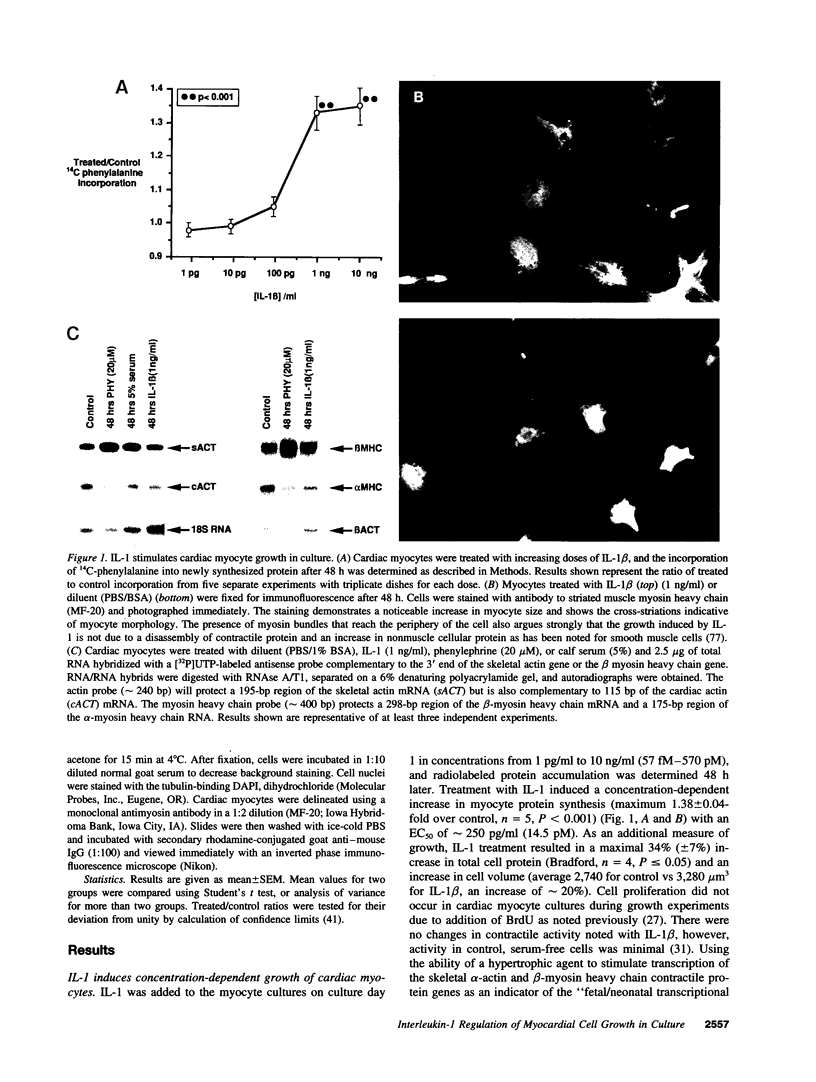
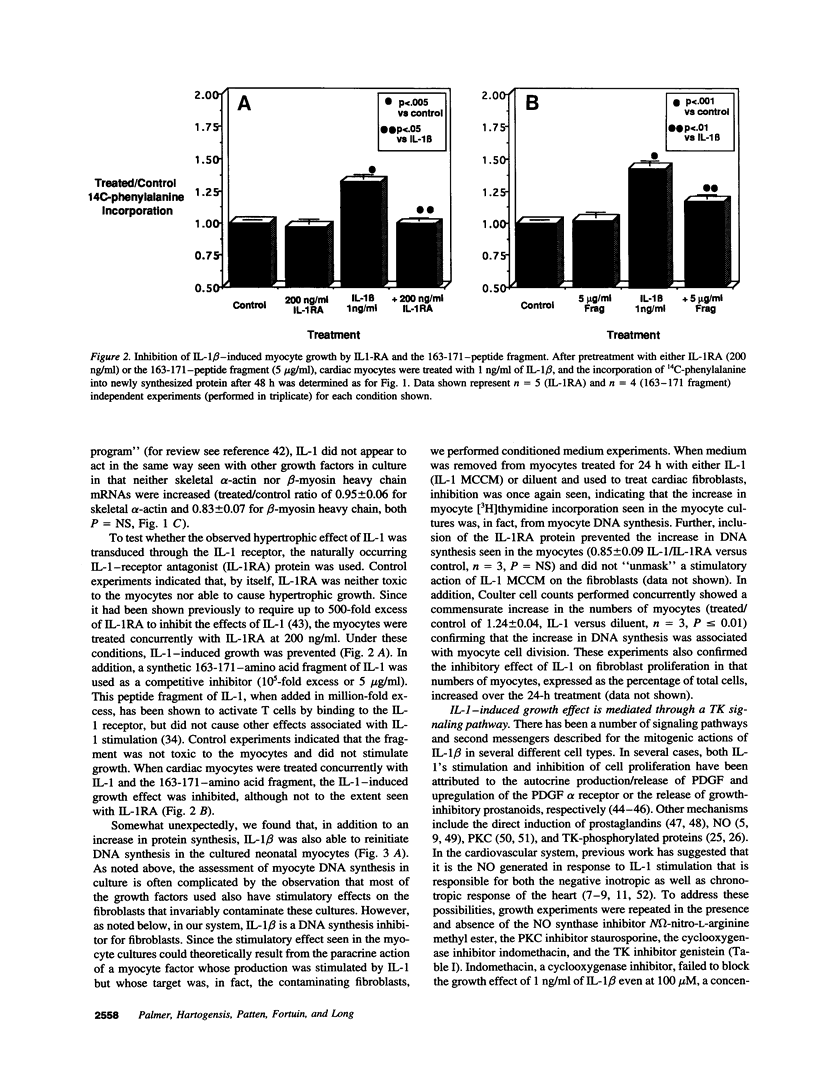
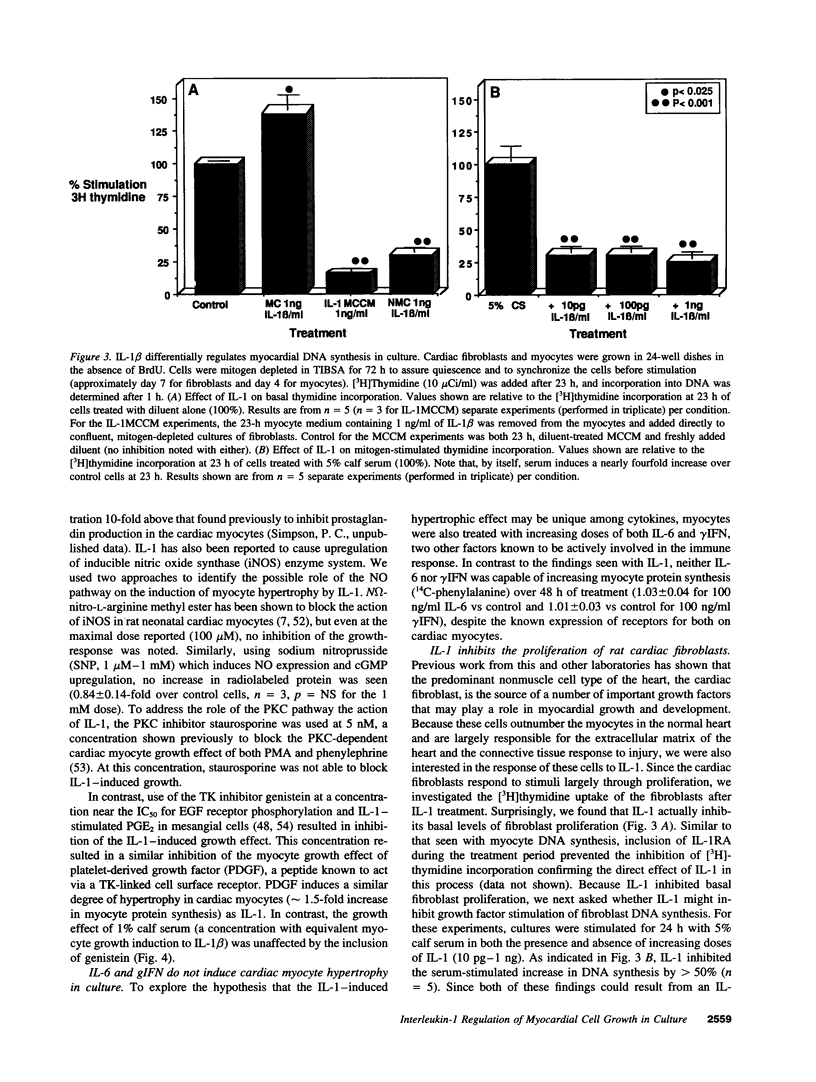
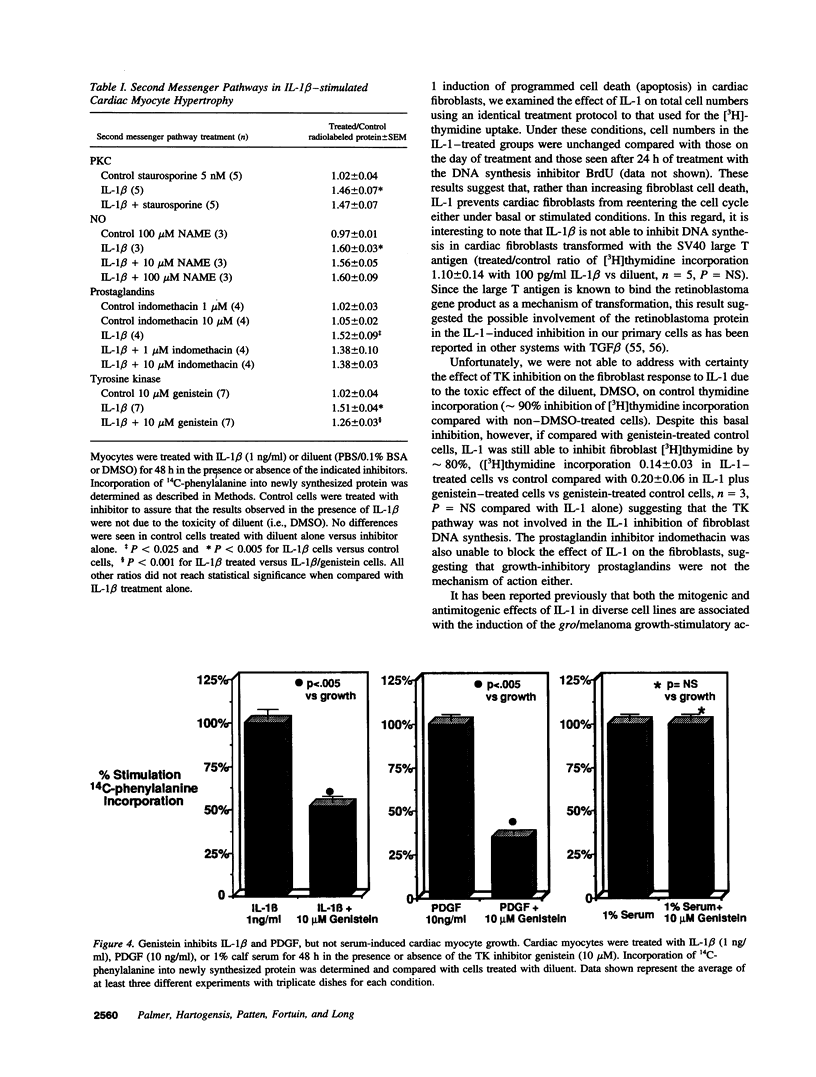
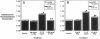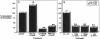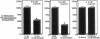Abstract
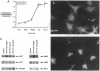
Interleukin-1 (IL-1), initially called "endogenous pyrogen," is primarily known as a mediator of inflammation. However, it also plays many other diverse physiologic roles including the stimulation and inhibition of both primary cells in culture and the interstitial and parenchymal cells of a number of organs including the heart. In the heart, IL-1 expression has traditionally been reported in situations where there is immunologic myocardial injury such as occurs during transplant rejection and congestive heart failure. For this reason, all of the effects of IL-1 have been presumed to be deleterious. Using a cell culture model which allows both the muscle cells (myocytes) and nonmuscle cells (fibroblasts) to be evaluated separately, we have found that IL-1 induces both cardiac myocyte hypertrophy and reinitiates myocyte DNA synthesis. In stark contrast, IL-1 exerts a potent anti-proliferative effect on cardiac fibroblasts. To our knowledge this is the first report concerning the differential effects of IL-1 on myocardial cell growth in culture and, given the inducible expression of IL-1 by myocardial cells during stress, underscores the importance of investigating the complex nature of the intracardiac cell-cell interactions that occur in the heart.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allo S. N., Carl L. L., Morgan H. E. Acceleration of growth of cultured cardiomyocytes and translocation of protein kinase C. Am J Physiol. 1992 Aug;263(2 Pt 1):C319–C325. doi: 10.1152/ajpcell.1992.263.2.C319. [DOI] [PubMed] [Google Scholar]
- Anisowicz A., Bardwell L., Sager R. Constitutive overexpression of a growth-regulated gene in transformed Chinese hamster and human cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7188–7192. doi: 10.1073/pnas.84.20.7188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend W. P., Welgus H. G., Thompson R. C., Eisenberg S. P. Biological properties of recombinant human monocyte-derived interleukin 1 receptor antagonist. J Clin Invest. 1990 May;85(5):1694–1697. doi: 10.1172/JCI114622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Kelly R. A., Marsden P. A., Smith T. W., Michel T. Control of cardiac muscle cell function by an endogenous nitric oxide signaling system. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):347–351. doi: 10.1073/pnas.90.1.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu-Longrois D., Simmons W. W., Pimental D., Malinski T. A., Kapturczak M., Taha Z., Lowenstein C. J., Davidoff A. J., Kelly R. A. Cytokine-inducible nitric oxide synthase (iNOS) expression in cardiac myocytes. Characterization and regulation of iNOS expression and detection of iNOS activity in single cardiac myocytes in vitro. J Biol Chem. 1994 Nov 4;269(44):27580–27588. [PubMed] [Google Scholar]
- Balligand J. L., Ungureanu D., Kelly R. A., Kobzik L., Pimental D., Michel T., Smith T. W. Abnormal contractile function due to induction of nitric oxide synthesis in rat cardiac myocytes follows exposure to activated macrophage-conditioned medium. J Clin Invest. 1993 May;91(5):2314–2319. doi: 10.1172/JCI116461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry W. H. Mechanisms of immune-mediated myocyte injury. Circulation. 1994 May;89(5):2421–2432. doi: 10.1161/01.cir.89.5.2421. [DOI] [PubMed] [Google Scholar]
- BenEzra D., Hemo I., Maftzir G. In vivo angiogenic activity of interleukins. Arch Ophthalmol. 1990 Apr;108(4):573–576. doi: 10.1001/archopht.1990.01070060121061. [DOI] [PubMed] [Google Scholar]
- Boraschi D., Volpini G., Villa L., Nencioni L., Scapigliati G., Nucci D., Antoni G., Matteucci G., Cioli F., Tagliabue A. A monoclonal antibody to the IL-1 beta peptide 163-171 blocks adjuvanticity but not pyrogenicity of IL-1 beta in vivo. J Immunol. 1989 Jul 1;143(1):131–134. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brown J. M., White C. W., Terada L. S., Grosso M. A., Shanley P. F., Mulvin D. W., Banerjee A., Whitman G. J., Harken A. H., Repine J. E. Interleukin 1 pretreatment decreases ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5026–5030. doi: 10.1073/pnas.87.13.5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Re F., Muzio M., Bertini R., Polentarutti N., Sironi M., Giri J. G., Dower S. K., Sims J. E., Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993 Jul 23;261(5120):472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Kwon G., Misko T. P., Rodi C. P., McDaniel M. L. Tyrosine kinase involvement in IL-1 beta-induced expression of iNOS by beta-cells purified from islets of Langerhans. Am J Physiol. 1994 Jul;267(1 Pt 1):C48–C54. doi: 10.1152/ajpcell.1994.267.1.C48. [DOI] [PubMed] [Google Scholar]
- Corbett J. A., Sweetland M. A., Lancaster J. R., Jr, McDaniel M. L. A 1-hour pulse with IL-1 beta induces formation of nitric oxide and inhibits insulin secretion by rat islets of Langerhans: evidence for a tyrosine kinase signaling mechanism. FASEB J. 1993 Feb 1;7(2):369–374. doi: 10.1096/fasebj.7.2.8440413. [DOI] [PubMed] [Google Scholar]
- Coyne D. W., Morrison A. R. Effect of the tyrosine kinase inhibitor, genistein, on interleukin-1 stimulated PGE2 production in mesangial cells. Biochem Biophys Res Commun. 1990 Dec 14;173(2):718–724. doi: 10.1016/s0006-291x(05)80094-4. [DOI] [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman M. J., Larsen C. P., Morris P. J. Cytokine gene transcription in vascularised organ grafts: analysis using semiquantitative polymerase chain reaction. J Exp Med. 1991 Aug 1;174(2):493–496. doi: 10.1084/jem.174.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy L. J., Goeddel D. V., Wong G. H. Tumor necrosis factor-alpha pretreatment is protective in a rat model of myocardial ischemia-reperfusion injury. Biochem Biophys Res Commun. 1992 Apr 30;184(2):1056–1059. doi: 10.1016/0006-291x(92)90698-k. [DOI] [PubMed] [Google Scholar]
- Eghbali M. Cellular origin and distribution of transforming growth factor-beta in the normal rat myocardium. Cell Tissue Res. 1989 Jun;256(3):553–558. doi: 10.1007/BF00225603. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Hoffman R. A., Shen L., Oddis C. V., Simmons R. L., Hattler B. G. Interleukin-6 (IL-6) as a mediator of stunned myocardium. Am J Cardiol. 1993 May 15;71(13):1231–1232. doi: 10.1016/0002-9149(93)90654-u. [DOI] [PubMed] [Google Scholar]
- Finkel M. S., Oddis C. V., Jacob T. D., Watkins S. C., Hattler B. G., Simmons R. L. Negative inotropic effects of cytokines on the heart mediated by nitric oxide. Science. 1992 Jul 17;257(5068):387–389. doi: 10.1126/science.1631560. [DOI] [PubMed] [Google Scholar]
- Frelin C. Serum growth factors for rat cardiac non-muscle cells in culture. J Mol Cell Cardiol. 1980 Dec;12(12):1329–1340. doi: 10.1016/0022-2828(80)90119-4. [DOI] [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. Interleukin 1 regulates heparin-binding growth factor 2 gene expression in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):296–300. doi: 10.1073/pnas.88.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giulian D., Woodward J., Young D. G., Krebs J. F., Lachman L. B. Interleukin-1 injected into mammalian brain stimulates astrogliosis and neovascularization. J Neurosci. 1988 Jul;8(7):2485–2490. doi: 10.1523/JNEUROSCI.08-07-02485.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han R. O., Ray P. E., Baughman K. L., Feldman A. M. Detection of interleukin and interleukin-receptor mRNA in human heart by polymerase chain reaction. Biochem Biophys Res Commun. 1991 Dec 16;181(2):520–523. doi: 10.1016/0006-291x(91)91219-3. [DOI] [PubMed] [Google Scholar]
- Hancock W., Mottram P. L., Purcell L. J., Han W. R., Pietersz G. A., McKenzie I. F. Prolonged survival of mouse cardiac allografts after CD4 or CD8 monoclonal antibody therapy is associated with selective intragraft cytokine protein expression: interleukin (IL)-4 and IL-10 but not IL-2 or interferon-gamma. Transplant Proc. 1993 Oct;25(5):2937–2938. [PubMed] [Google Scholar]
- Hosenpud J. D., Campbell S. M., Mendelson D. J. Interleukin-1-induced myocardial depression in an isolated beating heart preparation. J Heart Transplant. 1989 Nov-Dec;8(6):460–464. [PubMed] [Google Scholar]
- Hosenpud J. D., Campbell S. M., Pan G. Indirect inhibition of myocyte RNA and protein synthesis by interleukin-1. J Mol Cell Cardiol. 1990 Feb;22(2):213–225. doi: 10.1016/0022-2828(90)91117-p. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S. S., Rangnekar V. V., Sells S. F., Rangnekar V. M. Interleukin-1-inducible expression of gro-beta via NF-kappa B activation is dependent upon tyrosine kinase signaling. J Biol Chem. 1993 Aug 25;268(24):18018–18029. [PubMed] [Google Scholar]
- Kardami E., Fandrich R. R. Basic fibroblast growth factor in atria and ventricles of the vertebrate heart. J Cell Biol. 1989 Oct;109(4 Pt 1):1865–1875. doi: 10.1083/jcb.109.4.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardami E. Stimulation and inhibition of cardiac myocyte proliferation in vitro. Mol Cell Biochem. 1990 Feb 9;92(2):129–135. doi: 10.1007/BF00218130. [DOI] [PubMed] [Google Scholar]
- Katsuura G., Gottschall P. E., Dahl R. R., Arimura A. Interleukin-1 beta increases prostaglandin E2 in rat astrocyte cultures: modulatory effect of neuropeptides. Endocrinology. 1989 Jun;124(6):3125–3127. doi: 10.1210/endo-124-6-3125. [DOI] [PubMed] [Google Scholar]
- Kinugawa K., Takahashi T., Kohmoto O., Yao A., Aoyagi T., Momomura S., Hirata Y., Serizawa T. Nitric oxide-mediated effects of interleukin-6 on [Ca2+]i and cell contraction in cultured chick ventricular myocytes. Circ Res. 1994 Aug;75(2):285–295. doi: 10.1161/01.res.75.2.285. [DOI] [PubMed] [Google Scholar]
- Koga H., Mukawa J., Miyagi K., Higa Y., Nakasone S., Mekaru S., Ingram M. Human recombinant interleukin-1 beta-mediated growth inhibition of cultured malignant glioma cells. Neurol Med Chir (Tokyo) 1993 Jan;33(1):1–6. doi: 10.2176/nmc.33.1. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Lane J. R., Neumann D. A., Lafond-Walker A., Herskowitz A., Rose N. R. Role of IL-1 and tumor necrosis factor in coxsackie virus-induced autoimmune myocarditis. J Immunol. 1993 Aug 1;151(3):1682–1690. [PubMed] [Google Scholar]
- Li Y. H., Rozanski G. J. Effects of human recombinant interleukin-1 on electrical properties of guinea pig ventricular cells. Cardiovasc Res. 1993 Mar;27(3):525–530. doi: 10.1093/cvr/27.3.525. [DOI] [PubMed] [Google Scholar]
- Libby P., Friedman G. B., Salomon R. N. Cytokines as modulators of cell proliferation in fibrotic diseases. Am Rev Respir Dis. 1989 Oct;140(4):1114–1117. doi: 10.1164/ajrccm/140.4.1114. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libby P., Wyler D. J., Janicka M. W., Dinarello C. A. Differential effects of human interleukin-1 on growth of human fibroblasts and vascular smooth muscle cells. Arteriosclerosis. 1985 Mar-Apr;5(2):186–191. doi: 10.1161/01.atv.5.2.186. [DOI] [PubMed] [Google Scholar]
- Lompré A. M., Nadal-Ginard B., Mahdavi V. Expression of the cardiac ventricular alpha- and beta-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984 May 25;259(10):6437–6446. [PubMed] [Google Scholar]
- Long C. S., Hartogensis W. E., Simpson P. C. Beta-adrenergic stimulation of cardiac non-myocytes augments the growth-promoting activity of non-myocyte conditioned medium. J Mol Cell Cardiol. 1993 Aug;25(8):915–925. doi: 10.1006/jmcc.1993.1104. [DOI] [PubMed] [Google Scholar]
- Long C. S., Henrich C. J., Simpson P. C. A growth factor for cardiac myocytes is produced by cardiac nonmyocytes. Cell Regul. 1991 Dec;2(12):1081–1095. doi: 10.1091/mbc.2.12.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczin N., Papapetropoulos A., Catravas J. D. Tyrosine kinase inhibitors suppress endotoxin- and IL-1 beta-induced NO synthesis in aortic smooth muscle cells. Am J Physiol. 1993 Sep;265(3 Pt 2):H1014–H1018. doi: 10.1152/ajpheart.1993.265.3.H1014. [DOI] [PubMed] [Google Scholar]
- Martin M., Böl G. F., Eriksson A., Resch K., Brigelius-Flohé R. Interleukin-1-induced activation of a protein kinase co-precipitating with the type I interleukin-1 receptor in T cells. Eur J Immunol. 1994 Jul;24(7):1566–1571. doi: 10.1002/eji.1830240717. [DOI] [PubMed] [Google Scholar]
- Moses H. L. TGF-beta regulation of epithelial cell proliferation. Mol Reprod Dev. 1992 Jun;32(2):179–184. doi: 10.1002/mrd.1080320215. [DOI] [PubMed] [Google Scholar]
- Motro B., Itin A., Sachs L., Keshet E. Pattern of interleukin 6 gene expression in vivo suggests a role for this cytokine in angiogenesis. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3092–3096. doi: 10.1073/pnas.87.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognibene F. P., Rosenberg S. A., Lotze M., Skibber J., Parker M. M., Shelhamer J. H., Parrillo J. E. Interleukin-2 administration causes reversible hemodynamic changes and left ventricular dysfunction similar to those seen in septic shock. Chest. 1988 Oct;94(4):750–754. doi: 10.1378/chest.94.4.750. [DOI] [PubMed] [Google Scholar]
- Parker T. G., Packer S. E., Schneider M. D. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990 Feb;85(2):507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salamán C. R., ffrench-Mullen J. M. Interleukin-1 beta inhibits Ca2+ channel currents in hippocampal neurons through protein kinase C. Eur J Pharmacol. 1994 Jan 1;266(1):1–10. doi: 10.1016/0922-4106(94)90202-x. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Rangnekar V. V., Waheed S., Davies T. J., Toback F. G., Rangnekar V. M. Antimitogenic and mitogenic actions of interleukin-1 in diverse cell types are associated with induction of gro gene expression. J Biol Chem. 1991 Feb 5;266(4):2415–2422. [PubMed] [Google Scholar]
- Roberts A. B., Roche N. S., Winokur T. S., Burmester J. K., Sporn M. B. Role of transforming growth factor-beta in maintenance of function of cultured neonatal cardiac myocytes. Autocrine action and reversal of damaging effects of interleukin-1. J Clin Invest. 1992 Nov;90(5):2056–2062. doi: 10.1172/JCI116087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Vodovotz Y., Roche N. S., Sporn M. B., Nathan C. F. Role of nitric oxide in antagonistic effects of transforming growth factor-beta and interleukin-1 beta on the beating rate of cultured cardiac myocytes. Mol Endocrinol. 1992 Nov;6(11):1921–1930. doi: 10.1210/mend.6.11.1282674. [DOI] [PubMed] [Google Scholar]
- Rokosh D. G., Bailey B. A., Stewart A. F., Karns L. R., Long C. S., Simpson P. C. Distribution of alpha 1C-adrenergic receptor mRNA in adult rat tissues by RNase protection assay and comparison with alpha 1B and alpha 1D. Biochem Biophys Res Commun. 1994 May 16;200(3):1177–1184. doi: 10.1006/bbrc.1994.1575. [DOI] [PubMed] [Google Scholar]
- Sager R., Haskill S., Anisowicz A., Trask D., Pike M. C. GRO: a novel chemotactic cytokine. Adv Exp Med Biol. 1991;305:73–77. doi: 10.1007/978-1-4684-6009-4_9. [DOI] [PubMed] [Google Scholar]
- Schorb W., Booz G. W., Dostal D. E., Conrad K. M., Chang K. C., Baker K. M. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993 Jun;72(6):1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- Simpson P., McGrath A., Savion S. Myocyte hypertrophy in neonatal rat heart cultures and its regulation by serum and by catecholamines. Circ Res. 1982 Dec;51(6):787–801. doi: 10.1161/01.res.51.6.787. [DOI] [PubMed] [Google Scholar]
- Simpson P. Stimulation of hypertrophy of cultured neonatal rat heart cells through an alpha 1-adrenergic receptor and induction of beating through an alpha 1- and beta 1-adrenergic receptor interaction. Evidence for independent regulation of growth and beating. Circ Res. 1985 Jun;56(6):884–894. doi: 10.1161/01.res.56.6.884. [DOI] [PubMed] [Google Scholar]
- Sims J. E., Gayle M. A., Slack J. L., Alderson M. R., Bird T. A., Giri J. G., Colotta F., Re F., Mantovani A., Shanebeck K. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6155–6159. doi: 10.1073/pnas.90.13.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A. F., Rokosh D. G., Bailey B. A., Karns L. R., Chang K. C., Long C. S., Kariya K., Simpson P. C. Cloning of the rat alpha 1C-adrenergic receptor from cardiac myocytes. alpha 1C, alpha 1B, and alpha 1D mRNAs are present in cardiac myocytes but not in cardiac fibroblasts. Circ Res. 1994 Oct;75(4):796–802. doi: 10.1161/01.res.75.4.796. [DOI] [PubMed] [Google Scholar]
- Trinkle L. A., Beasley D., Moreland R. S. Interleukin-1 beta alters actin expression and inhibits contraction of rat thoracic aorta. Am J Physiol. 1992 Apr;262(4 Pt 1):C828–C833. doi: 10.1152/ajpcell.1992.262.4.C828. [DOI] [PubMed] [Google Scholar]
- Tsujino M., Hirata Y., Imai T., Kanno K., Eguchi S., Ito H., Marumo F. Induction of nitric oxide synthase gene by interleukin-1 beta in cultured rat cardiocytes. Circulation. 1994 Jul;90(1):375–383. doi: 10.1161/01.cir.90.1.375. [DOI] [PubMed] [Google Scholar]
- Weber K. T., Anversa P., Armstrong P. W., Brilla C. G., Burnett J. C., Jr, Cruickshank J. M., Devereux R. B., Giles T. D., Korsgaard N., Leier C. V. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol. 1992 Jul;20(1):3–16. doi: 10.1016/0735-1097(92)90130-f. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Swain J. L. Acidic fibroblast growth factor mRNA is expressed by cardiac myocytes in culture and the protein is localized to the extracellular matrix. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2683–2687. doi: 10.1073/pnas.86.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. J., Lovett M., Wong-Lee J., Moeller F., Kitamura M., Goralski T. J., Billingham M. E., Starnes V. A., Clayberger C. Cytokine gene expression in rejecting cardiac allografts. Transplantation. 1992 Aug;54(2):326–332. doi: 10.1097/00007890-199208000-00024. [DOI] [PubMed] [Google Scholar]
- Xie J. F., Stroumza J., Graves D. T. IL-1 down-regulates platelet-derived growth factor-alpha receptor gene expression at the transcriptional level in human osteoblastic cells. J Immunol. 1994 Jul 1;153(1):378–383. [PubMed] [Google Scholar]
- Zhao X. M., Frist W. H., Yeoh T. K., Miller G. G. Expression of cytokine genes in human cardiac allografts: correlation of IL-6 and transforming growth factor-beta (TGF-beta) with histological rejection. Clin Exp Immunol. 1993 Sep;93(3):448–451. doi: 10.1111/j.1365-2249.1993.tb08199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]