Abstract
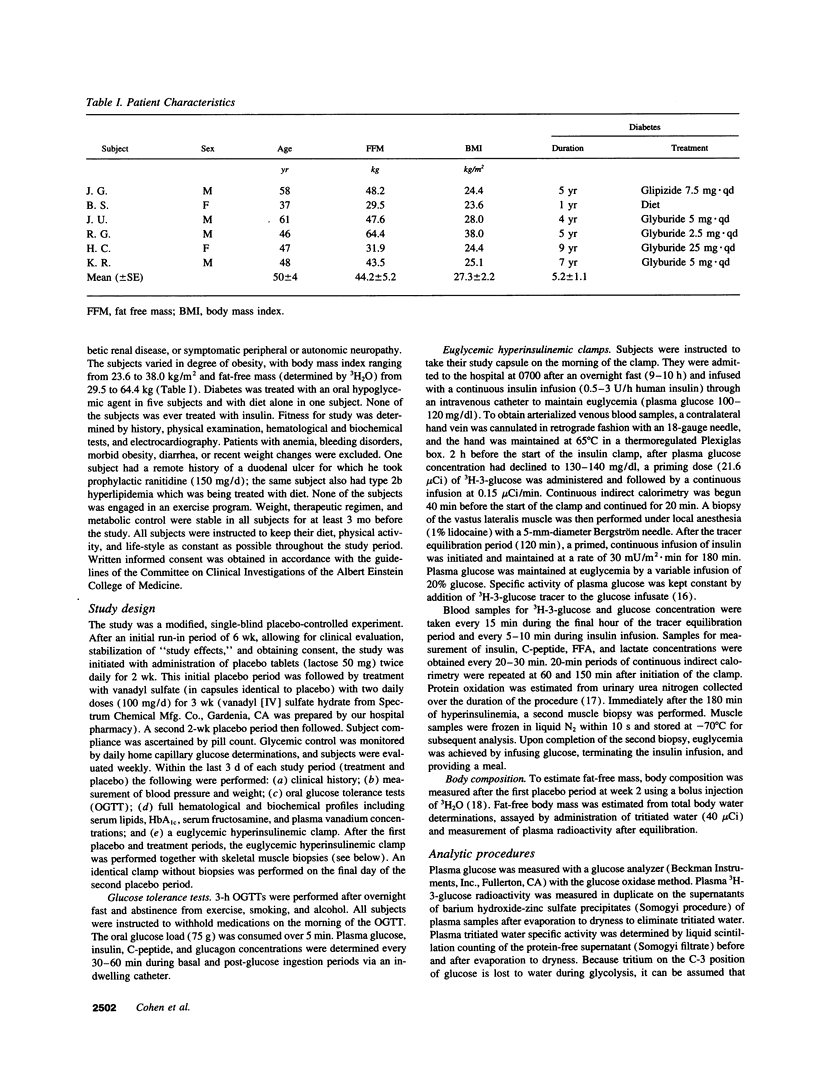
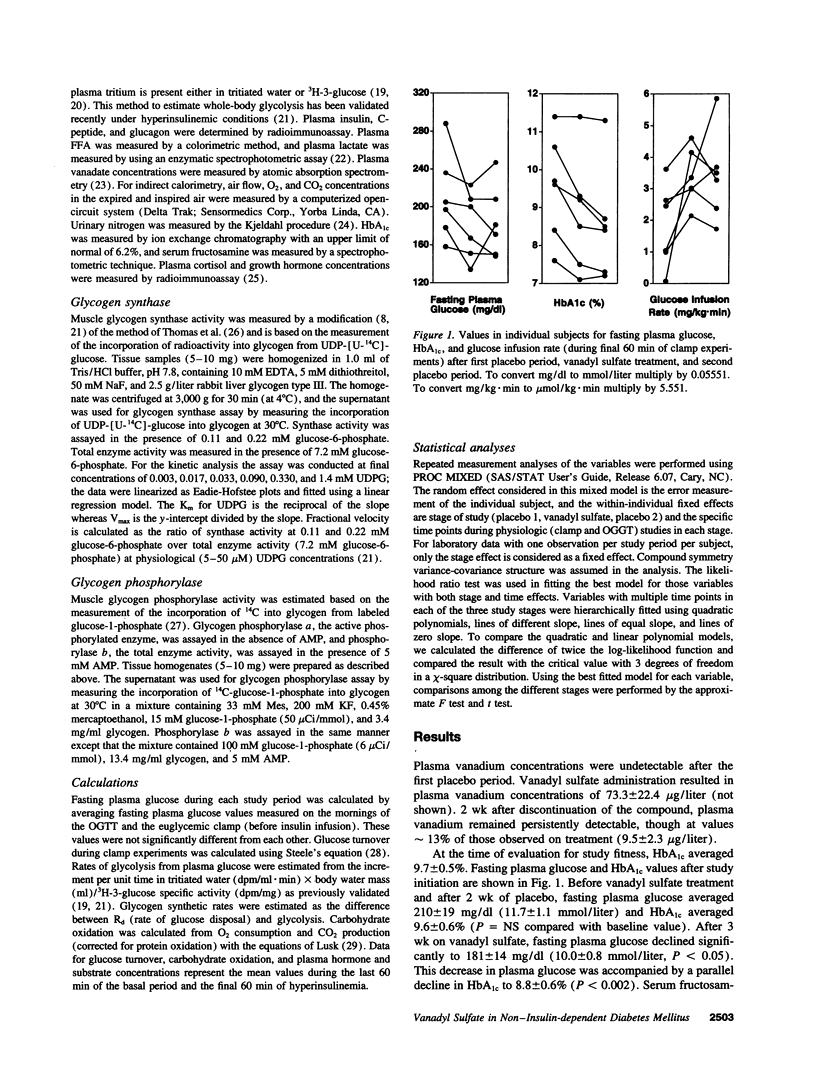
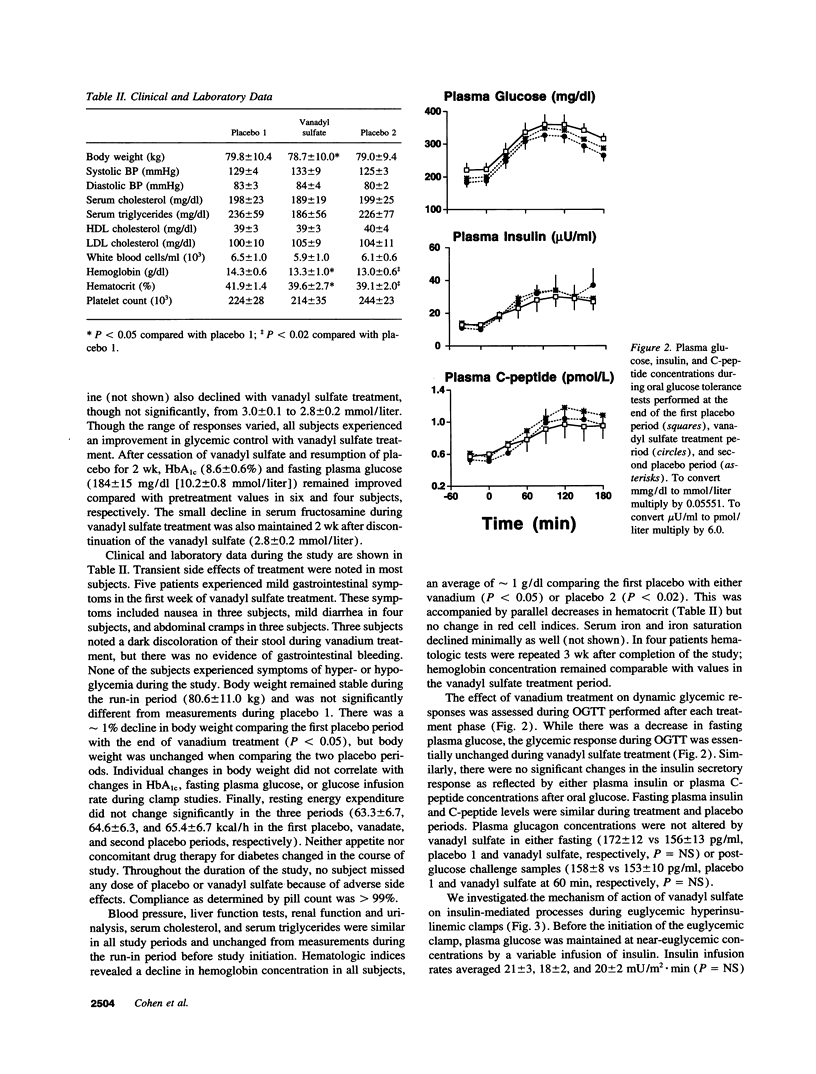
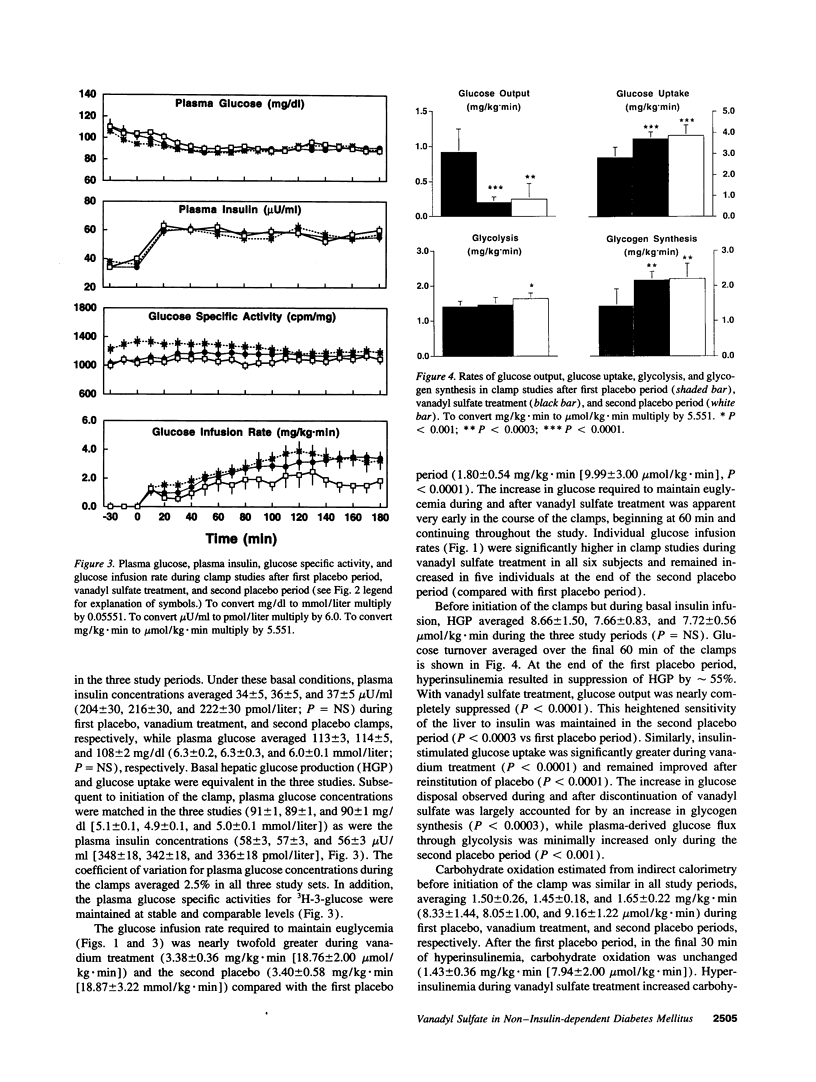
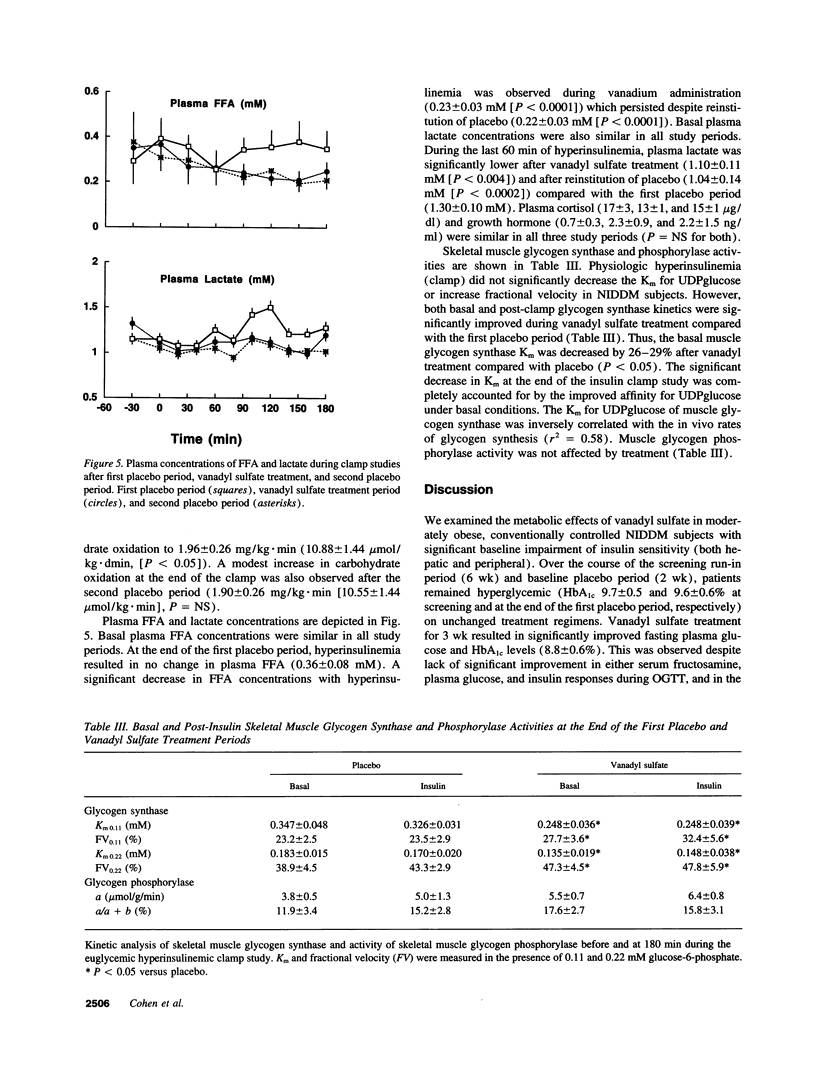
We examined the in vivo metabolic effects of vanadyl sulfate (VS) in non-insulin-dependent diabetes mellitus (NIDDM). Six NIDDM subjects treated with diet and/or sulfonylureas were examined at the end of three consecutive periods: placebo for 2 wk, VS (100 mg/d) for 3 wk, and placebo for 2 wk. Euglycemic hyperinsulinemic (30 mU/m2.min) clamps and oral glucose tolerance tests were performed at the end of each study period. Glycemic control at baseline was poor (fasting plasma glucose 210 +/- 19 mg/dl; HbA1c 9.6 +/- 0.6%) and improved after treatment (181 +/- 14 mg/dl [P < 0.05], 8.8 +/- 0.6%, [P < 0.002]); fasting and post-glucose tolerance test plasma insulin concentrations were unchanged. After VS, the glucose infusion rate during the clamp was increased (by approximately 88%, from 1.80 to 3.38 mg/kg.min, P < 0.0001). This improvement was due to both enhanced insulin-mediated stimulation of glucose uptake (rate of glucose disposal [Rd], +0.89 mg/kg.min) and increased inhibition of HGP (-0.74 mg/kg.min) (P < 0.0001 for both). Increased insulin-stimulated glycogen synthesis (+0.74 mg/kg.min, P < 0.0003) accounted for > 80% of the increased Rd after VS, and the improvement in insulin sensitivity was maintained after the second placebo period. The Km of skeletal muscle glycogen synthase was lowered by approximately 30% after VS treatment (P < 0.05). These results indicate that 3 wk of treatment with VS improves hepatic and peripheral insulin sensitivity in insulin-resistant NIDDM humans. These effects were sustained for up to 2 wk after discontinuation of VS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blondel O., Bailbe D., Portha B. In vivo insulin resistance in streptozotocin-diabetic rats--evidence for reversal following oral vanadate treatment. Diabetologia. 1989 Mar;32(3):185–190. doi: 10.1007/BF00265092. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983 Jun;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brichard S. M., Desbuquois B., Girard J. Vanadate treatment of diabetic rats reverses the impaired expression of genes involved in hepatic glucose metabolism: effects on glycolytic and gluconeogenic enzymes, and on glucose transporter GLUT2. Mol Cell Endocrinol. 1993 Feb;91(1-2):91–97. doi: 10.1016/0303-7207(93)90259-m. [DOI] [PubMed] [Google Scholar]
- Brichard S. M., Okitolonda W., Henquin J. C. Long term improvement of glucose homeostasis by vanadate treatment in diabetic rats. Endocrinology. 1988 Oct;123(4):2048–2053. doi: 10.1210/endo-123-4-2048. [DOI] [PubMed] [Google Scholar]
- Brichard S. M., Pottier A. M., Henquin J. C. Long term improvement of glucose homeostasis by vanadate in obese hyperinsulinemic fa/fa rats. Endocrinology. 1989 Nov;125(5):2510–2516. doi: 10.1210/endo-125-5-2510. [DOI] [PubMed] [Google Scholar]
- Butler P. C., Kryshak E. J., Schwenk W. F., Haymond M. W., Rizza R. A. Hepatic and extrahepatic responses to insulin in NIDDM and nondiabetic humans. Assessment in absence of artifact introduced by tritiated nonglucose contaminants. Diabetes. 1990 Feb;39(2):217–225. doi: 10.2337/diab.39.2.217. [DOI] [PubMed] [Google Scholar]
- CURRAN G. L. Effect of certain transition group elements on hepatic synthesis of cholesterol in the rat. J Biol Chem. 1954 Oct;210(2):765–770. [PubMed] [Google Scholar]
- Cam M. C., Pederson R. A., Brownsey R. W., McNeill J. H. Long-term effectiveness of oral vanadyl sulphate in streptozotocin-diabetic rats. Diabetologia. 1993 Mar;36(3):218–224. doi: 10.1007/BF00399953. [DOI] [PubMed] [Google Scholar]
- Clausen T., Andersen T. L., Stürup-Johansen M., Petkova O. The relationship between the transport of glucose and cations across cell membranes in isolated tissues. XI. The effect of vanadate on 45Ca-efflux and sugar transport in adipose tissue and skeletal muscle. Biochim Biophys Acta. 1981 Aug 20;646(2):261–267. doi: 10.1016/0005-2736(81)90332-1. [DOI] [PubMed] [Google Scholar]
- Cordera R., Andraghetti G., DeFronzo R. A., Rossetti L. Effect of in vivo vanadate treatment on insulin receptor tyrosine kinase activity in partially pancreatectomized diabetic rats. Endocrinology. 1990 Apr;126(4):2177–2183. doi: 10.1210/endo-126-4-2177. [DOI] [PubMed] [Google Scholar]
- D'Onofrio F., Le M. Q., Chiasson J. L., Srivastava A. K. Activation of mitogen activated protein (MAP) kinases by vanadate is independent of insulin receptor autophosphorylation. FEBS Lett. 1994 Mar 7;340(3):269–275. doi: 10.1016/0014-5793(94)80152-5. [DOI] [PubMed] [Google Scholar]
- DIMOND E. G., CARAVACA J., BENCHIMOL A. Vanadium, Excretion, toxicity, lipid effect in man. Am J Clin Nutr. 1963 Jan;12:49–53. doi: 10.1093/ajcn/12.1.49. [DOI] [PubMed] [Google Scholar]
- Dai S., McNeill J. H. One-year treatment of non-diabetic and streptozotocin-diabetic rats with vanadyl sulphate did not alter blood pressure or haematological indices. Pharmacol Toxicol. 1994 Feb;74(2):110–115. doi: 10.1111/j.1600-0773.1994.tb01084.x. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Simonson D., Ferrannini E. Hepatic and peripheral insulin resistance: a common feature of type 2 (non-insulin-dependent) and type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1982 Oct;23(4):313–319. doi: 10.1007/BF00253736. [DOI] [PubMed] [Google Scholar]
- Domingo J. L., Gomez M., Llobet J. M., Corbella J., Keen C. L. Oral vanadium administration to streptozotocin-diabetic rats has marked negative side-effects which are independent of the form of vanadium used. Toxicology. 1991 Mar 11;66(3):279–287. doi: 10.1016/0300-483x(91)90199-b. [DOI] [PubMed] [Google Scholar]
- Dubyak G. R., Kleinzeller A. The insulin-mimetic effects of vanadate in isolated rat adipocytes. Dissociation from effects of vanadate as a (Na+-K+)ATPase inhibitor. J Biol Chem. 1980 Jun 10;255(11):5306–5312. [PubMed] [Google Scholar]
- Ferrannini E. The theoretical bases of indirect calorimetry: a review. Metabolism. 1988 Mar;37(3):287–301. doi: 10.1016/0026-0495(88)90110-2. [DOI] [PubMed] [Google Scholar]
- Finegood D. T., Bergman R. N., Vranic M. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 1987 Aug;36(8):914–924. doi: 10.2337/diab.36.8.914. [DOI] [PubMed] [Google Scholar]
- Heyliger C. E., Tahiliani A. G., McNeill J. H. Effect of vanadate on elevated blood glucose and depressed cardiac performance of diabetic rats. Science. 1985 Mar 22;227(4693):1474–1477. doi: 10.1126/science.3156405. [DOI] [PubMed] [Google Scholar]
- Kubena L. F., Harvey R. B., Fletcher O. J., Phillips T. D., Mollenhauer H. H., Witzel D. A., Heidelbaugh N. D. Toxicity of ochratoxin A and vanadium to growing chicks. Poult Sci. 1985 Apr;64(4):620–628. doi: 10.3382/ps.0640620. [DOI] [PubMed] [Google Scholar]
- Madsen K. L., Porter V. M., Fedorak R. N. Oral vanadate reduces Na(+)-dependent glucose transport in rat small intestine. Diabetes. 1993 Aug;42(8):1126–1132. doi: 10.2337/diab.42.8.1126. [DOI] [PubMed] [Google Scholar]
- Malabu U. H., Dryden S., McCarthy H. D., Kilpatrick A., Williams G. Effects of chronic vanadate administration in the STZ-induced diabetic rat. The antihyperglycemic action of vanadate is attributable entirely to its suppression of feeding. Diabetes. 1994 Jan;43(1):9–15. doi: 10.2337/diab.43.1.9. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Farfel Z., Sack J., Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J Biol Chem. 1987 May 15;262(14):6658–6662. [PubMed] [Google Scholar]
- Miralpeix M., Decaux J. F., Kahn A., Bartrons R. Vanadate induction of L-type pyruvate kinase mRNA in adult rat hepatocytes in primary culture. Diabetes. 1991 Apr;40(4):462–464. doi: 10.2337/diab.40.4.462. [DOI] [PubMed] [Google Scholar]
- Mongold J. J., Cros G. H., Vian L., Tep A., Ramanadham S., Siou G., Diaz J., McNeill J. H., Serrano J. J. Toxicological aspects of vanadyl sulphate on diabetic rats: effects on vanadium levels and pancreatic B-cell morphology. Pharmacol Toxicol. 1990 Sep;67(3):192–198. doi: 10.1111/j.1600-0773.1990.tb00812.x. [DOI] [PubMed] [Google Scholar]
- Owusu-Yaw J., Cohen M. D., Fernando S. Y., Wei C. I. An assessment of the genotoxicity of vanadium. Toxicol Lett. 1990 Feb;50(2-3):327–336. doi: 10.1016/0378-4274(90)90026-i. [DOI] [PubMed] [Google Scholar]
- Pederson R. A., Ramanadham S., Buchan A. M., McNeill J. H. Long-term effects of vanadyl treatment on streptozocin-induced diabetes in rats. Diabetes. 1989 Nov;38(11):1390–1395. doi: 10.2337/diab.38.11.1390. [DOI] [PubMed] [Google Scholar]
- Ramanadham S., Mongold J. J., Brownsey R. W., Cros G. H., McNeill J. H. Oral vanadyl sulfate in treatment of diabetes mellitus in rats. Am J Physiol. 1989 Sep;257(3 Pt 2):H904–H911. doi: 10.1152/ajpheart.1989.257.3.H904. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Farrace S., Choi S. B., Giaccari A., Sloan L., Frontoni S., Katz M. S. Multiple metabolic effects of CGRP in conscious rats: role of glycogen synthase and phosphorylase. Am J Physiol. 1993 Jan;264(1 Pt 1):E1–10. doi: 10.1152/ajpendo.1993.264.1.E1. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A., Klein-Robbenhaar E., Vogel L. R. Insulinomimetic properties of trace elements and characterization of their in vivo mode of action. Diabetes. 1990 Oct;39(10):1243–1250. doi: 10.2337/diab.39.10.1243. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A. Relative contribution of glycogen synthesis and glycolysis to insulin-mediated glucose uptake. A dose-response euglycemic clamp study in normal and diabetic rats. J Clin Invest. 1990 Jun;85(6):1785–1792. doi: 10.1172/JCI114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lauglin M. R. Correction of chronic hyperglycemia with vanadate, but not with phlorizin, normalizes in vivo glycogen repletion and in vitro glycogen synthase activity in diabetic skeletal muscle. J Clin Invest. 1989 Sep;84(3):892–899. doi: 10.1172/JCI114250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti L., Lee Y. T., Ruiz J., Aldridge S. C., Shamoon H., Boden G. Quantitation of glycolysis and skeletal muscle glycogen synthesis in humans. Am J Physiol. 1993 Nov;265(5 Pt 1):E761–E769. doi: 10.1152/ajpendo.1993.265.5.E761. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Shulman G. I., Zawalich W., DeFronzo R. A. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest. 1987 Oct;80(4):1037–1044. doi: 10.1172/JCI113157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sabbioni E., Pozzi G., Pintar A., Casella L., Garattini S. Cellular retention, cytotoxicity and morphological transformation by vanadium(IV) and vanadium(V) in BALB/3T3 cell lines. Carcinogenesis. 1991 Jan;12(1):47–52. doi: 10.1093/carcin/12.1.47. [DOI] [PubMed] [Google Scholar]
- Shechter Y. Insulin-mimetic effects of vanadate. Possible implications for future treatment of diabetes. Diabetes. 1990 Jan;39(1):1–5. doi: 10.2337/diacare.39.1.1. [DOI] [PubMed] [Google Scholar]
- Sotsky M. J., Shilo S., Shamoon H. Regulation of counterregulatory hormone secretion in man during exercise and hypoglycemia. J Clin Endocrinol Metab. 1989 Jan;68(1):9–16. doi: 10.1210/jcem-68-1-9. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Schlender K. K., Larner J. A rapid filter paper assay for UDPglucose-glycogen glucosyltransferase, including an improved biosynthesis of UDP-14C-glucose. Anal Biochem. 1968 Oct 24;25(1):486–499. doi: 10.1016/0003-2697(68)90127-9. [DOI] [PubMed] [Google Scholar]
- Valera A., Rodriguez-Gil J. E., Bosch F. Vanadate treatment restores the expression of genes for key enzymes in the glucose and ketone bodies metabolism in the liver of diabetic rats. J Clin Invest. 1993 Jul;92(1):4–11. doi: 10.1172/JCI116580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yki-Järvinen H. Glucose toxicity. Endocr Rev. 1992 Aug;13(3):415–431. doi: 10.1210/edrv-13-3-415. [DOI] [PubMed] [Google Scholar]
- Younes M., Strubelt O. Vanadate-induced toxicity towards isolated perfused rat livers: the role of lipid peroxidation. Toxicology. 1991 Feb 11;66(1):63–74. doi: 10.1016/0300-483x(91)90178-4. [DOI] [PubMed] [Google Scholar]
- Young A. A., Bogardus C., Wolfe-Lopez D., Mott D. M. Muscle glycogen synthesis and disposition of infused glucose in humans with reduced rates of insulin-mediated carbohydrate storage. Diabetes. 1988 Mar;37(3):303–308. doi: 10.2337/diab.37.3.303. [DOI] [PubMed] [Google Scholar]
- Zaporowska H., Wasilewski W. Some selected peripheral blood and haemopoietic system indices in Wistar rats with chronic vanadium intoxication. Comp Biochem Physiol C. 1989;93(1):175–180. doi: 10.1016/0742-8413(89)90030-3. [DOI] [PubMed] [Google Scholar]
- al-Bayati M. A., Giri S. N., Raabe O. G. Time and dose-response study of the effects of vanadate in rats: changes in blood cells, serum enzymes, protein, cholesterol, glucose, calcium, and inorganic phosphate. J Environ Pathol Toxicol Oncol. 1990 Jul-Oct;10(4-5):206–213. [PubMed] [Google Scholar]