Abstract
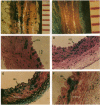
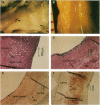
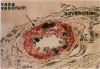
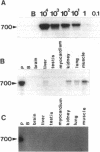
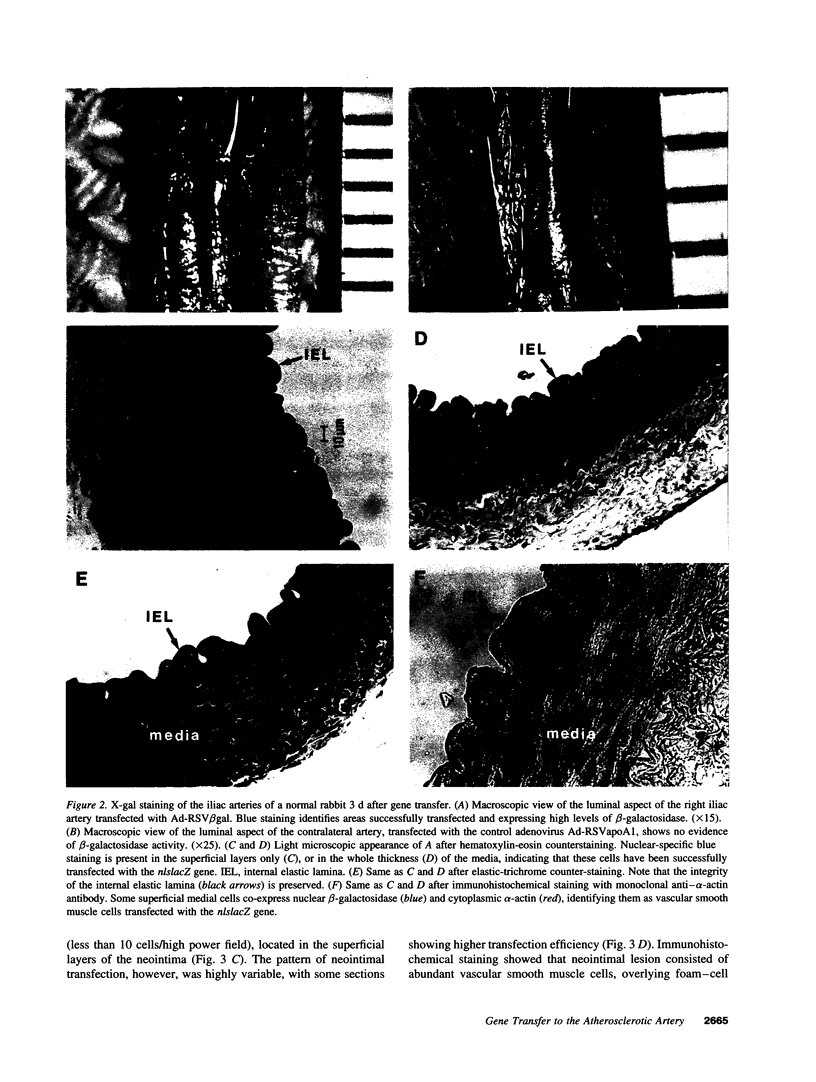
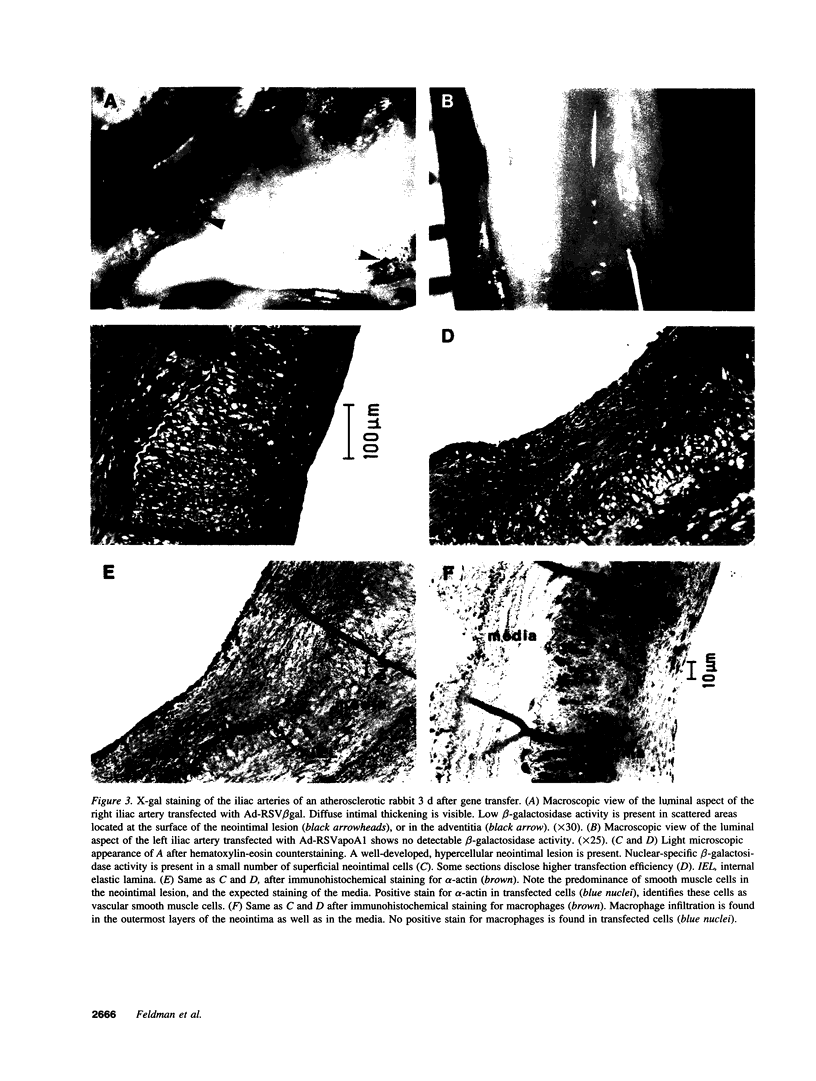
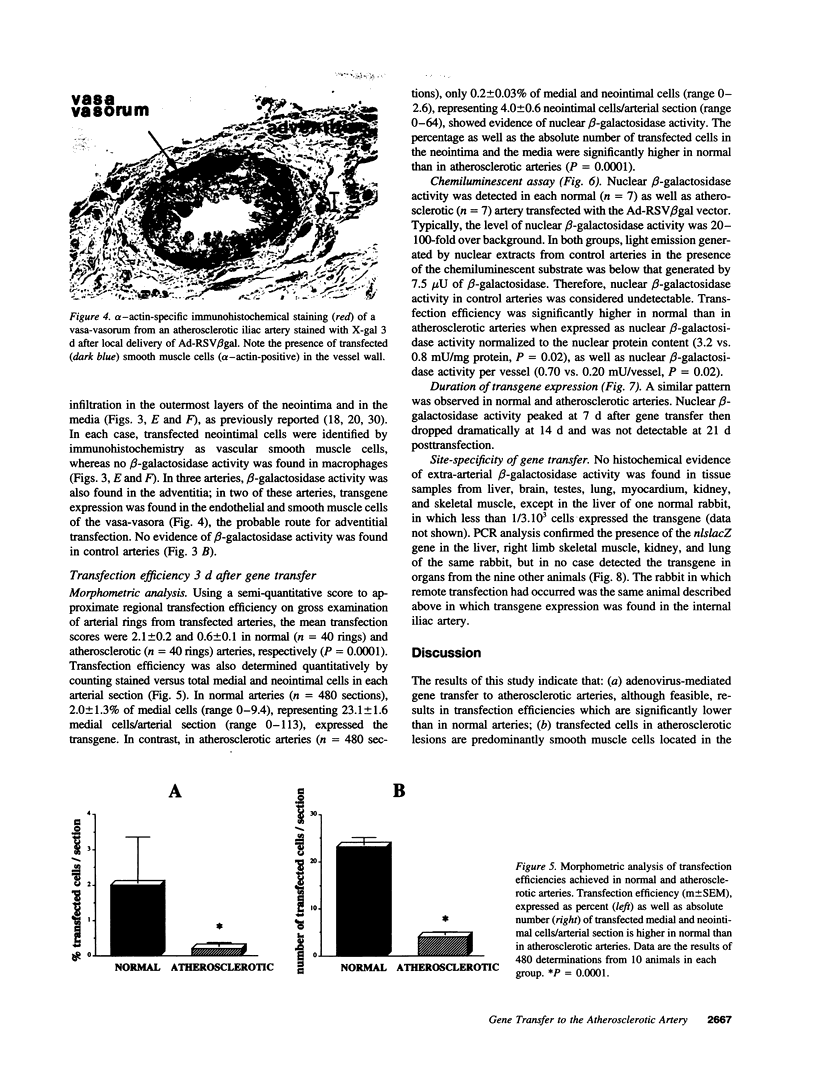
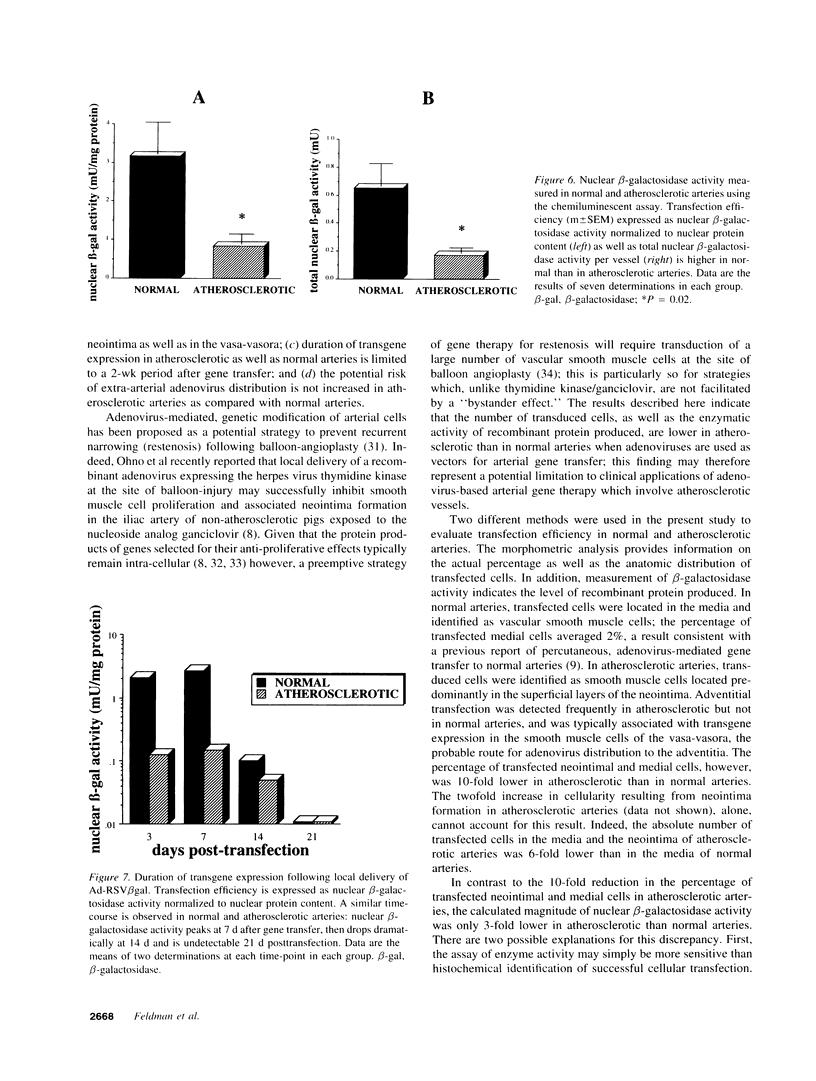
Recombinant adenoviruses are the most efficient vectors with which to perform arterial gene transfer. Previous in vivo studies of adenovirus-mediated arterial transfection, however, have been performed using normal or endothelium-denuded arteries. It is unclear whether these results can be extended to atherosclerotic arteries. Accordingly, this study was designed to (a) assess the feasibility of adenovirus-mediated gene transfer to atherosclerotic lesions, and (b) compare the transfection efficiency, anatomic distribution of transfected cells, and duration of transgene expression achieved in normal versus atherosclerotic arteries. A recombinant adenovirus including a nuclear-targeted beta-galactosidase gene was percutaneously delivered to the iliac artery of normal (n = 25) and atherosclerotic (n = 25) rabbits. Transgene expression, assessed by morphometric as well as chemiluminescent analyses, was documented in all normal and atherosclerotic arteries between 3 and 14 d after gene transfer, but was undetectable at later time points. Transfected cells were identified as smooth muscle cells located in the media of normal arteries, and in the neointima and the vasa-vasora of atherosclerotic arteries. Two percent of medial cells, but only 0.2% of medial and neointimal cells expressed the transgene in normal and atherosclerotic arteries, respectively (P = 0.0001). Similarly, nuclear beta-galactosidase activity was higher in normal than in atherosclerotic arteries (3.2 vs. 0.8 mU/mg protein, P = 0.02). These findings indicate that atherosclerosis reduces the transfection efficiency which can be achieved with adenoviral vectors, and thus constitutes a potential limitation to adenovirus-based, arterial gene therapy.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akli S., Caillaud C., Vigne E., Stratford-Perricaudet L. D., Poenaru L., Perricaudet M., Kahn A., Peschanski M. R. Transfer of a foreign gene into the brain using adenovirus vectors. Nat Genet. 1993 Mar;3(3):224–228. doi: 10.1038/ng0393-224. [DOI] [PubMed] [Google Scholar]
- BAUMGARTNER H. R., STUDER A. [Controlled over-dilatation of the abdominal aorta in normo- and hypercholesteremic rabbits]. Pathol Microbiol (Basel) 1963;26:129–148. [PubMed] [Google Scholar]
- Barger A. C., Beeuwkes R., 3rd, Lainey L. L., Silverman K. J. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984 Jan 19;310(3):175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- Barinaga M. Gene therapy for clogged arteries passes test in pigs. Science. 1994 Aug 5;265(5173):738–738. doi: 10.1126/science.8047879. [DOI] [PubMed] [Google Scholar]
- Block P. C., Baughman K. L., Pasternak R. C., Fallon J. T. Transluminal angioplasty: correlation of morphologic and angiographic findings in an experimental model. Circulation. 1980 Apr;61(4):778–785. doi: 10.1161/01.cir.61.4.778. [DOI] [PubMed] [Google Scholar]
- Chapman G. D., Lim C. S., Gammon R. S., Culp S. C., Desper J. S., Bauman R. P., Swain J. L., Stack R. S. Gene transfer into coronary arteries of intact animals with a percutaneous balloon catheter. Circ Res. 1992 Jul;71(1):27–33. doi: 10.1161/01.res.71.1.27. [DOI] [PubMed] [Google Scholar]
- Chen S. J., Wilson J. M., Muller D. W. Adenovirus-mediated gene transfer of soluble vascular cell adhesion molecule to porcine interposition vein grafts. Circulation. 1994 May;89(5):1922–1928. doi: 10.1161/01.cir.89.5.1922. [DOI] [PubMed] [Google Scholar]
- Engelhardt J. F., Yang Y., Stratford-Perricaudet L. D., Allen E. D., Kozarsky K., Perricaudet M., Yankaskas J. R., Wilson J. M. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993 May;4(1):27–34. doi: 10.1038/ng0593-27. [DOI] [PubMed] [Google Scholar]
- Epstein S. E., Speir E., Unger E. F., Guzman R. J., Finkel T. The basis of molecular strategies for treating coronary restenosis after angioplasty. J Am Coll Cardiol. 1994 May;23(6):1278–1288. doi: 10.1016/0735-1097(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Faxon D. P., Weber V. J., Haudenschild C., Gottsman S. B., McGovern W. A., Ryan T. J. Acute effects of transluminal angioplasty in three experimental models of atherosclerosis. Arteriosclerosis. 1982 Mar-Apr;2(2):125–133. doi: 10.1161/01.atv.2.2.125. [DOI] [PubMed] [Google Scholar]
- Flugelman M. Y., Jaklitsch M. T., Newman K. D., Casscells W., Bratthauer G. L., Dichek D. A. Low level in vivo gene transfer into the arterial wall through a perforated balloon catheter. Circulation. 1992 Mar;85(3):1110–1117. doi: 10.1161/01.cir.85.3.1110. [DOI] [PubMed] [Google Scholar]
- French B. A., Mazur W., Ali N. M., Geske R. S., Finnigan J. P., Rodgers G. P., Roberts R., Raizner A. E. Percutaneous transluminal in vivo gene transfer by recombinant adenovirus in normal porcine coronary arteries, atherosclerotic arteries, and two models of coronary restenosis. Circulation. 1994 Nov;90(5):2402–2413. doi: 10.1161/01.cir.90.5.2402. [DOI] [PubMed] [Google Scholar]
- Gao L., Wagner E., Cotten M., Agarwal S., Harris C., Rømer M., Miller L., Hu P. C., Curiel D. Direct in vivo gene transfer to airway epithelium employing adenovirus-polylysine-DNA complexes. Hum Gene Ther. 1993 Feb;4(1):17–24. doi: 10.1089/hum.1993.4.1-17. [DOI] [PubMed] [Google Scholar]
- Guzman R. J., Lemarchand P., Crystal R. G., Epstein S. E., Finkel T. Efficient and selective adenovirus-mediated gene transfer into vascular neointima. Circulation. 1993 Dec;88(6):2838–2848. doi: 10.1161/01.cir.88.6.2838. [DOI] [PubMed] [Google Scholar]
- Isner J. M., Feldman L. J. Gene therapy for arterial disease. Lancet. 1994 Dec 17;344(8938):1653–1654. doi: 10.1016/s0140-6736(94)90454-5. [DOI] [PubMed] [Google Scholar]
- Kozarsky K. F., McKinley D. R., Austin L. L., Raper S. E., Stratford-Perricaudet L. D., Wilson J. M. In vivo correction of low density lipoprotein receptor deficiency in the Watanabe heritable hyperlipidemic rabbit with recombinant adenoviruses. J Biol Chem. 1994 May 6;269(18):13695–13702. [PubMed] [Google Scholar]
- Le Gal La Salle G., Robert J. J., Berrard S., Ridoux V., Stratford-Perricaudet L. D., Perricaudet M., Mallet J. An adenovirus vector for gene transfer into neurons and glia in the brain. Science. 1993 Feb 12;259(5097):988–990. doi: 10.1126/science.8382374. [DOI] [PubMed] [Google Scholar]
- Leclerc G., Gal D., Takeshita S., Nikol S., Weir L., Isner J. M. Percutaneous arterial gene transfer in a rabbit model. Efficiency in normal and balloon-dilated atherosclerotic arteries. J Clin Invest. 1992 Sep;90(3):936–944. doi: 10.1172/JCI115970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. W., Trapnell B. C., Rade J. J., Virmani R., Dichek D. A. In vivo adenoviral vector-mediated gene transfer into balloon-injured rat carotid arteries. Circ Res. 1993 Nov;73(5):797–807. doi: 10.1161/01.res.73.5.797. [DOI] [PubMed] [Google Scholar]
- Lemarchand P., Jones M., Yamada I., Crystal R. G. In vivo gene transfer and expression in normal uninjured blood vessels using replication-deficient recombinant adenovirus vectors. Circ Res. 1993 May;72(5):1132–1138. doi: 10.1161/01.res.72.5.1132. [DOI] [PubMed] [Google Scholar]
- Losordo D. W., Rosenfield K., Pieczek A., Baker K., Harding M., Isner J. M. How does angioplasty work? Serial analysis of human iliac arteries using intravascular ultrasound. Circulation. 1992 Dec;86(6):1845–1858. doi: 10.1161/01.cir.86.6.1845. [DOI] [PubMed] [Google Scholar]
- March K. L., Madison J. E., Trapnell B. C. Pharmacokinetics of adenoviral vector-mediated gene delivery to vascular smooth muscle cells: modulation by poloxamer 407 and implications for cardiovascular gene therapy. Hum Gene Ther. 1995 Jan;6(1):41–53. doi: 10.1089/hum.1995.6.1-41. [DOI] [PubMed] [Google Scholar]
- Mastrangeli A., Danel C., Rosenfeld M. A., Stratford-Perricaudet L., Perricaudet M., Pavirani A., Lecocq J. P., Crystal R. G. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J Clin Invest. 1993 Jan;91(1):225–234. doi: 10.1172/JCI116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller D. W., Ellis S. G., Topol E. J. Experimental models of coronary artery restenosis. J Am Coll Cardiol. 1992 Feb;19(2):418–432. doi: 10.1016/0735-1097(92)90500-m. [DOI] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Nabel G. J. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990 Sep 14;249(4974):1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- Ohno T., Gordon D., San H., Pompili V. J., Imperiale M. J., Nabel G. J., Nabel E. G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994 Aug 5;265(5173):781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Plautz G., Nabel E. G., Nabel G. J. Introduction of vascular smooth muscle cells expressing recombinant genes in vivo. Circulation. 1991 Feb;83(2):578–583. doi: 10.1161/01.cir.83.2.578. [DOI] [PubMed] [Google Scholar]
- Quantin B., Perricaudet L. D., Tajbakhsh S., Mandel J. L. Adenovirus as an expression vector in muscle cells in vivo. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2581–2584. doi: 10.1073/pnas.89.7.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragot T., Vincent N., Chafey P., Vigne E., Gilgenkrantz H., Couton D., Cartaud J., Briand P., Kaplan J. C., Perricaudet M. Efficient adenovirus-mediated transfer of a human minidystrophin gene to skeletal muscle of mdx mice. Nature. 1993 Feb 18;361(6413):647–650. doi: 10.1038/361647a0. [DOI] [PubMed] [Google Scholar]
- Riessen R., Isner J. M. Prospects for site-specific delivery of pharmacologic and molecular therapies. J Am Coll Cardiol. 1994 Apr;23(5):1234–1244. doi: 10.1016/0735-1097(94)90616-5. [DOI] [PubMed] [Google Scholar]
- Riessen R., Rahimizadeh H., Blessing E., Takeshita S., Barry J. J., Isner J. M. Arterial gene transfer using pure DNA applied directly to a hydrogel-coated angioplasty balloon. Hum Gene Ther. 1993 Dec;4(6):749–758. doi: 10.1089/hum.1993.4.6-749. [DOI] [PubMed] [Google Scholar]
- Rome J. J., Shayani V., Flugelman M. Y., Newman K. D., Farb A., Virmani R., Dichek D. A. Anatomic barriers influence the distribution of in vivo gene transfer into the arterial wall. Modeling with microscopic tracer particles and verification with a recombinant adenoviral vector. Arterioscler Thromb. 1994 Jan;14(1):148–161. doi: 10.1161/01.atv.14.1.148. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Chu C. S., Seth P., Danel C., Banks T., Yoneyama K., Yoshimura K., Crystal R. G. Gene transfer to freshly isolated human respiratory epithelial cells in vitro using a replication-deficient adenovirus containing the human cystic fibrosis transmembrane conductance regulator cDNA. Hum Gene Ther. 1994 Mar;5(3):331–342. doi: 10.1089/hum.1994.5.3-331. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. A., Yoshimura K., Trapnell B. C., Yoneyama K., Rosenthal E. R., Dalemans W., Fukayama M., Bargon J., Stier L. E., Stratford-Perricaudet L. In vivo transfer of the human cystic fibrosis transmembrane conductance regulator gene to the airway epithelium. Cell. 1992 Jan 10;68(1):143–155. doi: 10.1016/0092-8674(92)90213-v. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Sanes J. R., Rubenstein J. L., Nicolas J. F. Use of a recombinant retrovirus to study post-implantation cell lineage in mouse embryos. EMBO J. 1986 Dec 1;5(12):3133–3142. doi: 10.1002/j.1460-2075.1986.tb04620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. D., French B. A. The advent of adenovirus. Gene therapy for cardiovascular disease. Circulation. 1993 Oct;88(4 Pt 1):1937–1942. doi: 10.1161/01.cir.88.4.1937. [DOI] [PubMed] [Google Scholar]
- Schreiber E., Matthias P., Müller M. M., Schaffner W. Rapid detection of octamer binding proteins with 'mini-extracts', prepared from a small number of cells. Nucleic Acids Res. 1989 Aug 11;17(15):6419–6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steg P. G., Feldman L. J., Scoazec J. Y., Tahlil O., Barry J. J., Boulechfar S., Ragot T., Isner J. M., Perricaudet M. Arterial gene transfer to rabbit endothelial and smooth muscle cells using percutaneous delivery of an adenoviral vector. Circulation. 1994 Oct;90(4):1648–1656. doi: 10.1161/01.cir.90.4.1648. [DOI] [PubMed] [Google Scholar]
- Stratford-Perricaudet L. D., Makeh I., Perricaudet M., Briand P. Widespread long-term gene transfer to mouse skeletal muscles and heart. J Clin Invest. 1992 Aug;90(2):626–630. doi: 10.1172/JCI115902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent N., Ragot T., Gilgenkrantz H., Couton D., Chafey P., Grégoire A., Briand P., Kaplan J. C., Kahn A., Perricaudet M. Long-term correction of mouse dystrophic degeneration by adenovirus-mediated transfer of a minidystrophin gene. Nat Genet. 1993 Oct;5(2):130–134. doi: 10.1038/ng1093-130. [DOI] [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Weidinger F. F., McLenachan J. M., Cybulsky M. I., Fallon J. T., Hollenberg N. K., Cooke J. P., Ganz P. Hypercholesterolemia enhances macrophage recruitment and dysfunction of regenerated endothelium after balloon injury of the rabbit iliac artery. Circulation. 1991 Aug;84(2):755–767. doi: 10.1161/01.cir.84.2.755. [DOI] [PubMed] [Google Scholar]
- Willard J. E., Landau C., Glamann D. B., Burns D., Jessen M. E., Pirwitz M. J., Gerard R. D., Meidell R. S. Genetic modification of the vessel wall. Comparison of surgical and catheter-based techniques for delivery of recombinant adenovirus. Circulation. 1994 May;89(5):2190–2197. doi: 10.1161/01.cir.89.5.2190. [DOI] [PubMed] [Google Scholar]