4) | C4—C5—C10—C9 | 179.1 (2) |
Symmetry codes: (i) −x+1, y, −z+1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FJ2151).
References
- Das, U., Kawase, M., Sakagami, H., Ideo, A., Shimada, J., Molnar, J., Barath, Z., Bata, Z. & Dimmock, J. R. (2008). Bioorg. Med. Chem.15, 3373–3380. [DOI] [PubMed]
- Du, Z.-Y., Zhang, K. & Ng, S. W. (2007). Acta Cryst. E63, o2595–o2596.
- Enraf–Nonius (1989). CAD-4 Software Enraf–Nonius, Delft, The Netherlands.
- Guilford, W. J., Shaw, K. J., Dallas, J. L., Koovakkat, S., Lee, W., Liang, A., Light, D. R., McCarrick, M. A., Whitlow, M., Ye, B. & Morrissey, M. M. (1999). J. Med. Chem.42, 5415–5425. [DOI] [PubMed]
- Harms, K. & Wocadlo, S. (1995). XCAD4 University of Marburg, Germany.
- North, A. C. T., Phillips, D. C. & Mathews, F. S. (1968). Acta Cryst. A24, 351–359.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Sun, Y.-F. & Cui, Y.-P. (2007). Acta Cryst. E63, o1932–o1933.
- Wei, J., Liang, G., Gai, Y. & Lu, J. (2008). Acta Cryst. E64, o1755. [DOI] [PMC free article] [PubMed]
- Wu, J., Shi, M. Q., Zhao, Y. X. & Wu, F. P. (2008). Dyes Pigments, 76, 690-695.
- Xue, J. Q., Zhao, Y. X., Wu, J. & Wu, F. P. (2008). J. Photochem. Photobiol. A: Chem.195, 261–266.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808029474/fj2151sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808029474/fj2151Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



