Abstract
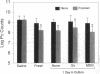
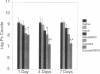
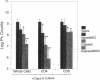
Pneumocystis carinii is a major opportunistic pathogen and a leading cause of morbidity in patients with AIDS. CD4+ cells have been shown to be important in host defenses against P. carinii, but the antigen(s) involved with this response have not been identified. We undertook the present study to determine whether the major surface glycoprotein (MSG) of P. carinii contains epitopes that can elicit a protective cellular immune response. Spleen cells and purified CD4+ cells isolated from Lewis rats, pulsed 1-4 d with MSG, and injected into corticosteroid-treated Lewis rats with pneumocystosis resulted in significant reduction in the P. carinii burden, as judged by organism quantitation and lung histology. The protective response demonstrated by the donor cells was dependent on previous exposure to P. carinii, cell concentration, and time of incubation with MSG. In addition, reconstitution with MSG-specific CD4+ cells resulted in an early hyperinflammatory response within the lungs of these animals with a high percentage of mortality. Thus, in this model, MSG can elicit an immune response mediated by CD4+ cells, which has a harmful as well as helpful effect on the host, and these responses occur despite the presence of corticosteroids.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck J. M., Warnock M. L., Curtis J. L., Sniezek M. J., Arraj-Peffer S. M., Kaltreider H. B., Shellito J. E. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am J Respir Cell Mol Biol. 1991 Aug;5(2):186–197. doi: 10.1165/ajrcmb/5.2.186. [DOI] [PubMed] [Google Scholar]
- Bowen D. L., Lane H. C., Fauci A. S. Immunopathogenesis of the acquired immunodeficiency syndrome. Ann Intern Med. 1985 Nov;103(5):704–709. doi: 10.7326/0003-4819-103-5-704. [DOI] [PubMed] [Google Scholar]
- Bozzette S. A., Sattler F. R., Chiu J., Wu A. W., Gluckstein D., Kemper C., Bartok A., Niosi J., Abramson I., Coffman J. A controlled trial of early adjunctive treatment with corticosteroids for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 1990 Nov 22;323(21):1451–1457. doi: 10.1056/NEJM199011223232104. [DOI] [PubMed] [Google Scholar]
- Brahim F. Marrow lymphocyte production during chronic hydrocortisone administration. J Reticuloendothel Soc. 1978 Feb;23(2):111–118. [PubMed] [Google Scholar]
- Claman H. N., Moorhead J. W., Benner W. H. Corticosteroids and lymphoid cells in vitro. I. Hydrocortisone lysis of human, guinea pig, and mouse thymus cells. J Lab Clin Med. 1971 Oct;78(4):499–507. [PubMed] [Google Scholar]
- Cushion M. T., Walzer P. D. Growth and serial passage of Pneumocystis carinii in the A549 cell line. Infect Immun. 1984 May;44(2):245–251. doi: 10.1128/iai.44.2.245-251.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeson E. E., Norin A. J., Veith F. J. Antigen-induced recruitment of circulating lymphocytes to the lungs and hilar lymph nodes of mice challenged intratracheally with alloantigens. Am Rev Respir Dis. 1982 Apr;125(4):453–459. doi: 10.1164/arrd.1982.125.4.453. [DOI] [PubMed] [Google Scholar]
- Fauci A. S., Dale D. C. The effect of in vivo hydrocortisone on subpopulations of human lymphocytes. J Clin Invest. 1974 Jan;53(1):240–246. doi: 10.1172/JCI107544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher D. J., Gigliotti F., Zauderer M., Harmsen A. G. Specific T-cell response to a Pneumocystis carinii surface glycoprotein (gp120) after immunization and natural infection. Infect Immun. 1991 Oct;59(10):3372–3376. doi: 10.1128/iai.59.10.3372-3376.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta T., Ueda K., Kyuwa S., Fujiwara K. Effect of T-cell transfer on Pneumocystis carinii infection in nude mice. Jpn J Exp Med. 1984 Apr;54(2):57–64. [PubMed] [Google Scholar]
- Gigliotti F., Ballou L. R., Hughes W. T., Mosley B. D. Purification and initial characterization of a ferret Pneumocystis carinii surface antigen. J Infect Dis. 1988 Oct;158(4):848–854. doi: 10.1093/infdis/158.4.848. [DOI] [PubMed] [Google Scholar]
- Gigliotti F., Stokes D. C., Cheatham A. B., Davis D. S., Hughes W. T. Development of murine monoclonal antibodies to Pneumocystis carinii. J Infect Dis. 1986 Aug;154(2):315–322. doi: 10.1093/infdis/154.2.315. [DOI] [PubMed] [Google Scholar]
- Graves D. C., McNabb S. J., Worley M. A., Downs T. D., Ivey M. H. Analyses of rat Pneumocystis carinii antigens recognized by human and rat antibodies by using western immunoblotting. Infect Immun. 1986 Oct;54(1):96–103. doi: 10.1128/iai.54.1.96-103.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmsen A. G., Stankiewicz M. Requirement for CD4+ cells in resistance to Pneumocystis carinii pneumonia in mice. J Exp Med. 1990 Sep 1;172(3):937–945. doi: 10.1084/jem.172.3.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori T., Hirata F., Hoffman T., Hizuta A., Herberman R. B. Inhibition of human natural killer (NK) activity and antibody dependent cellular cytotoxicity (ADCC) by lipomodulin, a phospholipase inhibitory protein. J Immunol. 1983 Aug;131(2):662–665. [PubMed] [Google Scholar]
- Henderson H. M., Deepe G. S., Jr Recognition of Histoplasma capsulatum yeast-cell antigens by human lymphocytes and human T-cell clones. J Leukoc Biol. 1992 May;51(5):432–436. doi: 10.1002/jlb.51.5.432. [DOI] [PubMed] [Google Scholar]
- Kim C. K., Foy J. M., Cushion M. T., Stanforth D., Linke M. J., Hendrix H. L., Walzer P. D. Comparison of histologic and quantitative techniques in evaluation of therapy for experimental Pneumocystis carinii pneumonia. Antimicrob Agents Chemother. 1987 Feb;31(2):197–201. doi: 10.1128/aac.31.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs J. A., Powell F., Edman J. C., Lundgren B., Martinez A., Drew B., Angus C. W. Multiple genes encode the major surface glycoprotein of Pneumocystis carinii. J Biol Chem. 1993 Mar 15;268(8):6034–6040. [PubMed] [Google Scholar]
- Linke M. J., Smulian A. G., Stringer J. R., Walzer P. D. Characterization of multiple unique cDNAs encoding the major surface glycoprotein of rat-derived Pneumocystis carinii. Parasitol Res. 1994;80(6):478–486. doi: 10.1007/BF00932694. [DOI] [PubMed] [Google Scholar]
- Masur H. Prevention and treatment of pneumocystis pneumonia. N Engl J Med. 1992 Dec 24;327(26):1853–1860. doi: 10.1056/NEJM199212243272606. [DOI] [PubMed] [Google Scholar]
- Montaner J. S., Lawson L. M., Levitt N., Belzberg A., Schechter M. T., Ruedy J. Corticosteroids prevent early deterioration in patients with moderately severe Pneumocystis carinii pneumonia and the acquired immunodeficiency syndrome (AIDS) Ann Intern Med. 1990 Jul 1;113(1):14–20. doi: 10.7326/0003-4819-113-1-14. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Rose R. M. Increased recovery of surfactant protein A in AIDS-related pneumonia. Am Rev Respir Dis. 1991 May;143(5 Pt 1):1072–1075. doi: 10.1164/ajrccm/143.5_Pt_1.1072. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Singleton F. M., Linke M. J., Walzer P. D. Regulation of surfactant phosphatidylcholine secretion from alveolar type II cells during Pneumocystis carinii pneumonia in the rat. J Clin Invest. 1993 Dec;92(6):2778–2782. doi: 10.1172/JCI116896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddell S. R., Watanabe K. S., Goodrich J. M., Li C. R., Agha M. E., Greenberg P. D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992 Jul 10;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- Roths J. B., Sidman C. L. Both immunity and hyperresponsiveness to Pneumocystis carinii result from transfer of CD4+ but not CD8+ T cells into severe combined immunodeficiency mice. J Clin Invest. 1992 Aug;90(2):673–678. doi: 10.1172/JCI115910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shellito J., Suzara V. V., Blumenfeld W., Beck J. M., Steger H. J., Ermak T. H. A new model of Pneumocystis carinii infection in mice selectively depleted of helper T lymphocytes. J Clin Invest. 1990 May;85(5):1686–1693. doi: 10.1172/JCI114621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunkin S. M., Stringer S. L., Stringer J. R. A tandem repeat of rat-derived Pneumocystis carinii genes encoding the major surface glycoprotein. J Eukaryot Microbiol. 1994 May-Jun;41(3):292–300. doi: 10.1111/j.1550-7408.1994.tb01509.x. [DOI] [PubMed] [Google Scholar]
- Theus S. A., Linke M. J., Andrews R. P., Walzer P. D. Proliferative and cytokine responses to a major surface glycoprotein of Pneumocystis carinii. Infect Immun. 1993 Nov;61(11):4703–4709. doi: 10.1128/iai.61.11.4703-4709.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Kitada K., Saito M., Egawa K., Nakamura Y. cDNA sequence diversity and genomic clusters of major surface glycoprotein genes of Pneumocystis carinii. J Infect Dis. 1993 Oct;168(4):979–985. doi: 10.1093/infdis/168.4.979. [DOI] [PubMed] [Google Scholar]
- Walzer P. D., Kim C. K., Linke M. J., Pogue C. L., Huerkamp M. J., Chrisp C. E., Lerro A. V., Wixson S. K., Hall E., Shultz L. D. Outbreaks of Pneumocystis carinii pneumonia in colonies of immunodeficient mice. Infect Immun. 1989 Jan;57(1):62–70. doi: 10.1128/iai.57.1.62-70.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D., LaBine M., Redington T. J., Cushion M. T. Lymphocyte changes during chronic administration of and withdrawal from corticosteroids: relation to Pneumocystis carinii pneumonia. J Immunol. 1984 Nov;133(5):2502–2508. [PubMed] [Google Scholar]
- Walzer P. D., LaBine M., Redington T. J., Cushion M. T. Predisposing factors in Pneumocystis carinii pneumonia: effects of tetracycline, protein malnutrition, and corticosteroids on hosts. Infect Immun. 1984 Dec;46(3):747–753. doi: 10.1128/iai.46.3.747-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walzer P. D. Pneumocystis carinii: recent advances in basic biology and their clinical application. AIDS. 1993 Oct;7(10):1293–1305. [PubMed] [Google Scholar]
- Walzer P. D., Rutledge M. E. Humoral immunity in experimental Pneumocystis carinii infection. I. Serum and bronchial lavage fluid antibody responses in rats. J Lab Clin Med. 1981 Jun;97(6):820–833. [PubMed] [Google Scholar]
- Walzer P. D., Stanforth D., Linke M. J., Cushion M. T. Pneumocystis carinii: immunoblotting and immunofluorescent analyses of serum antibodies during experimental rat infection and recovery. Exp Parasitol. 1987 Jun;63(3):319–328. doi: 10.1016/0014-4894(87)90179-2. [DOI] [PubMed] [Google Scholar]