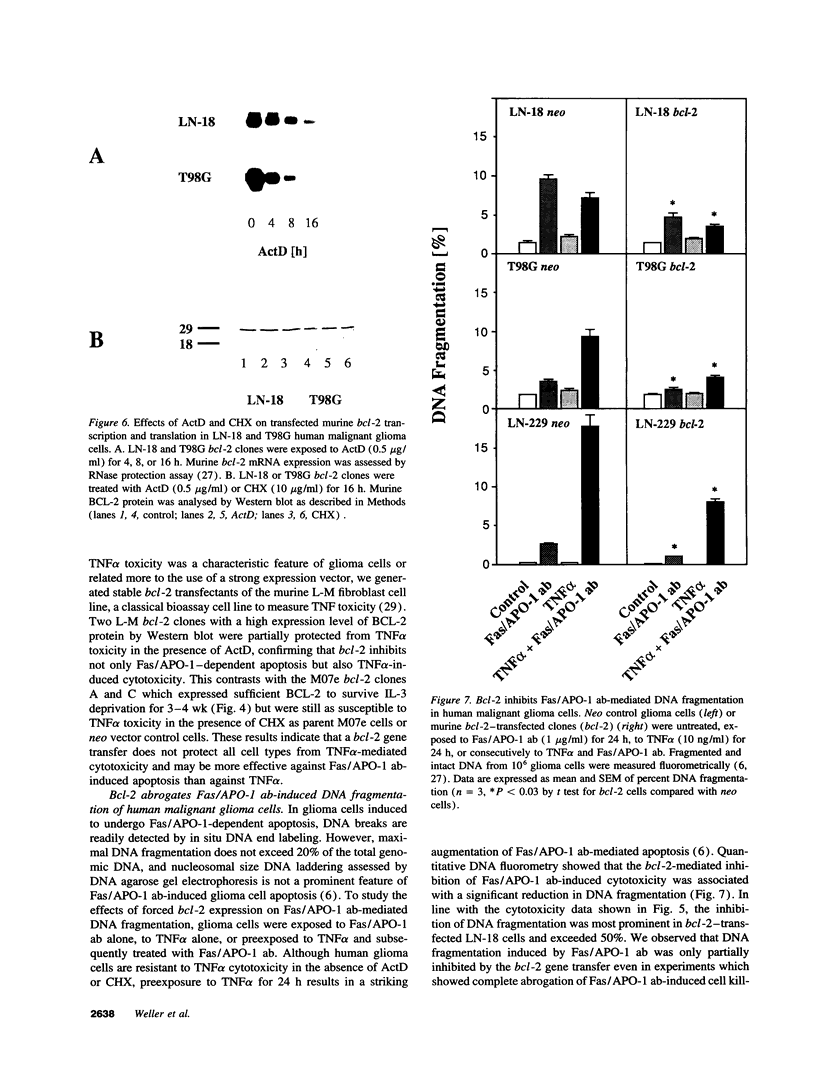
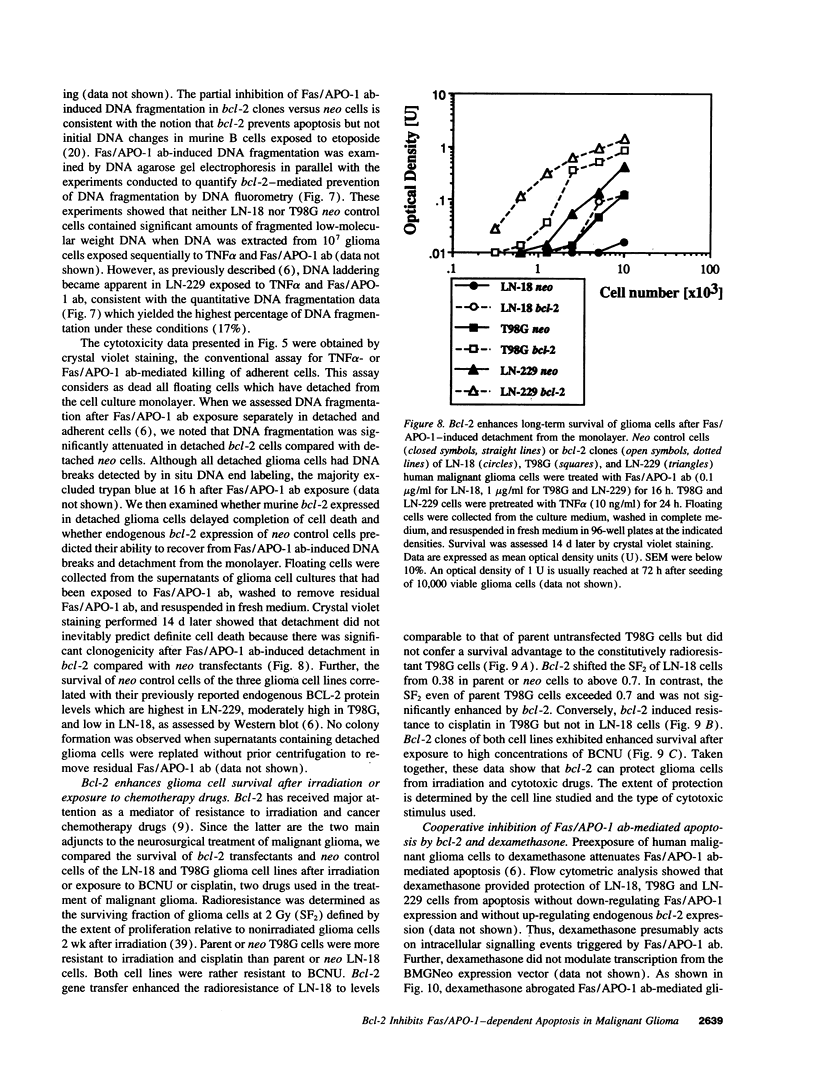
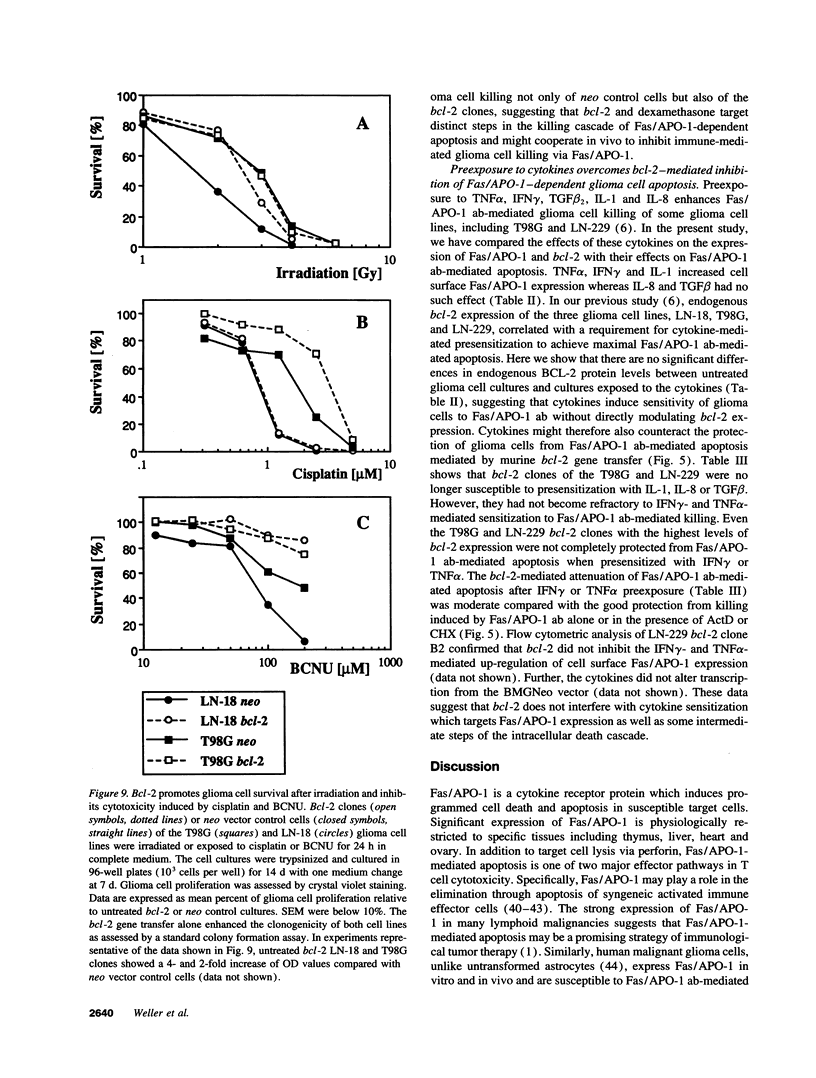
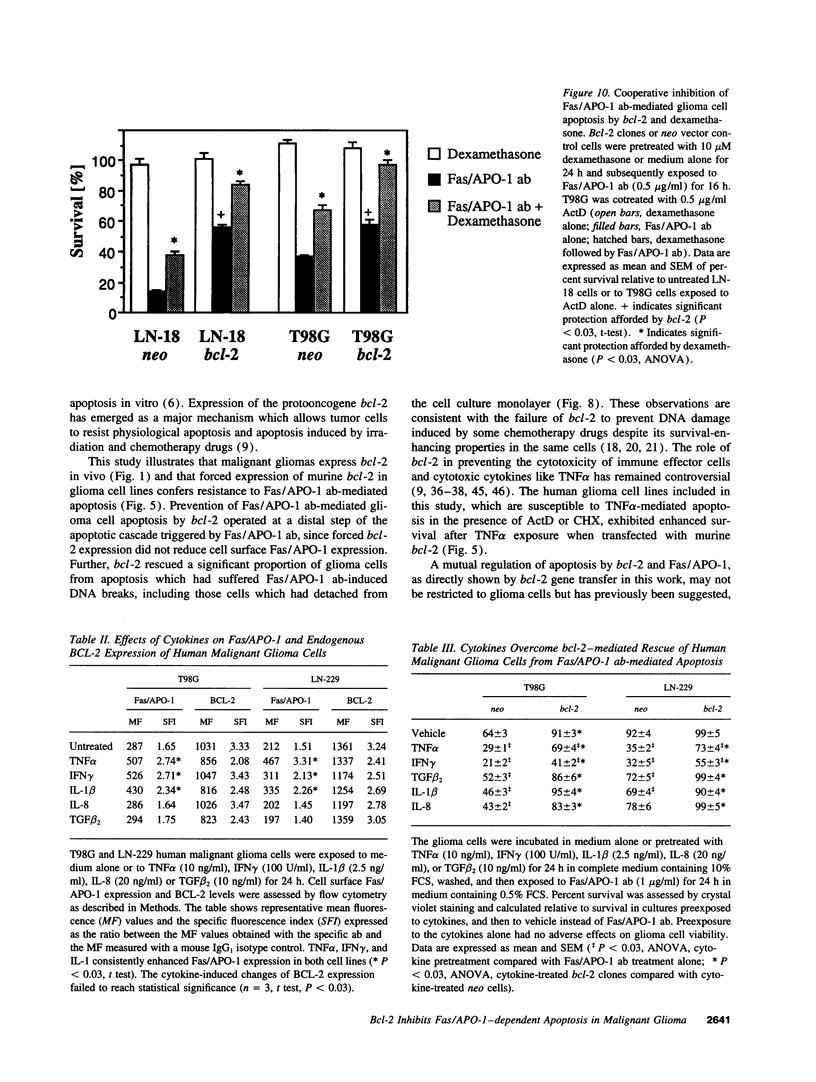
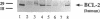Abstract
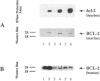
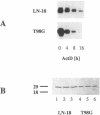
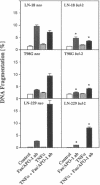
The majority of human malignant glioma cells express Fas/APO-1 and are susceptible to Fas/APO-1 antibody-mediated apoptosis in vitro. The sensitivity of Fas/APO-1-positive glioma cell lines to Fas/APO-1 antibody-mediated killing correlates inversely with the constitutive expression of the antiapoptotic protooncogene bcl-2. Here we report that BCL-2 protein expression of human glial tumors in vivo correlates with malignant transformation in that BCL-2 immunoreactive glioma cells were more abundant in WHO grade III/IV gliomas than in grade I/II gliomas. Fas/APO-1 antibody-sensitive human glioma cell lines stably transfected with a murine bcl-2 cDNA acquired resistance to Fas/APO-1 antibody-mediated apoptosis. Forced expression of bcl-2 also attenuated TNF alpha-mediated cytotoxicity of glioma cell lines in the presence of actinomycin D and cycloheximide and conferred partial protection from irradiation and the cancer chemotherapy drugs, cisplatin and BCNU. Preexposure of the glioma cell lines to the cytokines, IFN gamma and TNF alpha, which sensitize for Fas/APO-1-dependent killing, partially overcame bcl-2-mediated rescue from apoptosis, suggesting that multimodality immunotherapy involving cytokines and Fas/APO-1 targeting might eventually provide a promising approach to the treatment of human malignant gliomas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiello A., Delia D., Borrello M. G., Biassoni D., Giardini R., Fontanella E., Pezzella F., Pulford K., Pierotti M., Della Porta G. Flow cytometric detection of the mitochondrial BCL-2 protein in normal and neoplastic human lymphoid cells. Cytometry. 1992;13(5):502–509. doi: 10.1002/cyto.990130509. [DOI] [PubMed] [Google Scholar]
- Castle V. P., Heidelberger K. P., Bromberg J., Ou X., Dole M., Nuñez G. Expression of the apoptosis-suppressing protein bcl-2, in neuroblastoma is associated with unfavorable histology and N-myc amplification. Am J Pathol. 1993 Dec;143(6):1543–1550. [PMC free article] [PubMed] [Google Scholar]
- Cifone M. G., De Maria R., Roncaioli P., Rippo M. R., Azuma M., Lanier L. L., Santoni A., Testi R. Apoptotic signaling through CD95 (Fas/Apo-1) activates an acidic sphingomyelinase. J Exp Med. 1994 Oct 1;180(4):1547–1552. doi: 10.1084/jem.180.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins M. K., Marvel J., Malde P., Lopez-Rivas A. Interleukin 3 protects murine bone marrow cells from apoptosis induced by DNA damaging agents. J Exp Med. 1992 Oct 1;176(4):1043–1051. doi: 10.1084/jem.176.4.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng G., Podack E. R. Suppression of apoptosis in a cytotoxic T-cell line by interleukin 2-mediated gene transcription and deregulated expression of the protooncogene bcl-2. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2189–2193. doi: 10.1073/pnas.90.6.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher T. C., Milner A. E., Gregory C. D., Jackman A. L., Aherne G. W., Hartley J. A., Dive C., Hickman J. A. bcl-2 modulation of apoptosis induced by anticancer drugs: resistance to thymidylate stress is independent of classical resistance pathways. Cancer Res. 1993 Jul 15;53(14):3321–3326. [PubMed] [Google Scholar]
- Fontana A., Hengartner H., de Tribolet N., Weber E. Glioblastoma cells release interleukin 1 and factors inhibiting interleukin 2-mediated effects. J Immunol. 1984 Apr;132(4):1837–1844. [PubMed] [Google Scholar]
- Frei K., Piani D., Malipiero U. V., Van Meir E., de Tribolet N., Fontana A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) production by glioblastoma cells. Despite the presence of inducing signals GM-CSF is not expressed in vivo. J Immunol. 1992 May 15;148(10):3140–3146. [PubMed] [Google Scholar]
- Frei K., Siepl C., Groscurth P., Bodmer S., Schwerdel C., Fontana A. Antigen presentation and tumor cytotoxicity by interferon-gamma-treated microglial cells. Eur J Immunol. 1987 Sep;17(9):1271–1278. doi: 10.1002/eji.1830170909. [DOI] [PubMed] [Google Scholar]
- Färkkilä M., Jäskeläinen J., Kallio M., Blomstedt G., Raininko R., Virkkunen P., Paetau A., Sarelin H., Mäntylä M. Randomised, controlled study of intratumoral recombinant gamma-interferon treatment in newly diagnosed glioblastoma. Br J Cancer. 1994 Jul;70(1):138–141. doi: 10.1038/bjc.1994.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S. D., Goodhead D. T., Marsden S. J., Wright E. G., Krajewski S., Reed J. C., Korsmeyer S. J., Greaves M. Interleukin 7-dependent B lymphocyte precursor cells are ultrasensitive to apoptosis. J Exp Med. 1994 Jun 1;179(6):1789–1797. doi: 10.1084/jem.179.6.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada M., Krajewski S., Tanaka S., Cazals-Hatem D., Spengler B. A., Ross R. A., Biedler J. L., Reed J. C. Regulation of Bcl-2 oncoprotein levels with differentiation of human neuroblastoma cells. Cancer Res. 1993 Oct 15;53(20):4978–4986. [PubMed] [Google Scholar]
- Hennet T., Bertoni G., Richter C., Peterhans E. Expression of BCL-2 protein enhances the survival of mouse fibrosarcoid cells in tumor necrosis factor-mediated cytotoxicity. Cancer Res. 1993 Mar 15;53(6):1456–1460. [PubMed] [Google Scholar]
- Hockenbery D. M., Oltvai Z. N., Yin X. M., Milliman C. L., Korsmeyer S. J. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993 Oct 22;75(2):241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- Hockenbery D. M., Zutter M., Hickey W., Nahm M., Korsmeyer S. J. BCL2 protein is topographically restricted in tissues characterized by apoptotic cell death. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):6961–6965. doi: 10.1073/pnas.88.16.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug H., Enari M., Nagata S. No requirement of reactive oxygen intermediates in Fas-mediated apoptosis. FEBS Lett. 1994 Sep 12;351(3):311–313. doi: 10.1016/0014-5793(94)00852-3. [DOI] [PubMed] [Google Scholar]
- Itoh N., Tsujimoto Y., Nagata S. Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol. 1993 Jul 15;151(2):621–627. [PubMed] [Google Scholar]
- Itoh N., Yonehara S., Ishii A., Yonehara M., Mizushima S., Sameshima M., Hase A., Seto Y., Nagata S. The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991 Jul 26;66(2):233–243. doi: 10.1016/0092-8674(91)90614-5. [DOI] [PubMed] [Google Scholar]
- Iwai K., Miyawaki T., Takizawa T., Konno A., Ohta K., Yachie A., Seki H., Taniguchi N. Differential expression of bcl-2 and susceptibility to anti-Fas-mediated cell death in peripheral blood lymphocytes, monocytes, and neutrophils. Blood. 1994 Aug 15;84(4):1201–1208. [PubMed] [Google Scholar]
- Kamesaki S., Kamesaki H., Jorgensen T. J., Tanizawa A., Pommier Y., Cossman J. bcl-2 protein inhibits etoposide-induced apoptosis through its effects on events subsequent to topoisomerase II-induced DNA strand breaks and their repair. Cancer Res. 1993 Sep 15;53(18):4251–4256. [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kitada S., Takayama S., De Riel K., Tanaka S., Reed J. C. Reversal of chemoresistance of lymphoma cells by antisense-mediated reduction of bcl-2 gene expression. Antisense Res Dev. 1994 Summer;4(2):71–79. doi: 10.1089/ard.1994.4.71. [DOI] [PubMed] [Google Scholar]
- Kondo E., Yoshino T., Yamadori I., Matsuo Y., Kawasaki N., Minowada J., Akagi T. Expression of Bcl-2 protein and Fas antigen in non-Hodgkin's lymphomas. Am J Pathol. 1994 Aug;145(2):330–337. [PMC free article] [PubMed] [Google Scholar]
- Kägi D., Vignaux F., Ledermann B., Bürki K., Depraetere V., Nagata S., Hengartner H., Golstein P. Fas and perforin pathways as major mechanisms of T cell-mediated cytotoxicity. Science. 1994 Jul 22;265(5171):528–530. doi: 10.1126/science.7518614. [DOI] [PubMed] [Google Scholar]
- Leithäuser F., Dhein J., Mechtersheimer G., Koretz K., Brüderlein S., Henne C., Schmidt A., Debatin K. M., Krammer P. H., Möller P. Constitutive and induced expression of APO-1, a new member of the nerve growth factor/tumor necrosis factor receptor superfamily, in normal and neoplastic cells. Lab Invest. 1993 Oct;69(4):415–429. [PubMed] [Google Scholar]
- Mapara M. Y., Bargou R., Zugck C., Döhner H., Ustaoglu F., Jonker R. R., Krammer P. H., Dörken B. APO-1 mediated apoptosis or proliferation in human chronic B lymphocytic leukemia: correlation with bcl-2 oncogene expression. Eur J Immunol. 1993 Mar;23(3):702–708. doi: 10.1002/eji.1830230320. [DOI] [PubMed] [Google Scholar]
- Marvel J., Perkins G. R., Lopez Rivas A., Collins M. K. Growth factor starvation of bcl-2 overexpressing murine bone marrow cells induced refractoriness to IL-3 stimulation of proliferation. Oncogene. 1994 Apr;9(4):1117–1122. [PubMed] [Google Scholar]
- McDonnell T. J., Troncoso P., Brisbay S. M., Logothetis C., Chung L. W., Hsieh J. T., Tu S. M., Campbell M. L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992 Dec 15;52(24):6940–6944. [PubMed] [Google Scholar]
- Merry D. E., Veis D. J., Hickey W. F., Korsmeyer S. J. bcl-2 protein expression is widespread in the developing nervous system and retained in the adult PNS. Development. 1994 Feb;120(2):301–311. doi: 10.1242/dev.120.2.301. [DOI] [PubMed] [Google Scholar]
- Miescher S., Whiteside T. L., de Tribolet N., von Fliedner V. In situ characterization, clonogenic potential, and antitumor cytolytic activity of T lymphocytes infiltrating human brain cancers. J Neurosurg. 1988 Mar;68(3):438–448. doi: 10.3171/jns.1988.68.3.0438. [DOI] [PubMed] [Google Scholar]
- Miyashita T., Krajewski S., Krajewska M., Wang H. G., Lin H. K., Liebermann D. A., Hoffman B., Reed J. C. Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene. 1994 Jun;9(6):1799–1805. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. Bcl-2 oncoprotein blocks chemotherapy-induced apoptosis in a human leukemia cell line. Blood. 1993 Jan 1;81(1):151–157. [PubMed] [Google Scholar]
- Miyashita T., Reed J. C. bcl-2 gene transfer increases relative resistance of S49.1 and WEHI7.2 lymphoid cells to cell death and DNA fragmentation induced by glucocorticoids and multiple chemotherapeutic drugs. Cancer Res. 1992 Oct 1;52(19):5407–5411. [PubMed] [Google Scholar]
- Nuñez G., London L., Hockenbery D., Alexander M., McKearn J. P., Korsmeyer S. J. Deregulated Bcl-2 gene expression selectively prolongs survival of growth factor-deprived hemopoietic cell lines. J Immunol. 1990 May 1;144(9):3602–3610. [PubMed] [Google Scholar]
- Oehm A., Behrmann I., Falk W., Pawlita M., Maier G., Klas C., Li-Weber M., Richards S., Dhein J., Trauth B. C. Purification and molecular cloning of the APO-1 cell surface antigen, a member of the tumor necrosis factor/nerve growth factor receptor superfamily. Sequence identity with the Fas antigen. J Biol Chem. 1992 May 25;267(15):10709–10715. [PubMed] [Google Scholar]
- Owen-Schaub L. B., Radinsky R., Kruzel E., Berry K., Yonehara S. Anti-Fas on nonhematopoietic tumors: levels of Fas/APO-1 and bcl-2 are not predictive of biological responsiveness. Cancer Res. 1994 Mar 15;54(6):1580–1586. [PubMed] [Google Scholar]
- Owen-Schaub L. B., Yonehara S., Crump W. L., 3rd, Grimm E. A. DNA fragmentation and cell death is selectively triggered in activated human lymphocytes by Fas antigen engagement. Cell Immunol. 1992 Mar;140(1):197–205. doi: 10.1016/0008-8749(92)90187-t. [DOI] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Meister L., Tanaka S., Cuddy M., Yum S., Geyer C., Pleasure D. Differential expression of bcl2 protooncogene in neuroblastoma and other human tumor cell lines of neural origin. Cancer Res. 1991 Dec 15;51(24):6529–6538. [PubMed] [Google Scholar]
- Richardson B. C., Lalwani N. D., Johnson K. J., Marks R. M. Fas ligation triggers apoptosis in macrophages but not endothelial cells. Eur J Immunol. 1994 Nov;24(11):2640–2645. doi: 10.1002/eji.1830241111. [DOI] [PubMed] [Google Scholar]
- Rouvier E., Luciani M. F., Golstein P. Fas involvement in Ca(2+)-independent T cell-mediated cytotoxicity. J Exp Med. 1993 Jan 1;177(1):195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon M., Pilling D., Borthwick N. J., Viner N., Janossy G., Bacon P. A., Akbar A. N. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J Immunol. 1994 Apr;24(4):892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- Schulze-Osthoff K., Krammer P. H., Dröge W. Divergent signalling via APO-1/Fas and the TNF receptor, two homologous molecules involved in physiological cell death. EMBO J. 1994 Oct 3;13(19):4587–4596. doi: 10.1002/j.1460-2075.1994.tb06780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentman C. L., Shutter J. R., Hockenbery D., Kanagawa O., Korsmeyer S. J. bcl-2 inhibits multiple forms of apoptosis but not negative selection in thymocytes. Cell. 1991 Nov 29;67(5):879–888. doi: 10.1016/0092-8674(91)90361-2. [DOI] [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Suda T., Takahashi T., Golstein P., Nagata S. Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell. 1993 Dec 17;75(6):1169–1178. doi: 10.1016/0092-8674(93)90326-l. [DOI] [PubMed] [Google Scholar]
- Taghian A., Ramsay J., Allalunis-Turner J., Budach W., Gioioso D., Pardo F., Okunieff P., Bleehen N., Urtasun R., Suit H. Intrinsic radiation sensitivity may not be the major determinant of the poor clinical outcome of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1993 Jan 15;25(2):243–249. doi: 10.1016/0360-3016(93)90345-v. [DOI] [PubMed] [Google Scholar]
- Torigoe T., Millan J. A., Takayama S., Taichman R., Miyashita T., Reed J. C. Bcl-2 inhibits T-cell-mediated cytolysis of a leukemia cell line. Cancer Res. 1994 Sep 15;54(18):4851–4854. [PubMed] [Google Scholar]
- Trauth B. C., Klas C., Peters A. M., Matzku S., Möller P., Falk W., Debatin K. M., Krammer P. H. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989 Jul 21;245(4915):301–305. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B., Reed J. C., De Valck D., Grooten J., Miyashita T., Tanaka S., Beyaert R., Van Roy F., Fiers W. Effect of bcl-2 proto-oncogene expression on cellular sensitivity to tumor necrosis factor-mediated cytotoxicity. Oncogene. 1993 Apr;8(4):1075–1081. [PubMed] [Google Scholar]
- Vaux D. L., Aguila H. L., Weissman I. L. Bcl-2 prevents death of factor-deprived cells but fails to prevent apoptosis in targets of cell mediated killing. Int Immunol. 1992 Jul;4(7):821–824. doi: 10.1093/intimm/4.7.821. [DOI] [PubMed] [Google Scholar]
- Vignaux F., Golstein P. Fas-based lymphocyte-mediated cytotoxicity against syngeneic activated lymphocytes: a regulatory pathway? Eur J Immunol. 1994 Apr;24(4):923–927. doi: 10.1002/eji.1830240421. [DOI] [PubMed] [Google Scholar]
- Walton M. I., Whysong D., O'Connor P. M., Hockenbery D., Korsmeyer S. J., Kohn K. W. Constitutive expression of human Bcl-2 modulates nitrogen mustard and camptothecin induced apoptosis. Cancer Res. 1993 Apr 15;53(8):1853–1861. [PubMed] [Google Scholar]
- Weller M., Constam D. B., Malipiero U., Fontana A. Transforming growth factor-beta 2 induces apoptosis of murine T cell clones without down-regulating bcl-2 mRNA expression. Eur J Immunol. 1994 Jun;24(6):1293–1300. doi: 10.1002/eji.1830240608. [DOI] [PubMed] [Google Scholar]
- Weller M., Frei K., Groscurth P., Krammer P. H., Yonekawa Y., Fontana A. Anti-Fas/APO-1 antibody-mediated apoptosis of cultured human glioma cells. Induction and modulation of sensitivity by cytokines. J Clin Invest. 1994 Sep;94(3):954–964. doi: 10.1172/JCI117462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Fas antigen and p55 TNF receptor signal apoptosis through distinct pathways. J Immunol. 1994 Feb 15;152(4):1751–1755. [PubMed] [Google Scholar]
- Wrann M., Bodmer S., de Martin R., Siepl C., Hofer-Warbinek R., Frei K., Hofer E., Fontana A. T cell suppressor factor from human glioblastoma cells is a 12.5-kd protein closely related to transforming growth factor-beta. EMBO J. 1987 Jun;6(6):1633–1636. doi: 10.1002/j.1460-2075.1987.tb02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonehara S., Ishii A., Yonehara M. A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med. 1989 May 1;169(5):1747–1756. doi: 10.1084/jem.169.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida J., Wakabayashi T., Mizuno M., Sugita K., Yoshida T., Hori S., Mori T., Sato T., Karashima A., Kurisu K. Clinical effect of intra-arterial tumor necrosis factor-alpha for malignant glioma. J Neurosurg. 1992 Jul;77(1):78–83. doi: 10.3171/jns.1992.77.1.0078. [DOI] [PubMed] [Google Scholar]
- de Martin R., Haendler B., Hofer-Warbinek R., Gaugitsch H., Wrann M., Schlüsener H., Seifert J. M., Bodmer S., Fontana A., Hofer E. Complementary DNA for human glioblastoma-derived T cell suppressor factor, a novel member of the transforming growth factor-beta gene family. EMBO J. 1987 Dec 1;6(12):3673–3677. doi: 10.1002/j.1460-2075.1987.tb02700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]