Abstract
In the asymmetric unit of the title compound, C16H16N4O3·H2O, there are two symmetry-independent hydrazide molecules with almost identical geometries, and two independent water molecules. The dihedral angles between the two benzene rings in the two hydrazide molecules are 0.11 (5) and 0.77 (5)°. In one molecule, an intramolecular C—H⋯O hydrogen bond generates a ring of graph-set motif S(5). Intermolecular N—H⋯O, O—H⋯O, O—H⋯N and C—H⋯O hydrogen bonds and π–π stacking interactions between the benzene rings [centroid–centroid distances in the range 3.5021 (6)–3.6403 (6) Å] are observed, together with O⋯O [2.7226 (11) Å], O⋯N [2.7072 (10) Å] and N⋯O [2.7072 (10)–2.8582 (12) Å] short contacts. The hydrazine molecules are stacked along the b axis and adjacent molecules are linked by water molecules.
Related literature
For related literature on hydrazones, see: Rollas & Küçükgüzel (2007 ▶); Singh et al. (1992 ▶); Ergenç & Günay (1998 ▶); Durgun et al. (1993 ▶). For a related structure, see: Fun et al. (2008 ▶). For bond-length data, see: Allen et al. (1987 ▶). For graph-set analysis of hydrogen bonding, see: Bernstein et al. (1995 ▶).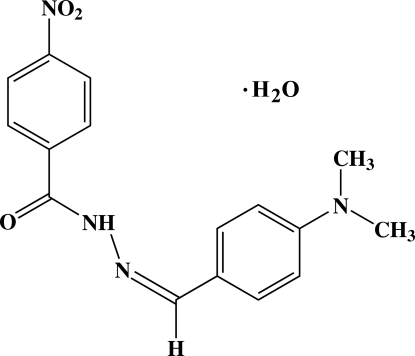
Experimental
Crystal data
C16H16N4O3·H2O
M r = 330.34
Triclinic,

a = 6.5866 (2) Å
b = 7.1337 (2) Å
c = 34.4059 (12) Å
α = 92.113 (2)°
β = 90.918 (2)°
γ = 107.816 (1)°
V = 1537.42 (8) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 100.0 (1) K
0.41 × 0.13 × 0.10 mm
Data collection
Bruker SMART APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2005 ▶) T min = 0.959, T max = 0.990
52996 measured reflections
11039 independent reflections
8656 reflections with I > 2σ(I)
R int = 0.028
Refinement
R[F 2 > 2σ(F 2)] = 0.048
wR(F 2) = 0.155
S = 1.07
11039 reflections
461 parameters
8 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.45 e Å−3
Δρmin = −0.36 e Å−3
Data collection: APEX2 (Bruker, 2005 ▶); cell refinement: APEX2; data reduction: SAINT (Bruker, 2005 ▶); program(s) used to solve structure: SHELXTL (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXTL; molecular graphics: SHELXTL; software used to prepare material for publication: SHELXTL and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808028328/is2331sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808028328/is2331Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N1A—H1NA⋯O1Wi | 0.850 (9) | 2.024 (10) | 2.8582 (11) | 166.8 (16) |
| O2W—H2W2⋯O1Wii | 0.839 (8) | 2.066 (9) | 2.8892 (12) | 166.9 (16) |
| O2W—H1W2⋯N2Biii | 0.838 (8) | 2.435 (11) | 3.1989 (12) | 151.8 (16) |
| O2W—H1W2⋯O1Biii | 0.838 (8) | 2.453 (13) | 3.1535 (11) | 141.6 (15) |
| O1W—H2W1⋯O1Aiv | 0.848 (9) | 1.907 (10) | 2.7227 (10) | 160.9 (18) |
| O1W—H2W1⋯N2Aiv | 0.848 (9) | 2.550 (16) | 3.1072 (11) | 124.2 (14) |
| N1B—H1NB⋯O2Wv | 0.859 (9) | 2.073 (9) | 2.9260 (12) | 171.5 (16) |
| O1W—H1W1⋯O1Biii | 0.842 (9) | 1.997 (9) | 2.8304 (12) | 170.2 (17) |
| C1A—H1AA⋯O1Wi | 0.93 | 2.49 | 3.3025 (13) | 146 |
| C8A—H8AA⋯O1Wi | 0.93 | 2.51 | 3.2886 (13) | 141 |
| C1B—H1BA⋯O2Wv | 0.93 | 2.41 | 3.3276 (13) | 169 |
| C5B—H5BA⋯O1B | 0.93 | 2.42 | 2.7555 (13) | 101 |
| C8B—H8BA⋯O2Wv | 0.93 | 2.48 | 3.2977 (14) | 147 |
| C15A—H15C⋯O2Bvi | 0.96 | 2.58 | 3.4738 (15) | 156 |
| C15B—H15F⋯O2Avii | 0.96 | 2.58 | 3.4773 (15) | 156 |
Symmetry codes: (i) 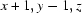 ; (ii)
; (ii)  ; (iii)
; (iii)  ; (iv)
; (iv)  ; (v)
; (v)  ; (vi)
; (vi)  ; (vii)
; (vii)  .
.
Acknowledgments
FHK and SRJ thank the Malaysian Government and Universiti Sains Malaysia for the Science Fund grant No. 305/PFIZIK/613312. SRJ thanks Universiti Sains Malaysia for a post-doctoral research fellowship.
supplementary crystallographic information
Comment
Hydrazones possessing an azometine —NHN═CH— proton constitute an important class of compounds for new drug development. Therefore, many researchers have synthesized these compounds as target structures and evaluated their biological activities. These observations have been the guides for the development of new hydrazones. Hydrazones containing an azometine —NHN═CH— proton are synthesized by heating the appropriate substituted hydrazines/hydrazides with aldehydes and ketones in solvents like ethanol, methanol, tetrahydrofuran, butanol, glacial acetic acid, ethanol-glacial acetic acid. Another synthetic route for the synthesis of hydrazones is the coupling of aryldiazonium salts with active hydrogen compounds (Rollas & Küçükgüzel, 2007). Hydrazide-hydrazones compounds are not only intermediates but they are also very effective organic compounds in their own right. When they are used as intermediates, coupling products can be synthesized by using the active hydrogen component of the —CONHN═CH— azometine group (Singh et al., 1992). N-Alkyl hydrazides can be synthesized by reduction of hydrazones with NaBH4 (Ergenç & Günay, 1998), substituted 1,3,4-oxadiazolines can be synthesized when hydrazones are heated in the presence of acetic anhydride (Durgun et al., 1993). Prompted by these review and in continuation of our work (Fun et al., 2008), we herein report the crystal structure of the title compound, (I).
There are two independent molecules (A and B) in the asymmetric unit of (I), with similar geometries (Fig. 1.) The bond lengths and angles are found to have normal values (Allen et al., 1987). The dihedral angle formed by the benzene (C1A–C6A) and (C9A–C14A) rings is 0.11 (5)° in molecule A and that between the benzene (C1B–C6B) and (C9B–C14B) rings is 0.77 (5)° in molecule B, indicating that they are coplanar. In molecule B, an intramolecular C—H···O hydrogen bond generates an S(5) ring motif (Bernstein et al., 1995).
The crystal packing is consolidated by N—H···O, O—H···O, O—H···N and C—H···O inter and intramolecular hydrogen bonding (Table 1). Furthermore, the packing is strengthened by π–π stacking interactions involving the benzene (C1A–C6A) (Cg1) and the symmetry related (C9B–C14B) ring (Cg4) [Cg1···Cg4i = 3.5021 Å; Cg1—Cg4ii = 3.6403 (6) Å; symmetry codes: (i) 2-x, 1-y, 1-z; (ii) 2-x, -y, 1-z] and the benzene (C9A–C14A) ring (Cg2) and the symmetry related (C9B–C14B) ring (Cg3) [Cg2···Cg3i = 3.6065 (6) Å; Cg2—Cg3ii = 3.5274 (6) Å; symmetry codes: (i) 2-x, 1-y, 1-z; (ii) 2-x, -y, 1-z] together with O···O = 2.7226 (11) Å, O···N = 2.7072 (10) Å and N···O = 2.7072 (10)–2.8582 (12) Å short contacts. In the crystal packing, the molecules are stacked along the b axis and the adjacent molecules are linked by water molecules to form an infinite one dimensional chain along the [010] direction.
Experimental
The title compound was obtained by refluxing 4-nitrrophenyl hydrazide (0.01 mol) and 4-(dimethylamino)benzaldehyde (0.01 mol) in ethanol (30 ml) with the addition of 3 drops of concentrated Sulfuric acid for 3 h. Excess ethanol was removed from the reaction mixture under reduced pressure. The solid product obtained was filtered, washed with water and dried. Crystals suitable for X-ray analysis were obtained from ethanol by slow evaporation.
Refinement
The amino and water H atoms were located in a difference map and refined with restraints of N—H = 0.85 (1) and O—H = 0.84 (1) Å. The remaining H atoms were positioned geometrically [C—H = 0.93 (aromatic) or 0.96 Å (methyl)] and refined using a riding model, with Uiso(H) = 1.2Ueq(aromatic C) and 1.5Ueq(methyl C). A rotating-group model was used for the methyl groups.
Figures
Fig. 1.
The molecular structure of the title compound, showing 50% probability displacement ellipsoids and the atom numbering scheme.
Fig. 2.
The crystal packing of the title compound, viewed down the a axis, showing infinite 1-D chains along the [010] direction.
Crystal data
| C16H16N4O3·H2O | Z = 4 |
| Mr = 330.34 | F(000) = 696 |
| Triclinic, P1 | Dx = 1.427 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.5866 (2) Å | Cell parameters from 9891 reflections |
| b = 7.1337 (2) Å | θ = 2.2–29.2° |
| c = 34.4059 (12) Å | µ = 0.11 mm−1 |
| α = 92.113 (2)° | T = 100 K |
| β = 90.918 (2)° | Block, red |
| γ = 107.816 (1)° | 0.41 × 0.13 × 0.10 mm |
| V = 1537.42 (8) Å3 |
Data collection
| Bruker SMART APEXII CCD area-detector diffractometer | 11039 independent reflections |
| Radiation source: fine-focus sealed tube | 8656 reflections with I > 2σ(I) |
| graphite | Rint = 0.028 |
| φ and ω scans | θmax = 32.5°, θmin = 0.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2005) | h = −9→9 |
| Tmin = 0.959, Tmax = 0.990 | k = −10→10 |
| 52996 measured reflections | l = −51→51 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.048 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.155 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.08 | w = 1/[σ2(Fo2) + (0.0736P)2 + 0.5006P] where P = (Fo2 + 2Fc2)/3 |
| 11039 reflections | (Δ/σ)max = 0.001 |
| 461 parameters | Δρmax = 0.45 e Å−3 |
| 8 restraints | Δρmin = −0.36 e Å−3 |
Special details
| Experimental. The data was collected with the Oxford Cyrosystem Cobra low-temperature attachment. |
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1A | 0.70471 (11) | −0.07582 (12) | 0.30351 (2) | 0.01918 (16) | |
| O2A | 1.23137 (15) | −0.24154 (16) | 0.46624 (3) | 0.0307 (2) | |
| O3A | 1.53442 (13) | −0.13214 (15) | 0.43817 (3) | 0.02754 (19) | |
| N1A | 0.99847 (13) | −0.03980 (13) | 0.26697 (2) | 0.01355 (15) | |
| N2A | 0.89267 (13) | −0.00979 (13) | 0.23380 (2) | 0.01426 (16) | |
| N3A | 1.33845 (15) | −0.17619 (14) | 0.43808 (3) | 0.01864 (17) | |
| N4A | 0.71656 (14) | 0.20609 (14) | 0.05749 (2) | 0.01700 (17) | |
| C1A | 1.24033 (15) | −0.04368 (15) | 0.33741 (3) | 0.01467 (17) | |
| H1AA | 1.3181 | 0.0119 | 0.3161 | 0.018* | |
| C2A | 1.34633 (15) | −0.06955 (15) | 0.37105 (3) | 0.01509 (18) | |
| H2AA | 1.4945 | −0.0333 | 0.3725 | 0.018* | |
| C3A | 1.22662 (15) | −0.15040 (14) | 0.40243 (3) | 0.01401 (17) | |
| C4A | 1.00511 (16) | −0.20815 (15) | 0.40145 (3) | 0.01574 (18) | |
| H4AA | 0.9283 | −0.2628 | 0.4229 | 0.019* | |
| C5A | 0.90143 (15) | −0.18208 (14) | 0.36758 (3) | 0.01475 (17) | |
| H5AA | 0.7532 | −0.2192 | 0.3663 | 0.018* | |
| C6A | 1.01742 (15) | −0.10065 (14) | 0.33537 (3) | 0.01233 (16) | |
| C7A | 0.89305 (15) | −0.07167 (14) | 0.30049 (3) | 0.01324 (17) | |
| C8A | 1.01208 (15) | 0.04600 (14) | 0.20449 (3) | 0.01353 (17) | |
| H8AA | 1.1575 | 0.0637 | 0.2074 | 0.016* | |
| C9A | 0.92940 (15) | 0.08245 (14) | 0.16715 (3) | 0.01250 (16) | |
| C10A | 0.71074 (15) | 0.03692 (14) | 0.15792 (3) | 0.01435 (17) | |
| H10A | 0.6115 | −0.0195 | 0.1764 | 0.017* | |
| C11A | 0.64051 (15) | 0.07458 (15) | 0.12178 (3) | 0.01456 (17) | |
| H11A | 0.4948 | 0.0421 | 0.1164 | 0.017* | |
| C12A | 0.78608 (15) | 0.16157 (14) | 0.09285 (3) | 0.01284 (17) | |
| C13A | 1.00565 (15) | 0.20405 (15) | 0.10204 (3) | 0.01502 (17) | |
| H13A | 1.1057 | 0.2589 | 0.0836 | 0.018* | |
| C14A | 1.07347 (15) | 0.16486 (15) | 0.13832 (3) | 0.01493 (17) | |
| H14A | 1.2190 | 0.1941 | 0.1437 | 0.018* | |
| C15A | 0.49080 (18) | 0.14199 (19) | 0.04745 (3) | 0.0236 (2) | |
| H15A | 0.4164 | 0.1962 | 0.0664 | 0.035* | |
| H15B | 0.4376 | 0.0007 | 0.0471 | 0.035* | |
| H15C | 0.4687 | 0.1865 | 0.0222 | 0.035* | |
| C16A | 0.86549 (18) | 0.26143 (18) | 0.02608 (3) | 0.0225 (2) | |
| H16A | 0.9824 | 0.3745 | 0.0344 | 0.034* | |
| H16B | 0.7939 | 0.2927 | 0.0039 | 0.034* | |
| H16C | 0.9185 | 0.1537 | 0.0192 | 0.034* | |
| O1B | 0.70699 (12) | 0.38412 (13) | 0.80186 (2) | 0.02177 (17) | |
| O2B | 1.25253 (15) | 0.27622 (16) | 0.96674 (3) | 0.0316 (2) | |
| O3B | 1.55367 (13) | 0.37835 (15) | 0.93778 (3) | 0.02784 (19) | |
| N1B | 1.01061 (13) | 0.49010 (12) | 0.76799 (2) | 0.01420 (16) | |
| N2B | 0.89598 (14) | 0.51210 (12) | 0.73516 (3) | 0.01539 (16) | |
| N3B | 1.35800 (15) | 0.33755 (14) | 0.93811 (3) | 0.01923 (18) | |
| N4B | 0.70414 (14) | 0.70854 (14) | 0.55794 (3) | 0.01718 (17) | |
| C1B | 1.25594 (15) | 0.46004 (15) | 0.83674 (3) | 0.01543 (18) | |
| H1BA | 1.3326 | 0.5104 | 0.8150 | 0.019* | |
| C2B | 1.36291 (16) | 0.43702 (15) | 0.87055 (3) | 0.01629 (18) | |
| H2BA | 1.5110 | 0.4714 | 0.8716 | 0.020* | |
| C3B | 1.24511 (16) | 0.36207 (14) | 0.90255 (3) | 0.01464 (17) | |
| C4B | 1.02391 (16) | 0.30698 (15) | 0.90212 (3) | 0.01653 (18) | |
| H4BA | 0.9482 | 0.2558 | 0.9239 | 0.020* | |
| C5B | 0.91904 (16) | 0.33052 (15) | 0.86818 (3) | 0.01572 (18) | |
| H5BA | 0.7708 | 0.2946 | 0.8672 | 0.019* | |
| C6B | 1.03316 (15) | 0.40749 (14) | 0.83543 (3) | 0.01277 (16) | |
| C7B | 0.90385 (15) | 0.42761 (14) | 0.80052 (3) | 0.01407 (17) | |
| C8B | 1.01092 (16) | 0.56375 (14) | 0.70506 (3) | 0.01526 (18) | |
| H8BA | 1.1568 | 0.5830 | 0.7074 | 0.018* | |
| C9B | 0.92289 (15) | 0.59328 (14) | 0.66764 (3) | 0.01363 (17) | |
| C10B | 0.70382 (15) | 0.54404 (14) | 0.65874 (3) | 0.01446 (17) | |
| H10B | 0.6059 | 0.4873 | 0.6774 | 0.017* | |
| C11B | 0.63122 (15) | 0.57871 (15) | 0.62253 (3) | 0.01458 (17) | |
| H11B | 0.4852 | 0.5445 | 0.6173 | 0.017* | |
| C12B | 0.77534 (15) | 0.66547 (14) | 0.59329 (3) | 0.01311 (17) | |
| C13B | 0.99531 (15) | 0.70948 (15) | 0.60209 (3) | 0.01521 (18) | |
| H13B | 1.0941 | 0.7631 | 0.5834 | 0.018* | |
| C14B | 1.06500 (15) | 0.67355 (14) | 0.63834 (3) | 0.01509 (18) | |
| H14B | 1.2108 | 0.7036 | 0.6435 | 0.018* | |
| C15B | 0.47861 (18) | 0.64283 (19) | 0.54800 (3) | 0.0242 (2) | |
| H15D | 0.4035 | 0.6948 | 0.5672 | 0.036* | |
| H15E | 0.4271 | 0.5014 | 0.5473 | 0.036* | |
| H15F | 0.4555 | 0.6886 | 0.5229 | 0.036* | |
| C16B | 0.85261 (18) | 0.76642 (18) | 0.52658 (3) | 0.0222 (2) | |
| H16D | 0.9674 | 0.8813 | 0.5349 | 0.033* | |
| H16E | 0.7797 | 0.7956 | 0.5043 | 0.033* | |
| H16F | 0.9088 | 0.6607 | 0.5198 | 0.033* | |
| O1W | 0.41887 (12) | 0.95772 (12) | 0.24824 (2) | 0.01787 (15) | |
| O2W | 0.53340 (12) | 0.38228 (13) | 0.24783 (2) | 0.02204 (17) | |
| H1NA | 1.1237 (17) | −0.049 (3) | 0.2650 (5) | 0.037 (4)* | |
| H2W2 | 0.485 (2) | 0.2591 (12) | 0.2452 (5) | 0.046 (5)* | |
| H1W2 | 0.433 (2) | 0.429 (2) | 0.2445 (5) | 0.042 (5)* | |
| H2W1 | 0.529 (2) | 0.962 (3) | 0.2618 (4) | 0.048 (5)* | |
| H1NB | 1.1465 (14) | 0.523 (2) | 0.7656 (5) | 0.037 (4)* | |
| H1W1 | 0.397 (3) | 0.860 (2) | 0.2324 (4) | 0.045 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1A | 0.0129 (3) | 0.0311 (4) | 0.0149 (3) | 0.0085 (3) | −0.0005 (2) | 0.0032 (3) |
| O2A | 0.0315 (4) | 0.0487 (6) | 0.0149 (4) | 0.0152 (4) | 0.0023 (3) | 0.0136 (4) |
| O3A | 0.0207 (4) | 0.0431 (5) | 0.0213 (4) | 0.0132 (4) | −0.0048 (3) | 0.0048 (4) |
| N1A | 0.0129 (3) | 0.0204 (4) | 0.0095 (3) | 0.0083 (3) | −0.0012 (3) | 0.0024 (3) |
| N2A | 0.0162 (4) | 0.0191 (4) | 0.0093 (4) | 0.0082 (3) | −0.0026 (3) | 0.0015 (3) |
| N3A | 0.0226 (4) | 0.0238 (4) | 0.0122 (4) | 0.0109 (3) | −0.0022 (3) | 0.0033 (3) |
| N4A | 0.0198 (4) | 0.0230 (4) | 0.0093 (4) | 0.0078 (3) | 0.0004 (3) | 0.0048 (3) |
| C1A | 0.0142 (4) | 0.0188 (4) | 0.0115 (4) | 0.0056 (3) | 0.0002 (3) | 0.0019 (3) |
| C2A | 0.0142 (4) | 0.0206 (4) | 0.0118 (4) | 0.0074 (3) | −0.0011 (3) | 0.0009 (3) |
| C3A | 0.0176 (4) | 0.0157 (4) | 0.0104 (4) | 0.0077 (3) | −0.0020 (3) | 0.0013 (3) |
| C4A | 0.0173 (4) | 0.0172 (4) | 0.0135 (4) | 0.0059 (3) | 0.0017 (3) | 0.0049 (3) |
| C5A | 0.0140 (4) | 0.0169 (4) | 0.0134 (4) | 0.0045 (3) | 0.0003 (3) | 0.0036 (3) |
| C6A | 0.0138 (4) | 0.0137 (4) | 0.0107 (4) | 0.0060 (3) | −0.0003 (3) | 0.0016 (3) |
| C7A | 0.0135 (4) | 0.0147 (4) | 0.0118 (4) | 0.0048 (3) | −0.0007 (3) | 0.0011 (3) |
| C8A | 0.0143 (4) | 0.0155 (4) | 0.0115 (4) | 0.0055 (3) | −0.0010 (3) | 0.0015 (3) |
| C9A | 0.0148 (4) | 0.0135 (4) | 0.0101 (4) | 0.0056 (3) | −0.0003 (3) | 0.0016 (3) |
| C10A | 0.0146 (4) | 0.0181 (4) | 0.0106 (4) | 0.0051 (3) | 0.0007 (3) | 0.0032 (3) |
| C11A | 0.0131 (4) | 0.0189 (4) | 0.0119 (4) | 0.0051 (3) | −0.0001 (3) | 0.0031 (3) |
| C12A | 0.0160 (4) | 0.0140 (4) | 0.0095 (4) | 0.0058 (3) | 0.0006 (3) | 0.0017 (3) |
| C13A | 0.0156 (4) | 0.0178 (4) | 0.0123 (4) | 0.0056 (3) | 0.0029 (3) | 0.0037 (3) |
| C14A | 0.0137 (4) | 0.0178 (4) | 0.0141 (4) | 0.0058 (3) | 0.0008 (3) | 0.0028 (3) |
| C15A | 0.0214 (5) | 0.0349 (6) | 0.0157 (5) | 0.0103 (4) | −0.0034 (4) | 0.0056 (4) |
| C16A | 0.0262 (5) | 0.0283 (5) | 0.0126 (5) | 0.0070 (4) | 0.0032 (4) | 0.0062 (4) |
| O1B | 0.0149 (3) | 0.0334 (4) | 0.0173 (4) | 0.0079 (3) | −0.0011 (3) | 0.0005 (3) |
| O2B | 0.0323 (5) | 0.0511 (6) | 0.0146 (4) | 0.0157 (4) | 0.0034 (3) | 0.0141 (4) |
| O3B | 0.0214 (4) | 0.0426 (5) | 0.0212 (4) | 0.0120 (4) | −0.0036 (3) | 0.0068 (4) |
| N1B | 0.0150 (4) | 0.0176 (4) | 0.0107 (4) | 0.0059 (3) | −0.0023 (3) | 0.0025 (3) |
| N2B | 0.0178 (4) | 0.0160 (4) | 0.0128 (4) | 0.0060 (3) | −0.0045 (3) | 0.0013 (3) |
| N3B | 0.0235 (4) | 0.0235 (4) | 0.0130 (4) | 0.0104 (3) | −0.0016 (3) | 0.0036 (3) |
| N4B | 0.0186 (4) | 0.0228 (4) | 0.0110 (4) | 0.0071 (3) | 0.0004 (3) | 0.0043 (3) |
| C1B | 0.0156 (4) | 0.0191 (4) | 0.0121 (4) | 0.0056 (3) | 0.0006 (3) | 0.0035 (3) |
| C2B | 0.0152 (4) | 0.0206 (4) | 0.0140 (4) | 0.0068 (3) | −0.0005 (3) | 0.0035 (3) |
| C3B | 0.0193 (4) | 0.0158 (4) | 0.0104 (4) | 0.0077 (3) | −0.0013 (3) | 0.0022 (3) |
| C4B | 0.0188 (4) | 0.0180 (4) | 0.0137 (4) | 0.0064 (3) | 0.0021 (3) | 0.0037 (3) |
| C5B | 0.0154 (4) | 0.0178 (4) | 0.0141 (4) | 0.0050 (3) | 0.0010 (3) | 0.0028 (3) |
| C6B | 0.0147 (4) | 0.0128 (4) | 0.0115 (4) | 0.0055 (3) | −0.0011 (3) | 0.0004 (3) |
| C7B | 0.0158 (4) | 0.0147 (4) | 0.0121 (4) | 0.0055 (3) | −0.0015 (3) | −0.0005 (3) |
| C8B | 0.0173 (4) | 0.0155 (4) | 0.0135 (4) | 0.0060 (3) | −0.0027 (3) | 0.0013 (3) |
| C9B | 0.0159 (4) | 0.0131 (4) | 0.0125 (4) | 0.0054 (3) | −0.0014 (3) | 0.0009 (3) |
| C10B | 0.0165 (4) | 0.0163 (4) | 0.0106 (4) | 0.0047 (3) | 0.0004 (3) | 0.0034 (3) |
| C11B | 0.0143 (4) | 0.0174 (4) | 0.0122 (4) | 0.0048 (3) | −0.0004 (3) | 0.0025 (3) |
| C12B | 0.0169 (4) | 0.0138 (4) | 0.0099 (4) | 0.0064 (3) | 0.0006 (3) | 0.0017 (3) |
| C13B | 0.0154 (4) | 0.0171 (4) | 0.0138 (4) | 0.0056 (3) | 0.0022 (3) | 0.0031 (3) |
| C14B | 0.0147 (4) | 0.0162 (4) | 0.0150 (4) | 0.0055 (3) | −0.0003 (3) | 0.0022 (3) |
| C15B | 0.0203 (5) | 0.0376 (6) | 0.0161 (5) | 0.0105 (4) | −0.0025 (4) | 0.0065 (4) |
| C16B | 0.0251 (5) | 0.0280 (5) | 0.0128 (5) | 0.0062 (4) | 0.0029 (4) | 0.0057 (4) |
| O1W | 0.0143 (3) | 0.0264 (4) | 0.0148 (3) | 0.0091 (3) | −0.0012 (3) | 0.0013 (3) |
| O2W | 0.0165 (3) | 0.0271 (4) | 0.0234 (4) | 0.0074 (3) | 0.0002 (3) | 0.0056 (3) |
Geometric parameters (Å, °)
| O1A—C7A | 1.2379 (11) | O3B—N3B | 1.2317 (12) |
| O2A—N3A | 1.2278 (12) | N1B—C7B | 1.3475 (13) |
| O3A—N3A | 1.2315 (12) | N1B—N2B | 1.3888 (11) |
| N1A—C7A | 1.3462 (12) | N1B—H1NB | 0.859 (9) |
| N1A—N2A | 1.3846 (11) | N2B—C8B | 1.2899 (13) |
| N1A—H1NA | 0.850 (9) | N3B—C3B | 1.4650 (13) |
| N2A—C8A | 1.2875 (12) | N4B—C12B | 1.3753 (12) |
| N3A—C3A | 1.4667 (12) | N4B—C15B | 1.4454 (14) |
| N4A—C12A | 1.3751 (12) | N4B—C16B | 1.4527 (14) |
| N4A—C15A | 1.4475 (14) | C1B—C2B | 1.3911 (13) |
| N4A—C16A | 1.4540 (13) | C1B—C6B | 1.3985 (13) |
| C1A—C2A | 1.3892 (13) | C1B—H1BA | 0.9300 |
| C1A—C6A | 1.3983 (13) | C2B—C3B | 1.3847 (14) |
| C1A—H1AA | 0.9300 | C2B—H2BA | 0.9300 |
| C2A—C3A | 1.3838 (13) | C3B—C4B | 1.3878 (14) |
| C2A—H2AA | 0.9300 | C4B—C5B | 1.3888 (14) |
| C3A—C4A | 1.3889 (13) | C4B—H4BA | 0.9300 |
| C4A—C5A | 1.3884 (13) | C5B—C6B | 1.3988 (13) |
| C4A—H4AA | 0.9300 | C5B—H5BA | 0.9300 |
| C5A—C6A | 1.3972 (13) | C6B—C7B | 1.4978 (13) |
| C5A—H5AA | 0.9300 | C8B—C9B | 1.4524 (13) |
| C6A—C7A | 1.4981 (13) | C8B—H8BA | 0.9300 |
| C8A—C9A | 1.4505 (13) | C9B—C14B | 1.4019 (13) |
| C8A—H8AA | 0.9300 | C9B—C10B | 1.4025 (13) |
| C9A—C14A | 1.4005 (13) | C10B—C11B | 1.3847 (13) |
| C9A—C10A | 1.4043 (13) | C10B—H10B | 0.9300 |
| C10A—C11A | 1.3835 (13) | C11B—C12B | 1.4189 (13) |
| C10A—H10A | 0.9300 | C11B—H11B | 0.9300 |
| C11A—C12A | 1.4167 (13) | C12B—C13B | 1.4107 (13) |
| C11A—H11A | 0.9300 | C13B—C14B | 1.3817 (13) |
| C12A—C13A | 1.4119 (13) | C13B—H13B | 0.9300 |
| C13A—C14A | 1.3857 (13) | C14B—H14B | 0.9300 |
| C13A—H13A | 0.9300 | C15B—H15D | 0.9600 |
| C14A—H14A | 0.9300 | C15B—H15E | 0.9600 |
| C15A—H15A | 0.9600 | C15B—H15F | 0.9600 |
| C15A—H15B | 0.9600 | C16B—H16D | 0.9600 |
| C15A—H15C | 0.9600 | C16B—H16E | 0.9600 |
| C16A—H16A | 0.9600 | C16B—H16F | 0.9600 |
| C16A—H16B | 0.9600 | O1W—H2W1 | 0.848 (9) |
| C16A—H16C | 0.9600 | O1W—H1W1 | 0.842 (9) |
| O1B—C7B | 1.2396 (12) | O2W—H2W2 | 0.839 (8) |
| O2B—N3B | 1.2297 (12) | O2W—H1W2 | 0.838 (8) |
| C7A—N1A—N2A | 118.95 (8) | C7B—N1B—H1NB | 125.9 (11) |
| C7A—N1A—H1NA | 122.1 (12) | N2B—N1B—H1NB | 115.4 (11) |
| N2A—N1A—H1NA | 118.7 (12) | C8B—N2B—N1B | 113.95 (8) |
| C8A—N2A—N1A | 114.85 (8) | O2B—N3B—O3B | 123.26 (9) |
| O2A—N3A—O3A | 123.44 (9) | O2B—N3B—C3B | 118.35 (9) |
| O2A—N3A—C3A | 118.16 (9) | O3B—N3B—C3B | 118.39 (9) |
| O3A—N3A—C3A | 118.40 (9) | C12B—N4B—C15B | 119.95 (8) |
| C12A—N4A—C15A | 119.77 (8) | C12B—N4B—C16B | 119.70 (9) |
| C12A—N4A—C16A | 119.76 (8) | C15B—N4B—C16B | 117.92 (9) |
| C15A—N4A—C16A | 117.83 (9) | C2B—C1B—C6B | 120.04 (9) |
| C2A—C1A—C6A | 120.39 (9) | C2B—C1B—H1BA | 120.0 |
| C2A—C1A—H1AA | 119.8 | C6B—C1B—H1BA | 120.0 |
| C6A—C1A—H1AA | 119.8 | C3B—C2B—C1B | 118.87 (9) |
| C3A—C2A—C1A | 118.55 (9) | C3B—C2B—H2BA | 120.6 |
| C3A—C2A—H2AA | 120.7 | C1B—C2B—H2BA | 120.6 |
| C1A—C2A—H2AA | 120.7 | C2B—C3B—C4B | 122.54 (9) |
| C2A—C3A—C4A | 122.60 (9) | C2B—C3B—N3B | 118.80 (9) |
| C2A—C3A—N3A | 118.59 (9) | C4B—C3B—N3B | 118.65 (9) |
| C4A—C3A—N3A | 118.82 (9) | C3B—C4B—C5B | 118.02 (9) |
| C5A—C4A—C3A | 118.18 (9) | C3B—C4B—H4BA | 121.0 |
| C5A—C4A—H4AA | 120.9 | C5B—C4B—H4BA | 121.0 |
| C3A—C4A—H4AA | 120.9 | C4B—C5B—C6B | 120.93 (9) |
| C4A—C5A—C6A | 120.71 (9) | C4B—C5B—H5BA | 119.5 |
| C4A—C5A—H5AA | 119.6 | C6B—C5B—H5BA | 119.5 |
| C6A—C5A—H5AA | 119.6 | C1B—C6B—C5B | 119.59 (9) |
| C5A—C6A—C1A | 119.57 (8) | C1B—C6B—C7B | 124.01 (9) |
| C5A—C6A—C7A | 117.17 (8) | C5B—C6B—C7B | 116.40 (8) |
| C1A—C6A—C7A | 123.22 (8) | O1B—C7B—N1B | 122.42 (9) |
| O1A—C7A—N1A | 123.57 (9) | O1B—C7B—C6B | 120.50 (9) |
| O1A—C7A—C6A | 120.16 (9) | N1B—C7B—C6B | 117.07 (8) |
| N1A—C7A—C6A | 116.26 (8) | N2B—C8B—C9B | 122.99 (9) |
| N2A—C8A—C9A | 122.77 (9) | N2B—C8B—H8BA | 118.5 |
| N2A—C8A—H8AA | 118.6 | C9B—C8B—H8BA | 118.5 |
| C9A—C8A—H8AA | 118.6 | C14B—C9B—C10B | 117.73 (9) |
| C14A—C9A—C10A | 117.64 (8) | C14B—C9B—C8B | 118.20 (9) |
| C14A—C9A—C8A | 118.88 (8) | C10B—C9B—C8B | 124.06 (9) |
| C10A—C9A—C8A | 123.48 (8) | C11B—C10B—C9B | 120.92 (9) |
| C11A—C10A—C9A | 121.09 (9) | C11B—C10B—H10B | 119.5 |
| C11A—C10A—H10A | 119.5 | C9B—C10B—H10B | 119.5 |
| C9A—C10A—H10A | 119.5 | C10B—C11B—C12B | 121.27 (9) |
| C10A—C11A—C12A | 121.35 (9) | C10B—C11B—H11B | 119.4 |
| C10A—C11A—H11A | 119.3 | C12B—C11B—H11B | 119.4 |
| C12A—C11A—H11A | 119.3 | N4B—C12B—C13B | 121.07 (9) |
| N4A—C12A—C13A | 121.29 (8) | N4B—C12B—C11B | 121.46 (9) |
| N4A—C12A—C11A | 121.36 (8) | C13B—C12B—C11B | 117.46 (8) |
| C13A—C12A—C11A | 117.34 (8) | C14B—C13B—C12B | 120.49 (9) |
| C14A—C13A—C12A | 120.62 (9) | C14B—C13B—H13B | 119.8 |
| C14A—C13A—H13A | 119.7 | C12B—C13B—H13B | 119.8 |
| C12A—C13A—H13A | 119.7 | C13B—C14B—C9B | 122.08 (9) |
| C13A—C14A—C9A | 121.94 (9) | C13B—C14B—H14B | 119.0 |
| C13A—C14A—H14A | 119.0 | C9B—C14B—H14B | 119.0 |
| C9A—C14A—H14A | 119.0 | N4B—C15B—H15D | 109.5 |
| N4A—C15A—H15A | 109.5 | N4B—C15B—H15E | 109.5 |
| N4A—C15A—H15B | 109.5 | H15D—C15B—H15E | 109.5 |
| H15A—C15A—H15B | 109.5 | N4B—C15B—H15F | 109.5 |
| N4A—C15A—H15C | 109.5 | H15D—C15B—H15F | 109.5 |
| H15A—C15A—H15C | 109.5 | H15E—C15B—H15F | 109.5 |
| H15B—C15A—H15C | 109.5 | N4B—C16B—H16D | 109.5 |
| N4A—C16A—H16A | 109.5 | N4B—C16B—H16E | 109.5 |
| N4A—C16A—H16B | 109.5 | H16D—C16B—H16E | 109.5 |
| H16A—C16A—H16B | 109.5 | N4B—C16B—H16F | 109.5 |
| N4A—C16A—H16C | 109.5 | H16D—C16B—H16F | 109.5 |
| H16A—C16A—H16C | 109.5 | H16E—C16B—H16F | 109.5 |
| H16B—C16A—H16C | 109.5 | H2W1—O1W—H1W1 | 106.7 (12) |
| C7B—N1B—N2B | 118.62 (8) | H2W2—O2W—H1W2 | 108.6 (12) |
| C7A—N1A—N2A—C8A | 170.93 (9) | C7B—N1B—N2B—C8B | −176.98 (9) |
| C6A—C1A—C2A—C3A | −0.75 (15) | C6B—C1B—C2B—C3B | −0.12 (15) |
| C1A—C2A—C3A—C4A | 0.60 (15) | C1B—C2B—C3B—C4B | 0.67 (16) |
| C1A—C2A—C3A—N3A | −179.48 (9) | C1B—C2B—C3B—N3B | 179.87 (9) |
| O2A—N3A—C3A—C2A | 177.47 (10) | O2B—N3B—C3B—C2B | 177.95 (10) |
| O3A—N3A—C3A—C2A | −2.85 (15) | O3B—N3B—C3B—C2B | −2.44 (15) |
| O2A—N3A—C3A—C4A | −2.60 (15) | O2B—N3B—C3B—C4B | −2.82 (15) |
| O3A—N3A—C3A—C4A | 177.07 (10) | O3B—N3B—C3B—C4B | 176.80 (10) |
| C2A—C3A—C4A—C5A | −0.35 (15) | C2B—C3B—C4B—C5B | −0.61 (16) |
| N3A—C3A—C4A—C5A | 179.73 (9) | N3B—C3B—C4B—C5B | −179.81 (9) |
| C3A—C4A—C5A—C6A | 0.25 (15) | C3B—C4B—C5B—C6B | 0.00 (15) |
| C4A—C5A—C6A—C1A | −0.42 (15) | C2B—C1B—C6B—C5B | −0.46 (15) |
| C4A—C5A—C6A—C7A | −178.04 (9) | C2B—C1B—C6B—C7B | 179.70 (9) |
| C2A—C1A—C6A—C5A | 0.68 (15) | C4B—C5B—C6B—C1B | 0.53 (15) |
| C2A—C1A—C6A—C7A | 178.15 (9) | C4B—C5B—C6B—C7B | −179.62 (9) |
| N2A—N1A—C7A—O1A | −0.01 (15) | N2B—N1B—C7B—O1B | 1.99 (15) |
| N2A—N1A—C7A—C6A | −179.29 (8) | N2B—N1B—C7B—C6B | −179.45 (8) |
| C5A—C6A—C7A—O1A | 16.42 (14) | C1B—C6B—C7B—O1B | −177.60 (10) |
| C1A—C6A—C7A—O1A | −161.10 (10) | C5B—C6B—C7B—O1B | 2.56 (14) |
| C5A—C6A—C7A—N1A | −164.26 (9) | C1B—C6B—C7B—N1B | 3.81 (14) |
| C1A—C6A—C7A—N1A | 18.21 (14) | C5B—C6B—C7B—N1B | −176.03 (9) |
| N1A—N2A—C8A—C9A | 179.26 (8) | N1B—N2B—C8B—C9B | 179.47 (9) |
| N2A—C8A—C9A—C14A | 172.55 (9) | N2B—C8B—C9B—C14B | 171.95 (9) |
| N2A—C8A—C9A—C10A | −8.37 (15) | N2B—C8B—C9B—C10B | −8.92 (16) |
| C14A—C9A—C10A—C11A | −0.81 (15) | C14B—C9B—C10B—C11B | −1.69 (14) |
| C8A—C9A—C10A—C11A | −179.90 (9) | C8B—C9B—C10B—C11B | 179.17 (9) |
| C9A—C10A—C11A—C12A | −0.44 (15) | C9B—C10B—C11B—C12B | −0.18 (15) |
| C15A—N4A—C12A—C13A | 173.52 (10) | C15B—N4B—C12B—C13B | 173.26 (10) |
| C16A—N4A—C12A—C13A | 12.30 (15) | C16B—N4B—C12B—C13B | 11.36 (15) |
| C15A—N4A—C12A—C11A | −7.65 (15) | C15B—N4B—C12B—C11B | −7.93 (15) |
| C16A—N4A—C12A—C11A | −168.87 (9) | C16B—N4B—C12B—C11B | −169.83 (9) |
| C10A—C11A—C12A—N4A | −177.42 (9) | C10B—C11B—C12B—N4B | −176.95 (9) |
| C10A—C11A—C12A—C13A | 1.46 (15) | C10B—C11B—C12B—C13B | 1.91 (15) |
| N4A—C12A—C13A—C14A | 177.65 (9) | N4B—C12B—C13B—C14B | 177.09 (9) |
| C11A—C12A—C13A—C14A | −1.23 (14) | C11B—C12B—C13B—C14B | −1.76 (14) |
| C12A—C13A—C14A—C9A | 0.00 (15) | C12B—C13B—C14B—C9B | −0.10 (15) |
| C10A—C9A—C14A—C13A | 1.04 (15) | C10B—C9B—C14B—C13B | 1.84 (15) |
| C8A—C9A—C14A—C13A | −179.83 (9) | C8B—C9B—C14B—C13B | −178.97 (9) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N1A—H1NA···O1Wi | 0.85 (1) | 2.02 (1) | 2.8582 (11) | 167.(2) |
| O2W—H2W2···O1Wii | 0.84 (1) | 2.07 (1) | 2.8892 (12) | 167.(2) |
| O2W—H1W2···N2Biii | 0.84 (1) | 2.44 (1) | 3.1989 (12) | 152.(2) |
| O2W—H1W2···O1Biii | 0.84 (1) | 2.45 (1) | 3.1535 (11) | 142.(2) |
| O1W—H2W1···O1Aiv | 0.85 (1) | 1.91 (1) | 2.7227 (10) | 161.(2) |
| O1W—H2W1···N2Aiv | 0.85 (1) | 2.55 (2) | 3.1072 (11) | 124.(1) |
| N1B—H1NB···O2Wv | 0.86 (1) | 2.07 (1) | 2.9260 (12) | 172.(2) |
| O1W—H1W1···O1Biii | 0.84 (1) | 2.00 (1) | 2.8304 (12) | 170.(2) |
| C1A—H1AA···O1Wi | 0.93 | 2.49 | 3.3025 (13) | 146 |
| C8A—H8AA···O1Wi | 0.93 | 2.51 | 3.2886 (13) | 141 |
| C1B—H1BA···O2Wv | 0.93 | 2.41 | 3.3276 (13) | 169 |
| C5B—H5BA···O1B | 0.93 | 2.42 | 2.7555 (13) | 101 |
| C8B—H8BA···O2Wv | 0.93 | 2.48 | 3.2977 (14) | 147 |
| C15A—H15C···O2Bvi | 0.96 | 2.58 | 3.4738 (15) | 156 |
| C15B—H15F···O2Avii | 0.96 | 2.58 | 3.4773 (15) | 156 |
Symmetry codes: (i) x+1, y−1, z; (ii) x, y−1, z; (iii) −x+1, −y+1, −z+1; (iv) x, y+1, z; (v) −x+2, −y+1, −z+1; (vi) x−1, y, z−1; (vii) x−1, y+1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IS2331).
References
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N. L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Durgun, B. B., Çapan, G., Ergenç, N. & Rollas, S. (1993). Pharmazie, 48, 942–943. [PubMed]
- Ergenç, N. & Günay, N. S. (1998). Eur. J. Med. Chem.33, 143–148.
- Fun, H.-K., Patil, P. S., Jebas, S. R., Sujith, K. V. & Kalluraya, B. (2008). Acta Cryst. E64, o1594–o1595. [DOI] [PMC free article] [PubMed]
- Rollas, S. & Küçükgüzel, S. G. (2007). Molecules, 12, 1910–1939. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Singh, V., Srivastava, V. K., Palit, G. & Shanker, K. (1992). Arzneim. Forsch. Drug. Res.42, 993–996. [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808028328/is2331sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808028328/is2331Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




