Abstract
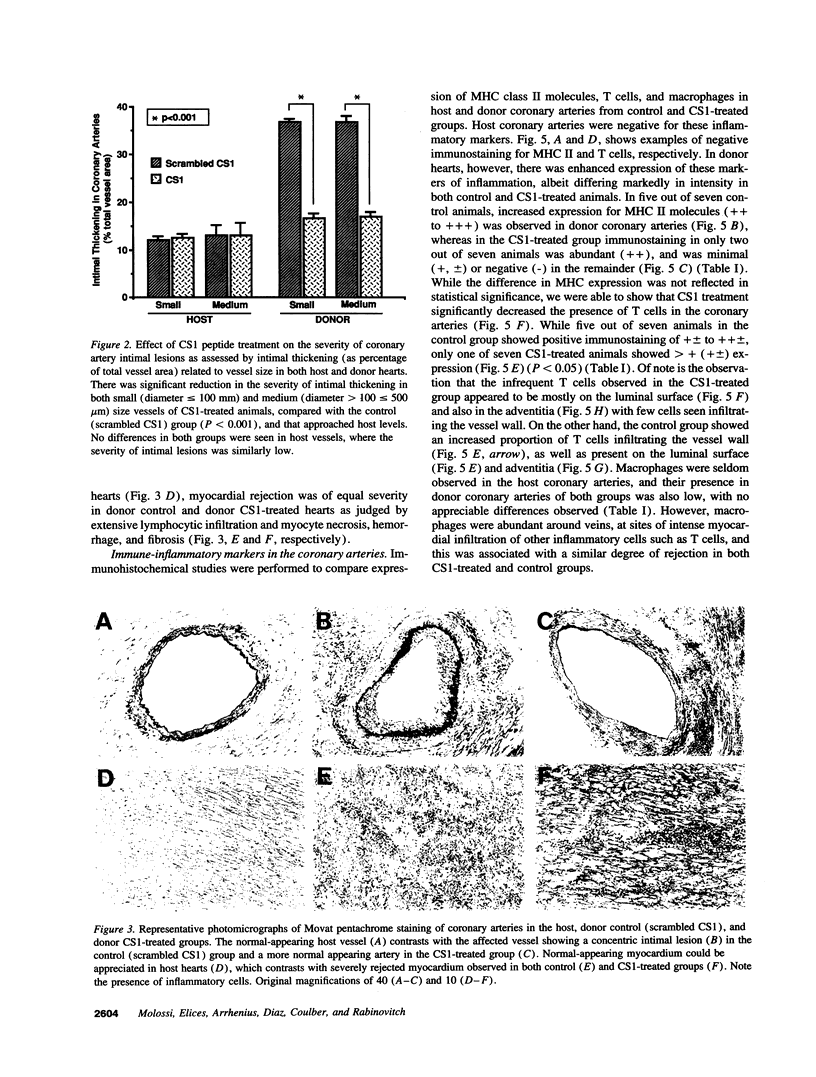
Graft arteriopathy, a leading cause of cardiac allograft failure, is associated with increased intimal smooth muscle cells, inflammatory cells, and accumulation of extracellular matrix. We hypothesized that cellular fibronectin plays a pivotal role in the progression of the allograft arteriopathy by directing the transendothelial trafficking of inflammatory cells through interaction of the connecting segment-1 (CS1) motif with the very late antigen-4 (VLA-4) integrin, and tested this in vivo using a blocking peptide. Cholesterol-fed rabbits underwent heterotopic cardiac transplantation without immunosuppression. The treatment group (n = 7) received a synthetic CS1 peptide (1 mg/kg per d, subcutaneously), and the controls (n = 7) received an inactive peptide (1 mg/kg per d, subcutaneously). At 7-8 d after transplantation, hearts were harvested and sectioned for morphometric analysis and immunohistochemical studies. We observed a > 50% decrease in the incidence (P < 0.001) and severity (P < 0.001) of donor coronary artery intimal thickening in the CS1-treated compared with the control group. These findings correlated with reduced infiltration of T cells (P < 0.05), a trend toward decreased expression of adhesion molecules (P < 0.06), and less accumulation of fibronectin (P < 0.03). Our data suggest that the VLA-4-fibronectin interaction is critical to the progression of the allograft arteriopathy by perpetuating the immune-inflammatory response in the vessel wall.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ager A., Humphries M. J. Use of synthetic peptides to probe lymphocyte--high endothelial cell interactions. Lymphocytes recognize a ligand on the endothelial surface which contains the CS1 adhesion motif. Int Immunol. 1990;2(10):921–928. doi: 10.1093/intimm/2.10.921. [DOI] [PubMed] [Google Scholar]
- Alonso D. R., Starek P. K., Minick C. R. Studies on the pathogenesis of atheroarteriosclerosis induced in rabbit cardiac allografts by the synergy of graft rejection and hypercholesterolemia. Am J Pathol. 1977 May;87(2):415–442. [PMC free article] [PubMed] [Google Scholar]
- Anderson T. J., Meredith I. T., Uehata A., Mudge G. H., Selwyn A. P., Ganz P., Yeung A. C. Functional significance of intimal thickening as detected by intravascular ultrasound early and late after cardiac transplantation. Circulation. 1993 Sep;88(3):1093–1100. doi: 10.1161/01.cir.88.3.1093. [DOI] [PubMed] [Google Scholar]
- Anwar A. R., Moqbel R., Walsh G. M., Kay A. B., Wardlaw A. J. Adhesion to fibronectin prolongs eosinophil survival. J Exp Med. 1993 Mar 1;177(3):839–843. doi: 10.1084/jem.177.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billingham M. E. Histopathology of graft coronary disease. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S38–S44. [PubMed] [Google Scholar]
- Billingham M. E. Some recent advances in cardiac pathology. Hum Pathol. 1979 Jul;10(4):367–386. doi: 10.1016/s0046-8177(79)80043-x. [DOI] [PubMed] [Google Scholar]
- Boudreau N., Turley E., Rabinovitch M. Fibronectin, hyaluronan, and a hyaluronan binding protein contribute to increased ductus arteriosus smooth muscle cell migration. Dev Biol. 1991 Feb;143(2):235–247. doi: 10.1016/0012-1606(91)90074-d. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Choi E. T., Engel L., Callow A. D., Sun S., Trachtenberg J., Santoro S., Ryan U. S. Inhibition of neointimal hyperplasia by blocking alpha V beta 3 integrin with a small peptide antagonist GpenGRGDSPCA. J Vasc Surg. 1994 Jan;19(1):125–134. doi: 10.1016/s0741-5214(94)70127-x. [DOI] [PubMed] [Google Scholar]
- Chuluyan H. E., Issekutz A. C. VLA-4 integrin can mediate CD11/CD18-independent transendothelial migration of human monocytes. J Clin Invest. 1993 Dec;92(6):2768–2777. doi: 10.1172/JCI116895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausell N., Molossi S., Rabinovitch M. Increased interleukin-1 beta and fibronectin expression are early features of the development of the postcardiac transplant coronary arteriopathy in piglets. Am J Pathol. 1993 Jun;142(6):1772–1786. [PMC free article] [PubMed] [Google Scholar]
- Clausell N., Molossi S., Sett S., Rabinovitch M. In vivo blockade of tumor necrosis factor-alpha in cholesterol-fed rabbits after cardiac transplant inhibits acute coronary artery neointimal formation. Circulation. 1994 Jun;89(6):2768–2779. doi: 10.1161/01.cir.89.6.2768. [DOI] [PubMed] [Google Scholar]
- Clausell N., Rabinovitch M. Upregulation of fibronectin synthesis by interleukin-1 beta in coronary artery smooth muscle cells is associated with the development of the post-cardiac transplant arteriopathy in piglets. J Clin Invest. 1993 Oct;92(4):1850–1858. doi: 10.1172/JCI116776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coito A. J., Binder J., de Sousa M., Kupiec-Weglinski J. W. The expression of extracellular matrix proteins during accelerated rejection of cardiac allografts in sensitized rats. Transplantation. 1994 Feb 27;57(4):599–605. [PubMed] [Google Scholar]
- Eich D. M., Nestler J. E., Johnson D. E., Dworkin G. H., Ko D., Wechsler A. S., Hess M. L. Inhibition of accelerated coronary atherosclerosis with dehydroepiandrosterone in the heterotopic rabbit model of cardiac transplantation. Circulation. 1993 Jan;87(1):261–269. doi: 10.1161/01.cir.87.1.261. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Elices M. J., Tsai V., Strahl D., Goel A. S., Tollefson V., Arrhenius T., Wayner E. A., Gaeta F. C., Fikes J. D., Firestein G. S. Expression and functional significance of alternatively spliced CS1 fibronectin in rheumatoid arthritis microvasculature. J Clin Invest. 1994 Jan;93(1):405–416. doi: 10.1172/JCI116975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foegh M. L. Accelerated cardiac transplant atherosclerosis/chronic rejection in rabbits: inhibition by angiopeptin. Transplant Proc. 1993 Apr;25(2):2095–2097. [PubMed] [Google Scholar]
- Foegh M. L., Khirabadi B. S., Chambers E., Amamoo S., Ramwell P. W. Inhibition of coronary artery transplant atherosclerosis in rabbits with angiopeptin, an octapeptide. Atherosclerosis. 1989 Aug;78(2-3):229–236. doi: 10.1016/0021-9150(89)90228-1. [DOI] [PubMed] [Google Scholar]
- Foegh M. L., Khirabadi B. S., Chambers E., Ramwell P. W. Peptide inhibition of accelerated transplant atherosclerosis. Transplant Proc. 1989 Aug;21(4):3674–3676. [PubMed] [Google Scholar]
- Garcia-Pardo A., Wayner E. A., Carter W. G., Ferreira O. C., Jr Human B lymphocytes define an alternative mechanism of adhesion to fibronectin. The interaction of the alpha 4 beta 1 integrin with the LHGPEILDVPST sequence of the type III connecting segment is sufficient to promote cell attachment. J Immunol. 1990 May 1;144(9):3361–3366. [PubMed] [Google Scholar]
- Gassel A. M., Radzun M. L., Hansmann H. J., Weyand M., Konertz W. Monocytes and macrophages in the rejection of human cardiac allografts. Transplant Proc. 1989 Feb;21(1 Pt 3):2514–2516. [PubMed] [Google Scholar]
- Gismondi A., Morrone S., Humphries M. J., Piccoli M., Frati L., Santoni A. Human natural killer cells express VLA-4 and VLA-5, which mediate their adhesion to fibronectin. J Immunol. 1991 Jan 1;146(1):384–392. [PubMed] [Google Scholar]
- Gould V. E., Martinez-Lacabe V., Virtanen I., Sahlin K. M., Schwartz M. M. Differential distribution of tenascin and cellular fibronectins in acute and chronic renal allograft rejection. Lab Invest. 1992 Jul;67(1):71–79. [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Hershkoviz R., Gilat D., Miron S., Mekori Y. A., Aderka D., Wallach D., Vlodavsky I., Cohen I. R., Lider O. Extracellular matrix induces tumour necrosis factor-alpha secretion by an interaction between resting rat CD4+ T cells and macrophages. Immunology. 1993 Jan;78(1):50–57. [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: a family of cell surface receptors. Cell. 1987 Feb 27;48(4):549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Johnson M. R. Transplant coronary disease: nonimmunologic risk factors. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S124–S132. [PubMed] [Google Scholar]
- Jonjic N., Jílek P., Bernasconi S., Peri G., Martìn-Padura I., Cenzuales S., Dejana E., Mantovani A. Molecules involved in the adhesion and cytotoxicity of activated monocytes on endothelial cells. J Immunol. 1992 Apr 1;148(7):2080–2083. [PubMed] [Google Scholar]
- Komoriya A., Green L. J., Mervic M., Yamada S. S., Yamada K. M., Humphries M. J. The minimal essential sequence for a major cell type-specific adhesion site (CS1) within the alternatively spliced type III connecting segment domain of fibronectin is leucine-aspartic acid-valine. J Biol Chem. 1991 Aug 15;266(23):15075–15079. [PubMed] [Google Scholar]
- Krensky A. M., Weiss A., Crabtree G., Davis M. M., Parham P. T-lymphocyte-antigen interactions in transplant rejection. N Engl J Med. 1990 Feb 22;322(8):510–517. doi: 10.1056/NEJM199002223220805. [DOI] [PubMed] [Google Scholar]
- Kuwahara M., Jacobsson J., Kuwahara M., Kagan E., Ramwell P. W., Foegh M. L. Coronary artery ultrastructural changes in cardiac transplant atherosclerosis in the rabbit. Transplantation. 1991 Nov;52(5):759–765. doi: 10.1097/00007890-199111000-00001. [DOI] [PubMed] [Google Scholar]
- Laffón A., García-Vicuña R., Humbría A., Postigo A. A., Corbí A. L., de Landázuri M. O., Sánchez-Madrid F. Upregulated expression and function of VLA-4 fibronectin receptors on human activated T cells in rheumatoid arthritis. J Clin Invest. 1991 Aug;88(2):546–552. doi: 10.1172/JCI115338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. Y., Cheung H. T. Basement membrane and its components on lymphocyte adhesion, migration, and proliferation. J Immunol. 1992 Nov 15;149(10):3174–3181. [PubMed] [Google Scholar]
- Libby P., Swanson S. J., Tanaka H., Murray A., Schoen F. J., Pober J. S. Immunopathology of coronary arteriosclerosis in transplanted hearts. J Heart Lung Transplant. 1992 May-Jun;11(3 Pt 2):S5–S6. [PubMed] [Google Scholar]
- Makarem R., Newham P., Askari J. A., Green L. J., Clements J., Edwards M., Humphries M. J., Mould A. P. Competitive binding of vascular cell adhesion molecule-1 and the HepII/IIICS domain of fibronectin to the integrin alpha 4 beta 1. J Biol Chem. 1994 Feb 11;269(6):4005–4011. [PubMed] [Google Scholar]
- Matsuyama T., Yamada A., Kay J., Yamada K. M., Akiyama S. K., Schlossman S. F., Morimoto C. Activation of CD4 cells by fibronectin and anti-CD3 antibody. A synergistic effect mediated by the VLA-5 fibronectin receptor complex. J Exp Med. 1989 Oct 1;170(4):1133–1148. doi: 10.1084/jem.170.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molossi S., Clausell N., Rabinovitch M. Reciprocal induction of tumor necrosis factor-alpha and interleukin-1 beta activity mediates fibronectin synthesis in coronary artery smooth muscle cells. J Cell Physiol. 1995 Apr;163(1):19–29. doi: 10.1002/jcp.1041630104. [DOI] [PubMed] [Google Scholar]
- Osborn L. Leukocyte adhesion to endothelium in inflammation. Cell. 1990 Jul 13;62(1):3–6. doi: 10.1016/0092-8674(90)90230-c. [DOI] [PubMed] [Google Scholar]
- Romanic A. M., Madri J. A. The induction of 72-kD gelatinase in T cells upon adhesion to endothelial cells is VCAM-1 dependent. J Cell Biol. 1994 Jun;125(5):1165–1178. doi: 10.1083/jcb.125.5.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Integrins. J Clin Invest. 1991 Jan;87(1):1–5. doi: 10.1172/JCI114957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon R. N., Hughes C. C., Schoen F. J., Payne D. D., Pober J. S., Libby P. Human coronary transplantation-associated arteriosclerosis. Evidence for a chronic immune reaction to activated graft endothelial cells. Am J Pathol. 1991 Apr;138(4):791–798. [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Shimizu Y., van Seventer G. A., Horgan K. J., Shaw S. Roles of adhesion molecules in T-cell recognition: fundamental similarities between four integrins on resting human T cells (LFA-1, VLA-4, VLA-5, VLA-6) in expression, binding, and costimulation. Immunol Rev. 1990 Apr;114:109–143. doi: 10.1111/j.1600-065x.1990.tb00563.x. [DOI] [PubMed] [Google Scholar]
- Smith C. H., Barker J. N., Lee T. H. Adhesion molecules in allergic inflammation. Am Rev Respir Dis. 1993 Dec;148(6 Pt 2):S75–S78. doi: 10.1164/ajrccm/148.6_Pt_2.S75. [DOI] [PubMed] [Google Scholar]
- Takeuchi T., Amano K., Sekine H., Koide J., Abe T. Upregulated expression and function of integrin adhesive receptors in systemic lupus erythematosus patients with vasculitis. J Clin Invest. 1993 Dec;92(6):3008–3016. doi: 10.1172/JCI116924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayner E. A., Kovach N. L. Activation-dependent recognition by hematopoietic cells of the LDV sequence in the V region of fibronectin. J Cell Biol. 1992 Jan;116(2):489–497. doi: 10.1083/jcb.116.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa M., Tilney N. L., Kupiec-Weglinski J. W. Recognition of self within self: specific lymphocyte positioning and the extracellular matrix. Immunol Today. 1991 Aug;12(8):262–266. doi: 10.1016/0167-5699(91)90123-B. [DOI] [PubMed] [Google Scholar]
- van Seventer G. A., Shimizu Y., Shaw S. Roles of multiple accessory molecules in T-cell activation. Curr Opin Immunol. 1991 Jun;3(3):294–303. doi: 10.1016/0952-7915(91)90027-x. [DOI] [PubMed] [Google Scholar]







