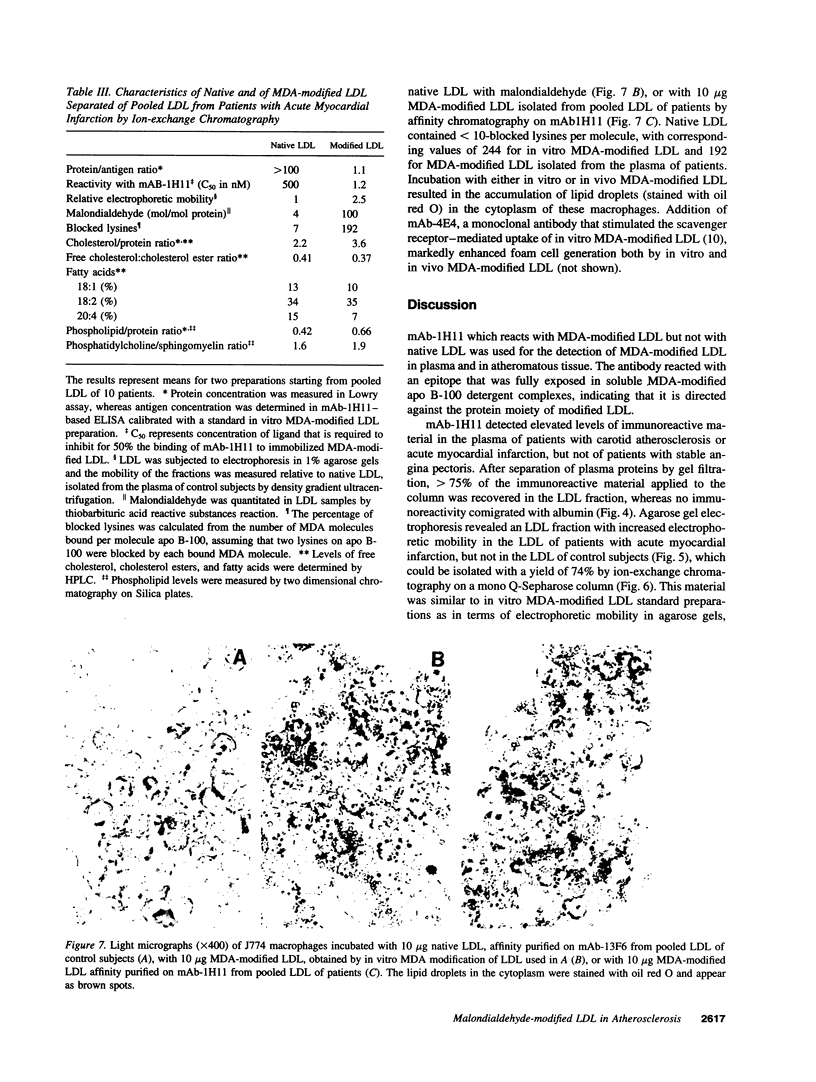
Abstract
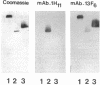
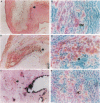
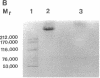
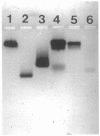
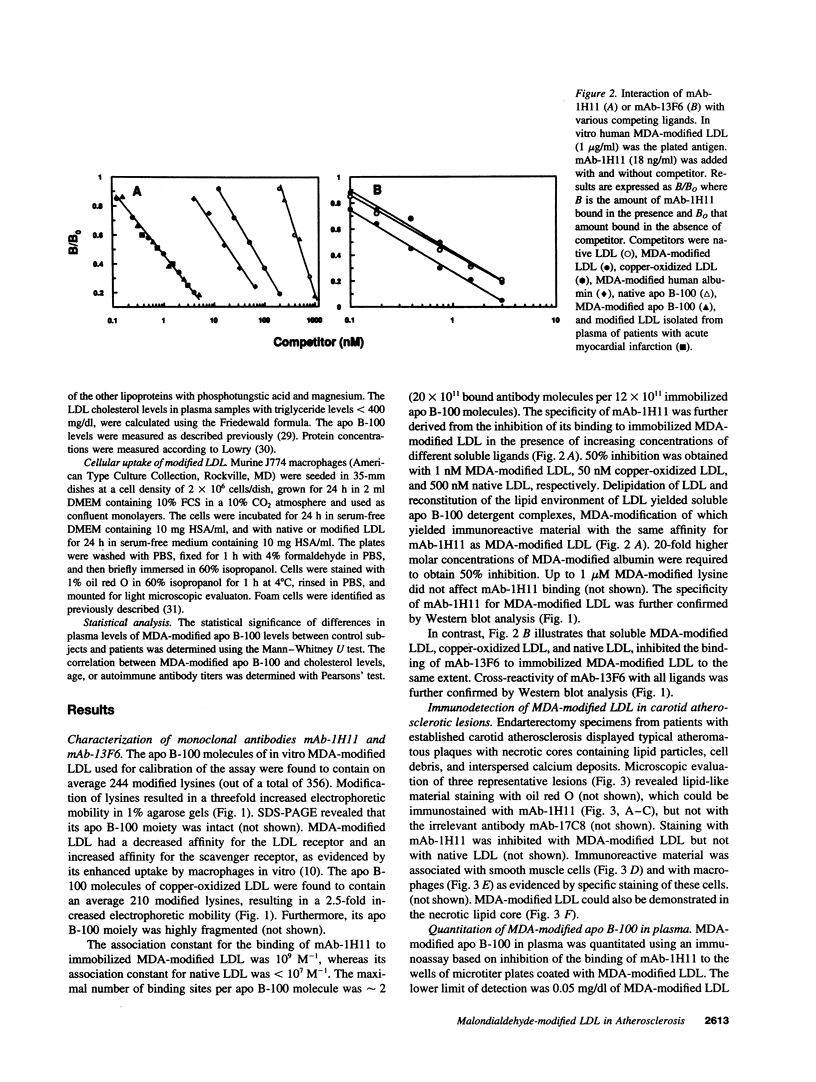
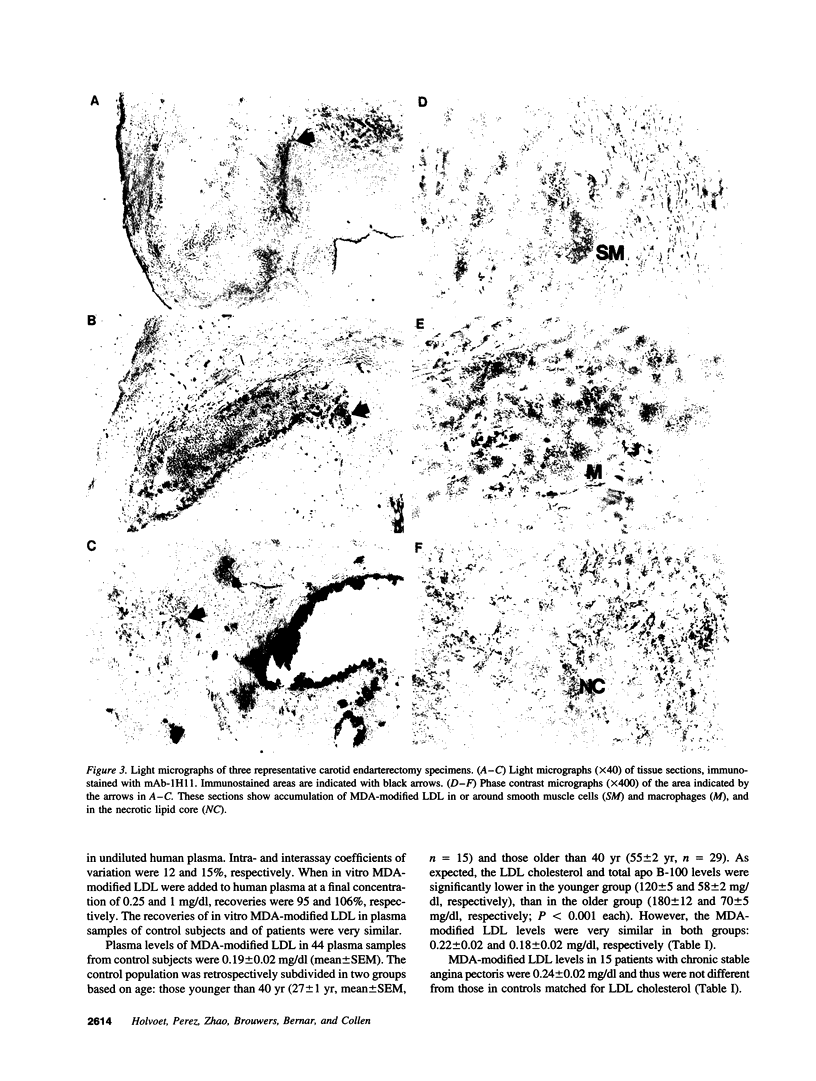
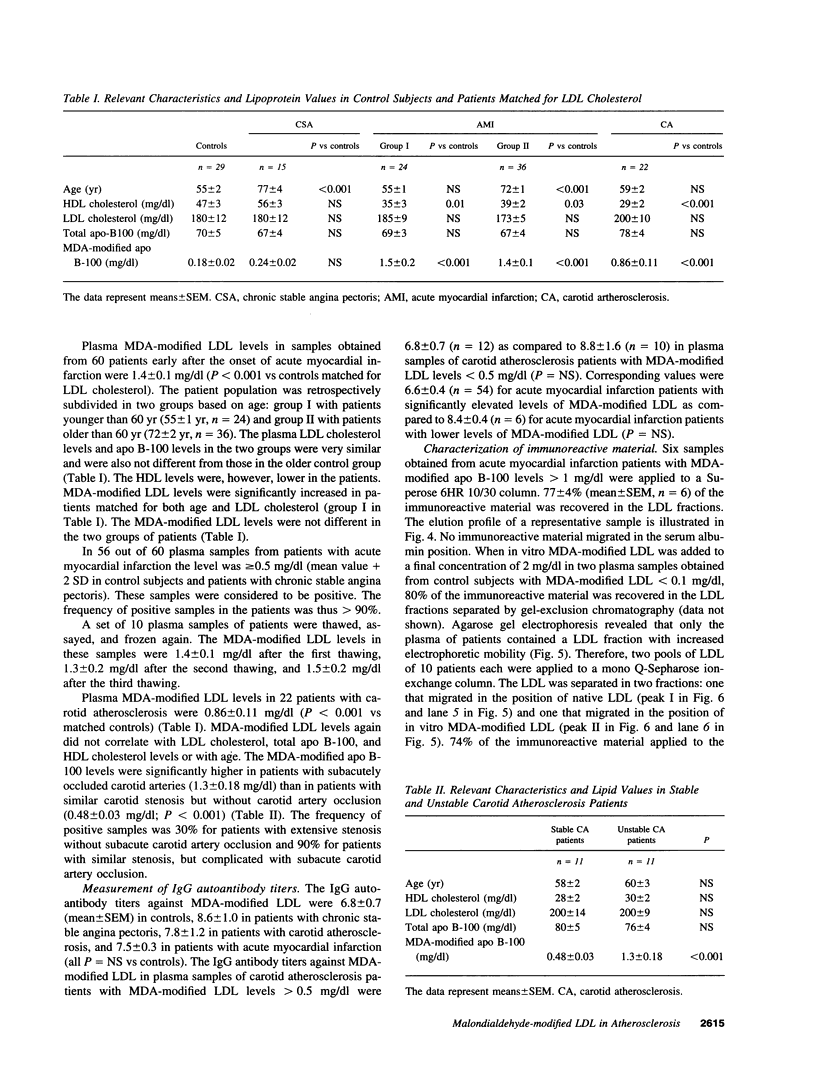
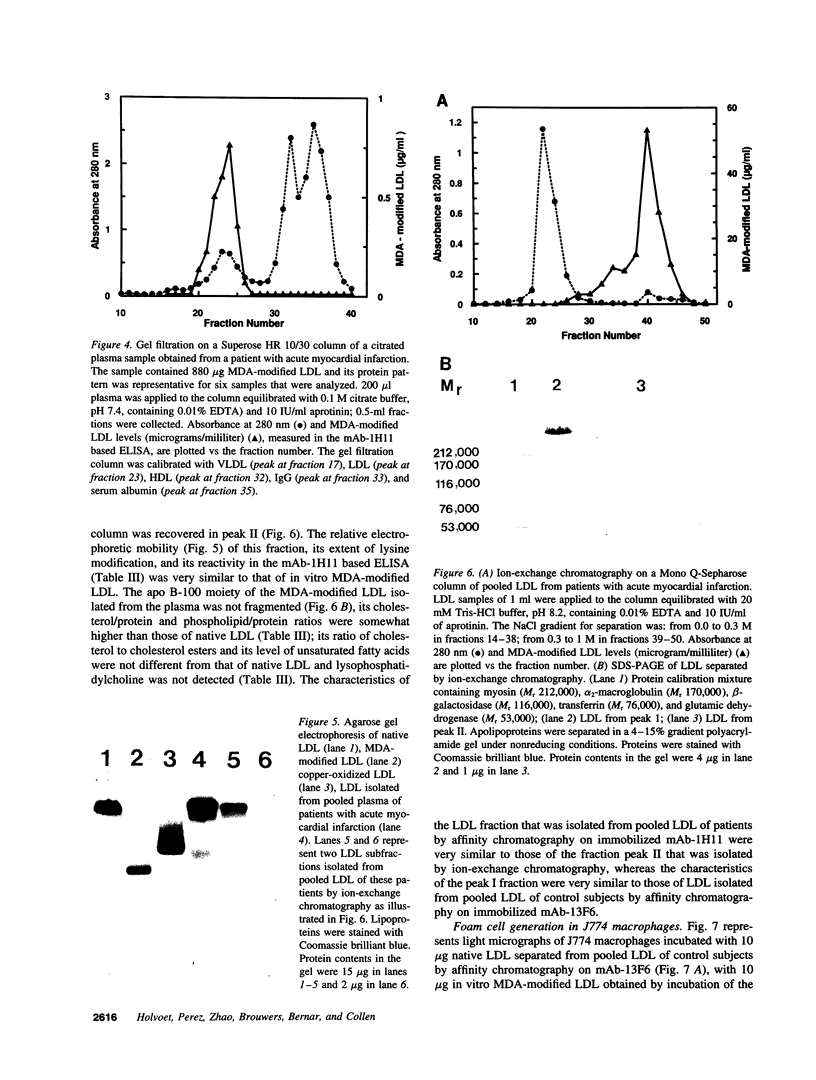
The murine monoclonal antibody mAb-1H11 raised against malondialdehyde (MDA)-modified LDL, was used to detect cross-reacting material in human atheromatous tissue and in plasma. MDA-modified LDL levels in plasma were 0.19 +/- 0.02 mg/dl (mean +/- SEM) in 44 control subjects, 0.24 +/- 0.02 mg/dl in 15 patients with chronic stable angina pectoris (P = NS vs LDL cholesterol matched controls), 1.4 +/- 0.1 mg/dl in 60 patients with acute myocardial infarction (P < 0.001 vs controls), and 0.86 +/- 0.11 mg/dl in 22 patients with carotid atherosclerosis (P < 0.001 vs controls). Modified LDL, isolated from pooled LDL of 10 patients, showed a higher electrophoretic mobility on agarose gels, a higher content of thiobarbituric acid reactive substances, and a higher cholesterol/protein ratio than native LDL and had a similar reactivity (antigen/protein ratio) in the assay as the in vitro MDA-modified LDL used for calibration. Its apo B-100 moiety was not fragmented. Uptake of this modified LDL by macrophages resulted in foam cell generation. In conclusion, elevated plasma levels of atherogenic MDA-modified LDL may be a marker for unstable atherosclerotic cardiovascular disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avogaro P., Bon G. B., Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis. 1988 Jan-Feb;8(1):79–87. [PubMed] [Google Scholar]
- Axén R., Porath J., Ernback S. Chemical coupling of peptides and proteins to polysaccharides by means of cyanogen halides. Nature. 1967 Jun 24;214(5095):1302–1304. doi: 10.1038/2141302a0. [DOI] [PubMed] [Google Scholar]
- Brousseau T., Clavey V., Bard J. M., Fruchart J. C. Sequential ultracentrifugation micromethod for separation of serum lipoproteins and assays of lipids, apolipoproteins, and lipoprotein particles. Clin Chem. 1993 Jun;39(6):960–964. [PubMed] [Google Scholar]
- Davies M. J., Richardson P. D., Woolf N., Katz D. R., Mann J. Risk of thrombosis in human atherosclerotic plaques: role of extracellular lipid, macrophage, and smooth muscle cell content. Br Heart J. 1993 May;69(5):377–381. doi: 10.1136/hrt.69.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Jürgens G., Quehenberger O., Koller E. Autoxidation of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res. 1987 May;28(5):495–509. [PubMed] [Google Scholar]
- Farber H. W., Barnett H. F. Differences in prostaglandin metabolism in cultured aortic and pulmonary arterial endothelial cells exposed to acute and chronic hypoxia. Circ Res. 1991 May;68(5):1446–1457. doi: 10.1161/01.res.68.5.1446. [DOI] [PubMed] [Google Scholar]
- Fogelman A. M., Shechter I., Seager J., Hokom M., Child J. S., Edwards P. A. Malondialdehyde alteration of low density lipoproteins leads to cholesteryl ester accumulation in human monocyte-macrophages. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2214–2218. doi: 10.1073/pnas.77.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu Rev Biochem. 1977;46:897–930. doi: 10.1146/annurev.bi.46.070177.004341. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fogelman A. M., Edwards P. A. Specificity of receptor-mediated recognition of malondialdehyde-modified low density lipoproteins. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1712–1716. doi: 10.1073/pnas.79.6.1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Haberland M. E., Olch C. L., Folgelman A. M. Role of lysines in mediating interaction of modified low density lipoproteins with the scavenger receptor of human monocyte macrophages. J Biol Chem. 1984 Sep 25;259(18):11305–11311. [PubMed] [Google Scholar]
- Hoff H. F., O'Neil J., Chisolm G. M., 3rd, Cole T. B., Quehenberger O., Esterbauer H., Jürgens G. Modification of low density lipoprotein with 4-hydroxynonenal induces uptake by macrophages. Arteriosclerosis. 1989 Jul-Aug;9(4):538–549. doi: 10.1161/01.atv.9.4.538. [DOI] [PubMed] [Google Scholar]
- Hoff H. F., O'Neil J. Lesion-derived low density lipoprotein and oxidized low density lipoprotein share a lability for aggregation, leading to enhanced macrophage degradation. Arterioscler Thromb. 1991 Sep-Oct;11(5):1209–1222. doi: 10.1161/01.atv.11.5.1209. [DOI] [PubMed] [Google Scholar]
- Holvoet P., Lijnen H. R., Collen D. A monoclonal antibody specific for Lys-plasminogen. Application to the study of the activation pathways of plasminogen in vivo. J Biol Chem. 1985 Oct 5;260(22):12106–12111. [PubMed] [Google Scholar]
- Holvoet P., Perez G., Bernar H., Brouwers E., Vanloo B., Rosseneu M., Collen D. Stimulation with a monoclonal antibody (mAb4E4) of scavenger receptor-mediated uptake of chemically modified low density lipoproteins by THP-1-derived macrophages enhances foam cell generation. J Clin Invest. 1994 Jan;93(1):89–98. doi: 10.1172/JCI116988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lynch S. M., Morrow J. D., Roberts L. J., 2nd, Frei B. Formation of non-cyclooxygenase-derived prostanoids (F2-isoprostanes) in plasma and low density lipoprotein exposed to oxidative stress in vitro. J Clin Invest. 1994 Mar;93(3):998–1004. doi: 10.1172/JCI117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow J. D., Awad J. A., Boss H. J., Blair I. A., Roberts L. J., 2nd Non-cyclooxygenase-derived prostanoids (F2-isoprostanes) are formed in situ on phospholipids. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10721–10725. doi: 10.1073/pnas.89.22.10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salonen J. T., Ylä-Herttuala S., Yamamoto R., Butler S., Korpela H., Salonen R., Nyyssönen K., Palinski W., Witztum J. L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992 Apr 11;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Schaffner T., Taylor K., Bartucci E. J., Fischer-Dzoga K., Beeson J. H., Glagov S., Wissler R. W. Arterial foam cells with distinctive immunomorphologic and histochemical features of macrophages. Am J Pathol. 1980 Jul;100(1):57–80. [PMC free article] [PubMed] [Google Scholar]
- Shimano H., Yamada N., Ishibashi S., Mokuno H., Mori N., Gotoda T., Harada K., Akanuma Y., Murase T., Yazaki Y. Oxidation-labile subfraction of human plasma low density lipoprotein isolated by ion-exchange chromatography. J Lipid Res. 1991 May;32(5):763–773. [PubMed] [Google Scholar]
- Siedel J., Schiefer S., Rosseneu M., Bergeaud R., De Keersgieter W., Pautz B., Vinaimont N., Ziegenhorn J. Immunoturbidimetric method for routine determinations of apolipoproteins A-I, A-II, and B in normo- and hyperlipemic sera compared with immunonephelometry. Clin Chem. 1988 Sep;34(9):1821–1825. [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J Lipid Res. 1988 Jun;29(6):745–753. [PubMed] [Google Scholar]
- Steele J. C., Jr, Reynolds J. A. Characterization of the apolipoprotein B polypeptide of human plasma low density lipoprotein in detergent and denaturation solutions. J Biol Chem. 1979 Mar 10;254(5):1633–1638. [PubMed] [Google Scholar]
- Steinberg D., Witztum J. L. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990 Dec 19;264(23):3047–3052. [PubMed] [Google Scholar]
- Steinbrecher U. P., Lougheed M. Scavenger receptor-independent stimulation of cholesterol esterification in macrophages by low density lipoprotein extracted from human aortic intima. Arterioscler Thromb. 1992 May;12(5):608–625. doi: 10.1161/01.atv.12.5.608. [DOI] [PubMed] [Google Scholar]
- Steinbrecher U. P. Oxidation of human low density lipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987 Mar 15;262(8):3603–3608. [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbrecher U. P., Witztum J. L., Parthasarathy S., Steinberg D. Decrease in reactive amino groups during oxidation or endothelial cell modification of LDL. Correlation with changes in receptor-mediated catabolism. Arteriosclerosis. 1987 Mar-Apr;7(2):135–143. doi: 10.1161/01.atv.7.2.135. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Inorganic and organic phosphate measurements in the nanomolar range. Anal Biochem. 1987 Feb 15;161(1):45–48. doi: 10.1016/0003-2697(87)90649-x. [DOI] [PubMed] [Google Scholar]
- Van Veldhoven P. P., Mannaerts G. P. Sphinganine 1-phosphate metabolism in cultured skin fibroblasts: evidence for the existence of a sphingosine phosphatase. Biochem J. 1994 May 1;299(Pt 3):597–601. doi: 10.1042/bj2990597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercaemst R., Union A., Rosseneu M., De Craene I., De Backer G., Kornitzer M. Quantitation of plasma free cholesterol and cholesteryl esters by high performance liquid chromatography. Study of a normal population. Atherosclerosis. 1989 Aug;78(2-3):245–250. doi: 10.1016/0021-9150(89)90230-x. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Butler S. W., Picard S., Steinberg D., Witztum J. L. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994 Jan;14(1):32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]