Abstract
The title compound, C25H24N2O3 was prepared by the reaction of (Z)-2-(1H-indol-3-ylmethylene)-1-azabicyclo[2.2.2]octan-3-one with methyl p-(bromomethyl)benzoate, under phase-transfer catalytic (PTC) conditions using triethylbenzylammonium chloride and 50% w/v aqueous NaOH solution in dichloromethane. The crystal structure indicates the presence of a double bond with Z geometry connecting the azabicyclic and indole groups.
Related literature
For related structures, see: Mason et al. (2003 ▶); Zarza et al. (1988 ▶). For related bond angles, see: Wilson (1992 ▶).
Experimental
Crystal data
C25H24N2O3
M r = 400.46
Triclinic,
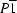
a = 9.8597 (3) Å
b = 10.3037 (3) Å
c = 11.3515 (4) Å
α = 106.6470 (14)°
β = 111.4372 (13)°
γ = 92.7863 (15)°
V = 1013.13 (6) Å3
Z = 2
Mo Kα radiation
μ = 0.09 mm−1
T = 90.0 (2) K
0.55 × 0.50 × 0.25 mm
Data collection
Nonius KappaCCD diffractometer
Absorption correction: multi-scan (SCALEPACK; Otwinowski & Minor, 1997 ▶) T min = 0.954, T max = 0.979
11866 measured reflections
3974 independent reflections
2132 reflections with I > 2σ(I)
R int = 0.068
Refinement
R[F 2 > 2σ(F 2)] = 0.054
wR(F 2) = 0.152
S = 0.97
3974 reflections
272 parameters
H-atom parameters constrained
Δρmax = 0.27 e Å−3
Δρmin = −0.25 e Å−3
Data collection: COLLECT (Nonius, 1998 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO-SMN (Otwinowski & Minor, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP in SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELX97 and local procedures.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808030018/om2262sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808030018/om2262Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This investigation was supported by NIH/National Cancer Institute grant PO1 CA104457.
supplementary crystallographic information
Comment
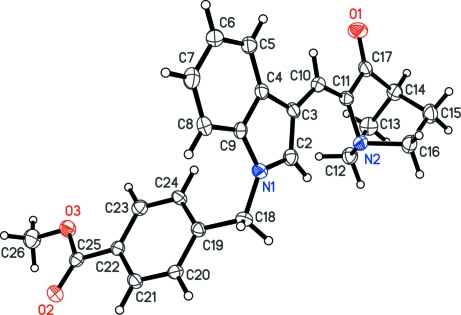
X-ray crystallography confirmed the molecular structure and the atom connectivity for the title compound, as illustrated in Fig. 1. The indole ring is planar with bond distances and angles comparable with those previously reported for other indole derivatives (Mason et al., 2003; Zarza, et al., 1988). The compound is the Z isomer, having the C11—C17 bond in a trans position with respect to the C3—C10 bond. The double bond (C10=C11) has a nearly planar atomic arrangement, since the r.m.s. deviation from the best plane passing through atoms N2, C11, C17, C10 and C3 is 0.0150 (14) Å. Deviations from ideal geometry are observed in the bond angles around atoms C3, C10 and C11. The C10=C11—C17 bond angle is close to the standard planar triangular value of 120°, whereas the C2=C3—C10, C3—C10=C11 and C10=C11—C17 bond angles are more distorted due to the strain induced by the C10=C11—C18=O1 conjugated double bond linkage. These bond angle deformations, which require little energy, are needed to release the intramolecular interactions between non-bonded atoms. In this molecule, the azabicyclic system presents very small distortions around atoms N2, C13, C14, C15, C16 and C17. The value of the C2—C3—C10—C11 torsion angle [-6.3 (4)°] indicates the deviation of the indole ring from the plane of the double bond connected to the azabicyclic ring. The C3—C10 bond length, when compared with the standard value for a single bond connecting a Car atom to a Csp2 atom (1.470 (15) Å; Wilson, 1992), suggests extensive conjugation, beginning at atom O1 and extending through to the indole ring. The bond angles in the azabicyclic system at C13, C14 and C15 are, on average, smaller than the standard tetrahedral value of 109.5°, while the bond angles at C12 and C16 are, on average, slightly larger than the ideal tetrahedral bond angle.
There are no significant intermolecular hydrogen-bonding interactions in the crystal structure. The packing is essentially stabilized via van der Waals forces.
Experimental
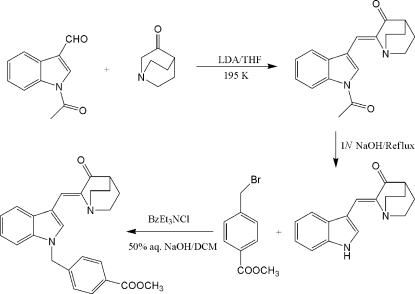
To a stirred solution of diisopropylamine (1.923 g, 19 mmol) in THF (20 ml) at 273 K under nitrogen was added a solution of 2.0 M n-butyllithium (9 ml, 18.8 mmol) and the mixture stirred at 273 K for 30 min. To this solution at 273 K, was added 1-aza-bicyclo[2.2.2]octan-3-one hydrochloride (1.5 g, 9.28 mmol) in one portion and stirring continued until the mixture completely dissolved (20 min). The temperature was lowered to 195 K and a solution of 1-acetyl-1H-indole-3-carboxaldehyde (1.722 g, 9.2 mmol) in THF (25 ml) was added dropwise. Stirring was continued for 30 min at this temperature and then for 90 min at 273 K. The reaction mixture was poured into saturated NaHCO3 at 273 K and the resulting solution was extracted with CHCl3 (3 x 15 ml). The combined organic extracts were dried over anhydrous Na2SO4 and evaporated to afford (Z)-2-(1-acetyl- 1H-indol-3-ylmethylene)-1-azabicyclo[2.2.2]octan-3-one, which was subsequently refluxed with sodium hydroxide solution (25 ml, 1 N) for 30 min. The reaction mixture was cooled to room temperature, and the yellow solid that separated was collected by filtration, washed with cold water and dried to afford the (Z)-2-(1H-indol-3-ylmethylene) -1-azabicyclo[2.2.2]octan-3-one.
To a stirred mixture of (Z)-2-(1H-indol-3-ylmethylene) -1-azabicyclo[2.2.2]octan-3-one (1.0 g, 3.96 mmol), 50% w/v aqueous NaOH solution (1.52 g, 19 mmol) and benzyltriethylammonium chloride (0.172 g, 0.75 mmol) in dichloromethane (DCM, 25 ml) at room temperature was added methyl-p-(bromomethyl)benzoate (1.0 g, 4.0 mmol) in one portion, then the reaction mixture was stirred vigorously for 1 hr until no (Z)-2-(1H-indol-3-ylmethylene)-1-azabicyclo[2.2.2] octan-3-one was detected by TLC. The organic layer was separated, washed exhaustively with water, dried with Na2SO4 and evaporated to afford the crude product. Crystallization from methanol gave a yellow crystalline product of compound (I) that was suitable for X-ray analysis. 1H NMR (CDCl3): δ 1.98–2.00 (m, 4H), 2.61 (p, 1H), 2.93–3.02 (m, 2H), 3.08–3.15 (m, 2H), 3.88 (s, 3H), 5.42 (s, 2H), 7.14–7.22 (m, 5H), 7.47 (s, 1H), 7.87 (d, J = 7.2 Hz, 2H), 7.90 (d, J = 7.2 Hz, 1H), 8.38 (s, 1H); 13C NMR (CDCl3): δ 27.1, 41.1, 48.1, 51.0, 52.7, 110.6, 111.5, 118.2, 119.7, 121.6, 123.3, 126.9, 127.1, 129.3, 130.2, 130.6, 134.6, 136.4, 141.3, 142.2, 166.9, 205.6.
Refinement
H atoms were found in difference Fourier maps and subsequently placed in idealized positions with constrained C—H distances of 0.98 Å (RCH3), 0.99 Å (R2CH2), 1.00 Å (R3CH) and 0.95 Å (CArH) with Uiso(H) values set to either 1.5Ueq (RCH3 only) or 1.2Ueq of the attached C atom.
Figures
Fig. 1.
A view of the molecule with the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level.
Fig. 2.
Reaction scheme.
Crystal data
| C25H24N2O3 | Z = 2 |
| Mr = 400.46 | F(000) = 424 |
| Triclinic, P1 | Dx = 1.313 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 9.8597 (3) Å | Cell parameters from 3599 reflections |
| b = 10.3037 (3) Å | θ = 1.0–27.5° |
| c = 11.3515 (4) Å | µ = 0.09 mm−1 |
| α = 106.6470 (14)° | T = 90 K |
| β = 111.4372 (13)° | Irregular block, colourless |
| γ = 92.7863 (15)° | 0.55 × 0.50 × 0.25 mm |
| V = 1013.13 (6) Å3 |
Data collection
| Nonius KappaCCD diffractometer | 3974 independent reflections |
| Radiation source: fine-focus sealed tube | 2132 reflections with I > 2σ(I) |
| graphite | Rint = 0.068 |
| Detector resolution: 18 pixels mm-1 | θmax = 26.0°, θmin = 2.0° |
| ω scans at fixed χ = 55° | h = −12→11 |
| Absorption correction: multi-scan (SCALEPACK; Otwinowski & Minor, 1997) | k = −12→12 |
| Tmin = 0.954, Tmax = 0.979 | l = −13→13 |
| 11866 measured reflections |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.054 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.152 | H-atom parameters constrained |
| S = 0.97 | w = 1/[σ2(Fo2) + (0.0735P)2] where P = (Fo2 + 2Fc2)/3 |
| 3974 reflections | (Δ/σ)max = 0.002 |
| 272 parameters | Δρmax = 0.27 e Å−3 |
| 0 restraints | Δρmin = −0.25 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.4526 (2) | 0.7860 (2) | 0.42974 (19) | 0.0196 (5) | |
| N2 | 0.7265 (2) | 0.6780 (2) | 0.19469 (19) | 0.0217 (5) | |
| O1 | 0.58592 (19) | 0.79547 (19) | −0.09183 (17) | 0.0349 (5) | |
| O2 | −0.05653 (18) | 0.23941 (17) | 0.44169 (17) | 0.0270 (5) | |
| O3 | −0.11508 (18) | 0.24052 (17) | 0.23058 (16) | 0.0274 (5) | |
| C2 | 0.5260 (3) | 0.7563 (2) | 0.3468 (2) | 0.0206 (6) | |
| H2 | 0.5975 | 0.6972 | 0.3523 | 0.025* | |
| C3 | 0.4822 (3) | 0.8236 (2) | 0.2539 (2) | 0.0204 (6) | |
| C4 | 0.3734 (3) | 0.9016 (2) | 0.2827 (2) | 0.0197 (6) | |
| C5 | 0.2910 (3) | 0.9933 (3) | 0.2293 (2) | 0.0229 (6) | |
| H5 | 0.3002 | 1.0142 | 0.1560 | 0.027* | |
| C6 | 0.1965 (3) | 1.0527 (3) | 0.2845 (3) | 0.0254 (6) | |
| H6 | 0.1414 | 1.1159 | 0.2495 | 0.031* | |
| C7 | 0.1802 (3) | 1.0215 (3) | 0.3912 (3) | 0.0277 (7) | |
| H7 | 0.1130 | 1.0629 | 0.4263 | 0.033* | |
| C8 | 0.2591 (3) | 0.9322 (2) | 0.4463 (2) | 0.0222 (6) | |
| H8 | 0.2475 | 0.9102 | 0.5182 | 0.027* | |
| C9 | 0.3569 (3) | 0.8754 (2) | 0.3919 (2) | 0.0194 (6) | |
| C10 | 0.5266 (3) | 0.8148 (3) | 0.1450 (2) | 0.0214 (6) | |
| H10 | 0.4756 | 0.8624 | 0.0864 | 0.026* | |
| C11 | 0.6295 (3) | 0.7491 (3) | 0.1140 (2) | 0.0208 (6) | |
| C12 | 0.7098 (3) | 0.5330 (3) | 0.1114 (3) | 0.0268 (7) | |
| H12A | 0.6084 | 0.4851 | 0.0846 | 0.032* | |
| H12B | 0.7800 | 0.4860 | 0.1652 | 0.032* | |
| C13 | 0.7389 (3) | 0.5234 (3) | −0.0153 (3) | 0.0319 (7) | |
| H13A | 0.8200 | 0.4708 | −0.0171 | 0.038* | |
| H13B | 0.6490 | 0.4752 | −0.0964 | 0.038* | |
| C14 | 0.7819 (3) | 0.6700 (3) | −0.0135 (2) | 0.0256 (6) | |
| H14 | 0.8014 | 0.6682 | −0.0944 | 0.031* | |
| C15 | 0.9188 (3) | 0.7419 (3) | 0.1160 (3) | 0.0314 (7) | |
| H15A | 0.9490 | 0.8368 | 0.1202 | 0.038* | |
| H15B | 1.0020 | 0.6915 | 0.1173 | 0.038* | |
| C16 | 0.8814 (3) | 0.7456 (3) | 0.2379 (3) | 0.0272 (7) | |
| H16A | 0.9500 | 0.6982 | 0.2926 | 0.033* | |
| H16B | 0.8952 | 0.8424 | 0.2945 | 0.033* | |
| C17 | 0.6562 (3) | 0.7455 (3) | −0.0068 (2) | 0.0236 (6) | |
| C18 | 0.4596 (3) | 0.7224 (3) | 0.5299 (2) | 0.0222 (6) | |
| H18A | 0.4622 | 0.7937 | 0.6110 | 0.027* | |
| H18B | 0.5527 | 0.6849 | 0.5552 | 0.027* | |
| C19 | 0.3302 (3) | 0.6077 (2) | 0.4828 (2) | 0.0184 (6) | |
| C20 | 0.3106 (3) | 0.5504 (3) | 0.5739 (2) | 0.0206 (6) | |
| H20 | 0.3792 | 0.5828 | 0.6646 | 0.025* | |
| C21 | 0.1933 (3) | 0.4476 (2) | 0.5343 (2) | 0.0214 (6) | |
| H21 | 0.1813 | 0.4099 | 0.5978 | 0.026* | |
| C22 | 0.0920 (3) | 0.3983 (2) | 0.4013 (2) | 0.0183 (6) | |
| C23 | 0.1106 (3) | 0.4535 (3) | 0.3092 (2) | 0.0221 (6) | |
| H23 | 0.0423 | 0.4200 | 0.2183 | 0.027* | |
| C24 | 0.2288 (3) | 0.5577 (2) | 0.3496 (2) | 0.0207 (6) | |
| H24 | 0.2409 | 0.5953 | 0.2861 | 0.025* | |
| C25 | −0.0330 (3) | 0.2863 (2) | 0.3636 (2) | 0.0208 (6) | |
| C26 | −0.2337 (3) | 0.1258 (3) | 0.1858 (3) | 0.0345 (7) | |
| H26A | −0.1959 | 0.0566 | 0.2275 | 0.052* | |
| H26B | −0.2716 | 0.0847 | 0.0882 | 0.052* | |
| H26C | −0.3137 | 0.1587 | 0.2117 | 0.052* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0217 (12) | 0.0210 (12) | 0.0198 (11) | 0.0051 (10) | 0.0104 (10) | 0.0090 (10) |
| N2 | 0.0193 (12) | 0.0247 (12) | 0.0204 (11) | 0.0035 (10) | 0.0062 (10) | 0.0086 (10) |
| O1 | 0.0373 (12) | 0.0493 (13) | 0.0296 (11) | 0.0136 (10) | 0.0170 (10) | 0.0235 (10) |
| O2 | 0.0263 (10) | 0.0302 (11) | 0.0287 (11) | 0.0038 (9) | 0.0131 (9) | 0.0131 (9) |
| O3 | 0.0243 (10) | 0.0293 (11) | 0.0234 (10) | −0.0036 (9) | 0.0053 (9) | 0.0076 (8) |
| C2 | 0.0196 (14) | 0.0180 (14) | 0.0250 (15) | 0.0046 (12) | 0.0105 (12) | 0.0059 (12) |
| C3 | 0.0188 (14) | 0.0230 (14) | 0.0203 (14) | −0.0009 (12) | 0.0079 (12) | 0.0092 (12) |
| C4 | 0.0174 (13) | 0.0189 (14) | 0.0209 (14) | −0.0009 (12) | 0.0064 (12) | 0.0058 (12) |
| C5 | 0.0209 (14) | 0.0250 (15) | 0.0214 (14) | 0.0007 (13) | 0.0072 (12) | 0.0078 (12) |
| C6 | 0.0239 (15) | 0.0237 (14) | 0.0306 (15) | 0.0050 (13) | 0.0109 (13) | 0.0113 (13) |
| C7 | 0.0215 (15) | 0.0276 (15) | 0.0343 (16) | 0.0041 (13) | 0.0138 (13) | 0.0068 (13) |
| C8 | 0.0211 (14) | 0.0208 (14) | 0.0241 (15) | −0.0015 (12) | 0.0109 (13) | 0.0046 (12) |
| C9 | 0.0147 (13) | 0.0180 (14) | 0.0199 (14) | −0.0027 (12) | 0.0030 (12) | 0.0040 (11) |
| C10 | 0.0185 (14) | 0.0221 (14) | 0.0221 (14) | 0.0020 (12) | 0.0059 (12) | 0.0080 (12) |
| C11 | 0.0200 (14) | 0.0245 (14) | 0.0176 (13) | 0.0011 (12) | 0.0067 (12) | 0.0077 (12) |
| C12 | 0.0257 (15) | 0.0233 (15) | 0.0303 (15) | 0.0039 (13) | 0.0112 (13) | 0.0071 (13) |
| C13 | 0.0362 (17) | 0.0305 (16) | 0.0279 (15) | 0.0064 (14) | 0.0147 (14) | 0.0050 (13) |
| C14 | 0.0269 (15) | 0.0337 (16) | 0.0207 (14) | 0.0060 (14) | 0.0145 (13) | 0.0085 (13) |
| C15 | 0.0232 (15) | 0.0402 (17) | 0.0320 (16) | 0.0006 (14) | 0.0134 (13) | 0.0111 (14) |
| C16 | 0.0216 (15) | 0.0319 (16) | 0.0267 (15) | 0.0032 (13) | 0.0092 (13) | 0.0083 (13) |
| C17 | 0.0217 (14) | 0.0268 (15) | 0.0215 (14) | −0.0020 (13) | 0.0080 (12) | 0.0083 (12) |
| C18 | 0.0246 (14) | 0.0255 (14) | 0.0198 (14) | 0.0056 (12) | 0.0093 (12) | 0.0112 (12) |
| C19 | 0.0200 (14) | 0.0180 (13) | 0.0208 (14) | 0.0053 (12) | 0.0110 (12) | 0.0072 (12) |
| C20 | 0.0198 (14) | 0.0256 (15) | 0.0201 (14) | 0.0081 (12) | 0.0086 (12) | 0.0113 (12) |
| C21 | 0.0235 (14) | 0.0239 (14) | 0.0242 (15) | 0.0076 (13) | 0.0143 (12) | 0.0118 (12) |
| C22 | 0.0186 (14) | 0.0187 (13) | 0.0213 (14) | 0.0079 (12) | 0.0108 (12) | 0.0073 (12) |
| C23 | 0.0226 (14) | 0.0232 (14) | 0.0193 (14) | 0.0051 (12) | 0.0078 (12) | 0.0056 (12) |
| C24 | 0.0234 (15) | 0.0241 (14) | 0.0196 (14) | 0.0041 (13) | 0.0118 (12) | 0.0100 (12) |
| C25 | 0.0212 (15) | 0.0215 (14) | 0.0246 (15) | 0.0103 (12) | 0.0116 (13) | 0.0101 (13) |
| C26 | 0.0282 (16) | 0.0317 (16) | 0.0336 (17) | −0.0053 (14) | 0.0063 (14) | 0.0058 (14) |
Geometric parameters (Å, °)
| N1—C2 | 1.364 (3) | C12—H12B | 0.9900 |
| N1—C9 | 1.392 (3) | C13—C14 | 1.541 (3) |
| N1—C18 | 1.449 (3) | C13—H13A | 0.9900 |
| N2—C11 | 1.450 (3) | C13—H13B | 0.9900 |
| N2—C12 | 1.486 (3) | C14—C17 | 1.508 (3) |
| N2—C16 | 1.487 (3) | C14—C15 | 1.536 (3) |
| O1—C17 | 1.228 (3) | C14—H14 | 1.0000 |
| O2—C25 | 1.208 (3) | C15—C16 | 1.549 (3) |
| O3—C25 | 1.349 (3) | C15—H15A | 0.9900 |
| O3—C26 | 1.453 (3) | C15—H15B | 0.9900 |
| C2—C3 | 1.377 (3) | C16—H16A | 0.9900 |
| C2—H2 | 0.9500 | C16—H16B | 0.9900 |
| C3—C10 | 1.435 (3) | C18—C19 | 1.517 (3) |
| C3—C4 | 1.447 (3) | C18—H18A | 0.9900 |
| C4—C5 | 1.401 (3) | C18—H18B | 0.9900 |
| C4—C9 | 1.404 (3) | C19—C20 | 1.392 (3) |
| C5—C6 | 1.378 (3) | C19—C24 | 1.397 (3) |
| C5—H5 | 0.9500 | C20—C21 | 1.375 (3) |
| C6—C7 | 1.398 (3) | C20—H20 | 0.9500 |
| C6—H6 | 0.9500 | C21—C22 | 1.394 (3) |
| C7—C8 | 1.374 (3) | C21—H21 | 0.9500 |
| C7—H7 | 0.9500 | C22—C23 | 1.386 (3) |
| C8—C9 | 1.392 (3) | C22—C25 | 1.492 (3) |
| C8—H8 | 0.9500 | C23—C24 | 1.389 (3) |
| C10—C11 | 1.343 (3) | C23—H23 | 0.9500 |
| C10—H10 | 0.9500 | C24—H24 | 0.9500 |
| C11—C17 | 1.478 (3) | C26—H26A | 0.9800 |
| C12—C13 | 1.544 (3) | C26—H26B | 0.9800 |
| C12—H12A | 0.9900 | C26—H26C | 0.9800 |
| C2—N1—C9 | 108.62 (19) | C17—C14—H14 | 111.3 |
| C2—N1—C18 | 125.6 (2) | C15—C14—H14 | 111.3 |
| C9—N1—C18 | 125.4 (2) | C13—C14—H14 | 111.3 |
| C11—N2—C12 | 109.37 (18) | C14—C15—C16 | 109.1 (2) |
| C11—N2—C16 | 107.79 (19) | C14—C15—H15A | 109.9 |
| C12—N2—C16 | 108.0 (2) | C16—C15—H15A | 109.9 |
| C25—O3—C26 | 114.8 (2) | C14—C15—H15B | 109.9 |
| N1—C2—C3 | 110.7 (2) | C16—C15—H15B | 109.9 |
| N1—C2—H2 | 124.7 | H15A—C15—H15B | 108.3 |
| C3—C2—H2 | 124.7 | N2—C16—C15 | 111.3 (2) |
| C2—C3—C10 | 128.3 (2) | N2—C16—H16A | 109.4 |
| C2—C3—C4 | 105.9 (2) | C15—C16—H16A | 109.4 |
| C10—C3—C4 | 125.7 (2) | N2—C16—H16B | 109.4 |
| C5—C4—C9 | 118.2 (2) | C15—C16—H16B | 109.4 |
| C5—C4—C3 | 134.6 (2) | H16A—C16—H16B | 108.0 |
| C9—C4—C3 | 107.2 (2) | O1—C17—C11 | 125.4 (2) |
| C6—C5—C4 | 119.1 (2) | O1—C17—C14 | 123.8 (2) |
| C6—C5—H5 | 120.4 | C11—C17—C14 | 110.8 (2) |
| C4—C5—H5 | 120.4 | N1—C18—C19 | 113.29 (19) |
| C5—C6—C7 | 121.2 (2) | N1—C18—H18A | 108.9 |
| C5—C6—H6 | 119.4 | C19—C18—H18A | 108.9 |
| C7—C6—H6 | 119.4 | N1—C18—H18B | 108.9 |
| C8—C7—C6 | 121.4 (2) | C19—C18—H18B | 108.9 |
| C8—C7—H7 | 119.3 | H18A—C18—H18B | 107.7 |
| C6—C7—H7 | 119.3 | C20—C19—C24 | 118.6 (2) |
| C7—C8—C9 | 116.9 (2) | C20—C19—C18 | 119.7 (2) |
| C7—C8—H8 | 121.5 | C24—C19—C18 | 121.6 (2) |
| C9—C8—H8 | 121.5 | C21—C20—C19 | 121.0 (2) |
| N1—C9—C8 | 129.2 (2) | C21—C20—H20 | 119.5 |
| N1—C9—C4 | 107.7 (2) | C19—C20—H20 | 119.5 |
| C8—C9—C4 | 123.2 (2) | C20—C21—C22 | 120.3 (2) |
| C11—C10—C3 | 129.5 (2) | C20—C21—H21 | 119.9 |
| C11—C10—H10 | 115.3 | C22—C21—H21 | 119.9 |
| C3—C10—H10 | 115.3 | C23—C22—C21 | 119.5 (2) |
| C10—C11—N2 | 124.3 (2) | C23—C22—C25 | 122.4 (2) |
| C10—C11—C17 | 122.2 (2) | C21—C22—C25 | 118.1 (2) |
| N2—C11—C17 | 113.4 (2) | C22—C23—C24 | 120.1 (2) |
| N2—C12—C13 | 111.7 (2) | C22—C23—H23 | 120.0 |
| N2—C12—H12A | 109.3 | C24—C23—H23 | 120.0 |
| C13—C12—H12A | 109.3 | C23—C24—C19 | 120.6 (2) |
| N2—C12—H12B | 109.3 | C23—C24—H24 | 119.7 |
| C13—C12—H12B | 109.3 | C19—C24—H24 | 119.7 |
| H12A—C12—H12B | 107.9 | O2—C25—O3 | 123.6 (2) |
| C14—C13—C12 | 108.8 (2) | O2—C25—C22 | 124.4 (2) |
| C14—C13—H13A | 109.9 | O3—C25—C22 | 111.9 (2) |
| C12—C13—H13A | 109.9 | O3—C26—H26A | 109.5 |
| C14—C13—H13B | 109.9 | O3—C26—H26B | 109.5 |
| C12—C13—H13B | 109.9 | H26A—C26—H26B | 109.5 |
| H13A—C13—H13B | 108.3 | O3—C26—H26C | 109.5 |
| C17—C14—C15 | 107.8 (2) | H26A—C26—H26C | 109.5 |
| C17—C14—C13 | 107.2 (2) | H26B—C26—H26C | 109.5 |
| C15—C14—C13 | 107.9 (2) | ||
| C9—N1—C2—C3 | 0.0 (3) | C12—C13—C14—C15 | 57.9 (3) |
| C18—N1—C2—C3 | 173.2 (2) | C17—C14—C15—C16 | 56.2 (3) |
| N1—C2—C3—C10 | −176.5 (2) | C13—C14—C15—C16 | −59.3 (3) |
| N1—C2—C3—C4 | 0.3 (3) | C11—N2—C16—C15 | −58.5 (3) |
| C2—C3—C4—C5 | 177.7 (3) | C12—N2—C16—C15 | 59.6 (3) |
| C10—C3—C4—C5 | −5.4 (4) | C14—C15—C16—N2 | 0.6 (3) |
| C2—C3—C4—C9 | −0.5 (3) | C10—C11—C17—O1 | −2.9 (4) |
| C10—C3—C4—C9 | 176.4 (2) | N2—C11—C17—O1 | 178.2 (2) |
| C9—C4—C5—C6 | −0.7 (3) | C10—C11—C17—C14 | 177.6 (2) |
| C3—C4—C5—C6 | −178.7 (3) | N2—C11—C17—C14 | −1.3 (3) |
| C4—C5—C6—C7 | −0.9 (4) | C15—C14—C17—O1 | 123.5 (3) |
| C5—C6—C7—C8 | 1.0 (4) | C13—C14—C17—O1 | −120.5 (3) |
| C6—C7—C8—C9 | 0.6 (4) | C15—C14—C17—C11 | −57.0 (3) |
| C2—N1—C9—C8 | 179.3 (2) | C13—C14—C17—C11 | 58.9 (3) |
| C18—N1—C9—C8 | 6.0 (4) | C2—N1—C18—C19 | −98.3 (3) |
| C2—N1—C9—C4 | −0.4 (3) | C9—N1—C18—C19 | 73.8 (3) |
| C18—N1—C9—C4 | −173.6 (2) | N1—C18—C19—C20 | −170.9 (2) |
| C7—C8—C9—N1 | 178.2 (2) | N1—C18—C19—C24 | 8.9 (3) |
| C7—C8—C9—C4 | −2.3 (3) | C24—C19—C20—C21 | −0.6 (4) |
| C5—C4—C9—N1 | −178.0 (2) | C18—C19—C20—C21 | 179.2 (2) |
| C3—C4—C9—N1 | 0.5 (3) | C19—C20—C21—C22 | 0.3 (4) |
| C5—C4—C9—C8 | 2.3 (3) | C20—C21—C22—C23 | 0.2 (3) |
| C3—C4—C9—C8 | −179.1 (2) | C20—C21—C22—C25 | 179.8 (2) |
| C2—C3—C10—C11 | −6.3 (4) | C21—C22—C23—C24 | −0.4 (3) |
| C4—C3—C10—C11 | 177.4 (2) | C25—C22—C23—C24 | 180.0 (2) |
| C3—C10—C11—N2 | −4.2 (4) | C22—C23—C24—C19 | 0.1 (4) |
| C3—C10—C11—C17 | 177.0 (2) | C20—C19—C24—C23 | 0.4 (3) |
| C12—N2—C11—C10 | 123.9 (3) | C18—C19—C24—C23 | −179.4 (2) |
| C16—N2—C11—C10 | −118.9 (3) | C26—O3—C25—O2 | −1.8 (3) |
| C12—N2—C11—C17 | −57.2 (3) | C26—O3—C25—C22 | 176.60 (19) |
| C16—N2—C11—C17 | 60.0 (3) | C23—C22—C25—O2 | −176.8 (2) |
| C11—N2—C12—C13 | 55.9 (3) | C21—C22—C25—O2 | 3.6 (4) |
| C16—N2—C12—C13 | −61.2 (3) | C23—C22—C25—O3 | 4.8 (3) |
| N2—C12—C13—C14 | 1.8 (3) | C21—C22—C25—O3 | −174.8 (2) |
| C12—C13—C14—C17 | −58.0 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: OM2262).
References
- Mason, M. R., Barnard, T. S., Segla, M. F., Xie, B. & Kirschbaum, K. (2003). J. Chem. Crystallogr 33, 531–540.
- Nonius (1998). COLLECT Nonius, BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr and R. M. Sweet, pp. 307–326. New York: Academic Press.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wilson, A. J. C. (1992). International Tables for X-ray Crystallography, Vol. C, Table 9.5.1.1. Dordrecht: Kluwer Academic Publishers.
- Zarza, P. M., Gill, P., Díaz González, M. C., Martin Reyes, M. G., Arrieta, J. M., Nastopoulos, V., Germain, G. & Debaerdemaeker, T. (1988). Acta Cryst C44, 678–681.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808030018/om2262sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808030018/om2262Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report