Abstract
The title compound, K+·C6H9N4O4 −·H2O, crystallizes with the K atoms located on special positions related by pseudocentres of symmetry. Each K atom is coordinated by six O-atom donors. The N and water H atoms are involved in inter- and intramolecular N—H⋯O, N—H⋯N and O—H⋯O hydrogen bonding. The data indicate inversion twinning.
Related literature
For related literature, see: Apreyan & Petrosyan (2008 ▶); Apreyan et al. (2008a
▶,b
▶); Karapetyan (2008a
▶,b
▶); Karapetyan et al. (2007 ▶); Kurtz & Perry (1968 ▶); Paul et al. (1961 ▶); Petrosyan et al. (2005 ▶).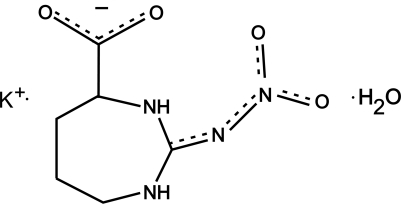
Experimental
Crystal data
K+·C6H9N4O4 −·H2O
M r = 258.29
Orthorhombic,

a = 7.3883 (15) Å
b = 10.087 (2) Å
c = 29.031 (6) Å
V = 2163.5 (8) Å3
Z = 8
Mo Kα radiation
μ = 0.51 mm−1
T = 293 (2) K
0.21 × 0.14 × 0.11 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: none
5030 measured reflections
3141 independent reflections
1736 reflections with I > 2σ(I)
R int = 0.030
3 standard reflections every 400 reflections intensity decay: none
Refinement
R[F 2 > 2σ(F 2)] = 0.065
wR(F 2) = 0.193
S = 1.04
3141 reflections
154 parameters
3 restraints
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.42 e Å−3
Δρmin = −0.39 e Å−3
Absolute structure: Flack (1983 ▶), 1350 Friedel pairs
Flack parameter: 0.48 (20)
Data collection: DATCOL in CAD-4 Software (Enraf–Nonius, 1988 ▶); cell refinement: LS in CAD-4 Software; data reduction: HELENA (Spek, 1997 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808023817/hg2410sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808023817/hg2410Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O5—H11⋯O1i | 0.87 (2) | 2.09 (5) | 2.885 (5) | 153 (10) |
| O5—H10⋯O3ii | 0.856 (19) | 2.03 (4) | 2.793 (4) | 148 (6) |
| N2—H9⋯N3iii | 0.86 | 2.39 | 3.080 (4) | 138 |
| N1—H2⋯O3 | 0.86 | 2.09 | 2.561 (5) | 114 |
Symmetry codes: (i)  ; (ii)
; (ii)  ; (iii)
; (iii)  .
.
Acknowledgments
The author expresses his thanks to Dr R. A. Apreyan and Dr A. M. Petrosyan for providing the crystals and to Dr R. A. Tamazyan for valuable discussion of the results.
supplementary crystallographic information
Comment
Cyclic L-2-nitrimino-1,3-diazepane-4-carboxylic acid (L-NIDCA), produced by elimination of amine from L-nitroarginine (Paul et al., 1961) , may generate new non-linear optical materials like L-nitroarginine itself [Apreyan et al. (2008a, 2008b); Karapetyan et al.(2007); Petrosyan et al. (2005); the crystal structures of L-NIDCA and its monohydrate have been recently reported [Karapetyan (2008a, 2008b)].
This paper presents a structural study of the potassium salt of L-NIDCA monohydrate. The structure was solved and refined in the orthorhombic unit cell with I222 space group. The choice of the non-centric space group was based on the generation of second harmonic observed on a powder sample (YAG:Nd laser, Kurtz-Perry method [Kurtz & Perry,1968]). In this structure, two independent potassium cations occupy special positions. These potassium atoms are located about pseudo-inversion centers, which is most likely the reason for the presence of high level pseudosymmetry in the structure. Both potassium cations are coordinated by six oxygen atoms with K···O bond lengths in the ranges 2.712 (5)-2.815 (7) Å for K1 and 2.642 (5)-2.783 (6) Å for K2.
A view of the asymmetric unit is shown in Fig. 1. The high value of Ueq of atom C3 of the 1,3-diazepane ring compared to those of its neighbors indicates potential disorder of this atom. In the crystal structure, the nitrogen-bound H atoms and the water H atoms are involved in N—H···O, N—H···N and O—H···O hydrogen bonding (Table 1), one of them being intra- and the other three intermolecular, linking anions and water molecules in infinite layers parallel to the bc plane (Fig. 2).
Experimental
The title compound was synthesized from a mixture of aqueous solutions containing L-nitroarginine (2 g, Sigma-Aldrich) and KOH (0.512 g) at room temperature. Single crystals of the title compound were obtained by slow evaporation of the solution. At 97° C decomposition of the crystals was observed.
Refinement
The data set was collected in a full sphere of reciprocal space. Space group I222 was chosen on the basis of the powder second harmonic of YAG:Nb laser generation property of the crystals of the title compound. In spite of the fact that all H atoms appear in difference Fourier maps in reasonable positions, they became unacceptable after refinement. Because of this, all the H atoms except those belonging to the water molecule were placed in geometrically calculated positions and included in the refinement in a riding model approximation, with Uiso(H) = 1.2Ueq(carrier atom). The positions of the H atoms of the water molecule were located in difference Fourier maps and included in the refinement with fixed O—H (0.85 Å), H···H (1.35 Å) distances and isotropic temperature parameters Uiso(H) = 1.4Ueq(O). The absolute configuration has been determined using L-nitroarginine of known absolute configuration.
Figures
Fig. 1.
A perspective view of the asymmetric unit, showing the atomic numbering and displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
Packing of the molecules. For clarity, only the donors in the original molecule and their corresponding acceptors are labelled. Symmetry codes are: (i) x, y - 1, z; (ii)-x + 1/2, y - 1/2, -z + 1/2; (iii) x, 2 - y, 1 - z. Dashed lines indicate hydrogen bonds. H atoms not involved in hydrogen bonding have been omitted.
Crystal data
| K+·C6H9N4O4−·H2O | F(000) = 1072 |
| Mr = 258.29 | Dx = 1.586 Mg m−3 |
| Orthorhombic, I222 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: I 2 2 | Cell parameters from 24 reflections |
| a = 7.3883 (15) Å | θ = 14–16° |
| b = 10.087 (2) Å | µ = 0.51 mm−1 |
| c = 29.031 (6) Å | T = 293 K |
| V = 2163.5 (8) Å3 | Prism, yellow |
| Z = 8 | 0.21 × 0.14 × 0.11 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.030 |
| Radiation source: fine-focus sealed tube | θmax = 30.0°, θmin = 2.1° |
| graphite | h = −10→10 |
| ω/2θ scans | k = −13→13 |
| 5030 measured reflections | l = −38→38 |
| 3141 independent reflections | 3 standard reflections every 400 reflections |
| 1726 reflections with I > 2σ(I) | intensity decay: none |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.065 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.193 | w = 1/[σ2(Fo2) + (0.0676P)2 + 6.1136P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.04 | (Δ/σ)max < 0.001 |
| 3141 reflections | Δρmax = 0.43 e Å−3 |
| 154 parameters | Δρmin = −0.39 e Å−3 |
| 3 restraints | Absolute structure: Flack (1983), 1350 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.48 (20) |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| K1 | 0.5000 | 0.5000 | 0.26313 (7) | 0.0481 (5) | |
| K2 | 0.0000 | 0.5000 | 0.26268 (8) | 0.0536 (5) | |
| O1 | 0.2429 (10) | 0.6833 (3) | 0.28276 (9) | 0.0510 (8) | |
| O2 | 0.2513 (10) | 0.8993 (3) | 0.29658 (9) | 0.0618 (10) | |
| O3 | 0.2476 (15) | 1.1286 (3) | 0.37039 (10) | 0.0905 (16) | |
| O4 | 0.2389 (10) | 1.2557 (3) | 0.42935 (11) | 0.0723 (12) | |
| O5 | 0.2461 (12) | 0.3267 (3) | 0.30317 (11) | 0.0644 (10) | |
| N1 | 0.2755 (14) | 0.8784 (3) | 0.38382 (11) | 0.0630 (19) | |
| H2 | 0.3373 | 0.9320 | 0.3670 | 0.076* | |
| N2 | 0.2659 (10) | 0.8275 (3) | 0.46137 (11) | 0.0583 (14) | |
| H9 | 0.3224 | 0.8526 | 0.4858 | 0.070* | |
| N3 | 0.2516 (11) | 1.0441 (3) | 0.44244 (10) | 0.0490 (10) | |
| N4 | 0.2478 (12) | 1.1416 (3) | 0.41290 (11) | 0.0526 (10) | |
| C1 | 0.2419 (12) | 0.7820 (4) | 0.30906 (13) | 0.0433 (11) | |
| C2 | 0.2101 (7) | 0.7544 (4) | 0.36059 (14) | 0.0397 (12) | |
| H1 | 0.0794 | 0.7470 | 0.3657 | 0.048* | |
| C3 | 0.294 (2) | 0.6381 (6) | 0.37834 (19) | 0.118 (5) | |
| H4 | 0.4240 | 0.6514 | 0.3793 | 0.142* | |
| H3 | 0.2697 | 0.5645 | 0.3578 | 0.142* | |
| C4 | 0.2262 (15) | 0.6022 (4) | 0.42740 (17) | 0.069 (2) | |
| H5 | 0.1150 | 0.5526 | 0.4232 | 0.082* | |
| H6 | 0.3139 | 0.5404 | 0.4399 | 0.082* | |
| C5 | 0.1931 (9) | 0.6894 (5) | 0.46054 (17) | 0.0601 (19) | |
| H7 | 0.2319 | 0.6481 | 0.4891 | 0.072* | |
| H8 | 0.0624 | 0.6974 | 0.4624 | 0.072* | |
| C6 | 0.2498 (15) | 0.9152 (4) | 0.42687 (13) | 0.0475 (10) | |
| H10 | 0.288 (8) | 0.279 (5) | 0.3251 (13) | 0.07 (2)* | |
| H11 | 0.288 (14) | 0.287 (8) | 0.2790 (13) | 0.16 (5)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| K1 | 0.0547 (12) | 0.0388 (11) | 0.0507 (11) | 0.0026 (10) | 0.000 | 0.000 |
| K2 | 0.0566 (12) | 0.0404 (12) | 0.0637 (13) | −0.0021 (11) | 0.000 | 0.000 |
| O1 | 0.070 (2) | 0.0423 (15) | 0.0409 (14) | 0.000 (3) | 0.008 (3) | −0.0129 (13) |
| O2 | 0.115 (3) | 0.0374 (15) | 0.0326 (13) | 0.013 (4) | −0.002 (4) | 0.0002 (11) |
| O3 | 0.202 (5) | 0.0350 (16) | 0.0343 (15) | −0.001 (5) | −0.009 (5) | 0.0075 (12) |
| O4 | 0.136 (4) | 0.0279 (13) | 0.0534 (18) | 0.006 (4) | −0.007 (4) | −0.0024 (13) |
| O5 | 0.103 (3) | 0.0470 (17) | 0.0436 (17) | −0.009 (4) | −0.003 (4) | 0.0143 (15) |
| N1 | 0.134 (6) | 0.0286 (16) | 0.0266 (16) | −0.021 (4) | 0.012 (3) | −0.0014 (12) |
| N2 | 0.115 (4) | 0.0309 (16) | 0.0291 (15) | −0.017 (3) | 0.012 (4) | −0.0006 (13) |
| N3 | 0.087 (3) | 0.0286 (14) | 0.0312 (15) | 0.007 (4) | 0.006 (4) | 0.0001 (12) |
| N4 | 0.088 (3) | 0.0310 (16) | 0.0393 (17) | 0.005 (4) | 0.014 (4) | 0.0017 (13) |
| C1 | 0.057 (3) | 0.039 (2) | 0.0339 (18) | 0.007 (4) | −0.010 (3) | −0.0022 (15) |
| C2 | 0.049 (3) | 0.0352 (19) | 0.0344 (19) | 0.004 (2) | −0.002 (2) | −0.0046 (16) |
| C3 | 0.267 (17) | 0.043 (3) | 0.043 (3) | 0.046 (7) | −0.002 (6) | 0.008 (2) |
| C4 | 0.125 (6) | 0.029 (2) | 0.052 (2) | −0.013 (4) | −0.008 (5) | 0.0091 (18) |
| C5 | 0.098 (6) | 0.037 (2) | 0.045 (3) | 0.001 (3) | 0.004 (3) | 0.011 (2) |
| C6 | 0.079 (3) | 0.0335 (17) | 0.0298 (17) | −0.002 (5) | 0.001 (5) | −0.0016 (14) |
Geometric parameters (Å, °)
| K1—O1 | 2.712 (5) | O4—N4 | 1.247 (4) |
| K1—O1i | 2.712 (5) | O5—H10 | 0.856 (19) |
| K1—O2ii | 2.736 (6) | O5—H11 | 0.87 (2) |
| K1—O2iii | 2.736 (6) | N1—C6 | 1.318 (5) |
| K1—O5i | 2.815 (7) | N1—C2 | 1.502 (6) |
| K1—O5 | 2.815 (7) | N1—H2 | 0.8600 |
| K1—C1ii | 3.524 (5) | N2—C6 | 1.342 (5) |
| K1—C1iii | 3.524 (5) | N2—C5 | 1.494 (7) |
| K1—K2 | 3.6942 (8) | N2—H9 | 0.8600 |
| K1—K2iv | 3.6942 (8) | N3—N4 | 1.305 (4) |
| K1—H11 | 2.70 (11) | N3—C6 | 1.377 (5) |
| K2—O1 | 2.642 (5) | C1—C2 | 1.540 (6) |
| K2—O1v | 2.642 (5) | C1—K1viii | 3.524 (5) |
| K2—O2vi | 2.714 (6) | C2—C3 | 1.423 (9) |
| K2—O2ii | 2.714 (6) | C2—H1 | 0.9800 |
| K2—O5 | 2.783 (6) | C3—C4 | 1.552 (9) |
| K2—O5v | 2.783 (6) | C3—H4 | 0.9700 |
| K2—K1vii | 3.6942 (8) | C3—H3 | 0.9700 |
| K2—H11 | 3.06 (6) | C4—C5 | 1.326 (7) |
| O1—C1 | 1.255 (4) | C4—H5 | 0.9700 |
| O2—C1 | 1.239 (5) | C4—H6 | 0.9700 |
| O2—K2viii | 2.714 (6) | C5—H7 | 0.9700 |
| O2—K1viii | 2.736 (6) | C5—H8 | 0.9700 |
| O3—N4 | 1.241 (4) | ||
| O1—K1—O1i | 155.74 (15) | O2ii—K2—K1vii | 132.78 (15) |
| O1—K1—O2ii | 84.89 (16) | O5—K2—K1vii | 130.69 (16) |
| O1i—K1—O2ii | 110.81 (14) | O5v—K2—K1vii | 49.08 (15) |
| O1—K1—O2iii | 110.81 (14) | O1—K2—K1 | 47.16 (12) |
| O1i—K1—O2iii | 84.89 (16) | O1v—K2—K1 | 132.72 (12) |
| O2ii—K1—O2iii | 101.4 (2) | O2vi—K2—K1 | 132.78 (15) |
| O1—K1—O5i | 87.51 (15) | O2ii—K2—K1 | 47.57 (13) |
| O1i—K1—O5i | 82.52 (14) | O5—K2—K1 | 49.08 (15) |
| O2ii—K1—O5i | 160.81 (11) | O5v—K2—K1 | 130.69 (16) |
| O2iii—K1—O5i | 65.09 (14) | K1vii—K2—K1 | 179.60 (13) |
| O1—K1—O5 | 82.52 (14) | O1—K2—H11 | 89 (2) |
| O1i—K1—O5 | 87.51 (15) | O1v—K2—H11 | 87 (2) |
| O2ii—K1—O5 | 65.09 (14) | O2vi—K2—H11 | 146.2 (10) |
| O2iii—K1—O5 | 160.81 (11) | O2ii—K2—H11 | 50.6 (8) |
| O5i—K1—O5 | 131.23 (19) | O5—K2—H11 | 16.1 (6) |
| O1—K1—C1ii | 101.25 (17) | O5v—K2—H11 | 146.1 (6) |
| O1i—K1—C1ii | 93.15 (14) | K1vii—K2—H11 | 134 (2) |
| O2ii—K1—C1ii | 17.69 (15) | K1—K2—H11 | 46 (2) |
| O2iii—K1—C1ii | 101.48 (14) | C1—O1—K2 | 133.3 (6) |
| O5i—K1—C1ii | 166.12 (17) | C1—O1—K1 | 132.3 (5) |
| O5—K1—C1ii | 61.33 (12) | K2—O1—K1 | 87.26 (8) |
| O1—K1—C1iii | 93.15 (14) | C1—O2—K2viii | 125.5 (5) |
| O1i—K1—C1iii | 101.25 (17) | C1—O2—K1viii | 120.1 (6) |
| O2ii—K1—C1iii | 101.48 (14) | K2viii—O2—K1viii | 85.34 (8) |
| O2iii—K1—C1iii | 17.69 (15) | K2—O5—K1 | 82.59 (8) |
| O5i—K1—C1iii | 61.33 (12) | K2—O5—H10 | 155 (4) |
| O5—K1—C1iii | 166.12 (17) | K1—O5—H10 | 114 (5) |
| C1ii—K1—C1iii | 107.02 (17) | K2—O5—H11 | 101 (5) |
| O1—K1—K2 | 45.59 (12) | K1—O5—H11 | 74 (7) |
| O1i—K1—K2 | 134.53 (12) | H10—O5—H11 | 102 (3) |
| O2ii—K1—K2 | 47.09 (13) | C6—N1—C2 | 127.9 (6) |
| O2iii—K1—K2 | 132.56 (15) | C6—N1—H2 | 116.0 |
| O5i—K1—K2 | 131.90 (15) | C2—N1—H2 | 116.0 |
| O5—K1—K2 | 48.33 (15) | C6—N2—C5 | 124.8 (5) |
| C1ii—K1—K2 | 59.38 (14) | C6—N2—H9 | 117.6 |
| C1iii—K1—K2 | 120.34 (15) | C5—N2—H9 | 117.6 |
| O1—K1—K2iv | 134.53 (12) | N4—N3—C6 | 119.7 (3) |
| O1i—K1—K2iv | 45.59 (12) | O3—N4—O4 | 118.6 (3) |
| O2ii—K1—K2iv | 132.56 (15) | O3—N4—N3 | 125.0 (3) |
| O2iii—K1—K2iv | 47.09 (13) | O4—N4—N3 | 116.4 (3) |
| O5i—K1—K2iv | 48.33 (15) | O2—C1—O1 | 125.4 (4) |
| O5—K1—K2iv | 131.90 (15) | O2—C1—C2 | 117.8 (3) |
| C1ii—K1—K2iv | 120.34 (15) | O1—C1—C2 | 116.6 (4) |
| C1iii—K1—K2iv | 59.38 (14) | O2—C1—K1viii | 42.2 (4) |
| K2—K1—K2iv | 179.60 (13) | O1—C1—K1viii | 97.8 (3) |
| O1—K1—H11 | 95.8 (12) | C2—C1—K1viii | 127.8 (4) |
| O1i—K1—H11 | 80.1 (15) | C3—C2—N1 | 112.6 (5) |
| O2ii—K1—H11 | 54.4 (13) | C3—C2—C1 | 115.8 (5) |
| O2iii—K1—H11 | 142.9 (4) | N1—C2—C1 | 103.6 (4) |
| O5i—K1—H11 | 144.2 (12) | C3—C2—H1 | 108.2 |
| O5—K1—H11 | 17.9 (4) | N1—C2—H1 | 108.2 |
| C1ii—K1—H11 | 46.4 (10) | C1—C2—H1 | 108.2 |
| C1iii—K1—H11 | 153.2 (8) | C2—C3—C4 | 112.6 (8) |
| K2—K1—H11 | 54.5 (15) | C2—C3—H4 | 109.1 |
| K2iv—K1—H11 | 125.5 (15) | C4—C3—H4 | 109.1 |
| O1—K2—O1v | 154.51 (16) | C2—C3—H3 | 109.1 |
| O1—K2—O2vi | 109.73 (14) | C4—C3—H3 | 109.1 |
| O1v—K2—O2vi | 86.68 (16) | H4—C3—H3 | 107.8 |
| O1—K2—O2ii | 86.68 (16) | C5—C4—C3 | 124.8 (4) |
| O1v—K2—O2ii | 109.73 (14) | C5—C4—H5 | 106.1 |
| O2vi—K2—O2ii | 101.3 (2) | C3—C4—H5 | 106.1 |
| O1—K2—O5 | 84.41 (15) | C5—C4—H6 | 106.1 |
| O1v—K2—O5 | 84.89 (16) | C3—C4—H6 | 106.1 |
| O2vi—K2—O5 | 160.93 (12) | H5—C4—H6 | 106.3 |
| O2ii—K2—O5 | 65.81 (15) | C4—C5—N2 | 124.3 (5) |
| O1—K2—O5v | 84.89 (16) | C4—C5—H7 | 106.3 |
| O1v—K2—O5v | 84.41 (15) | N2—C5—H7 | 106.3 |
| O2vi—K2—O5v | 65.81 (15) | C4—C5—H8 | 106.3 |
| O2ii—K2—O5v | 160.93 (12) | N2—C5—H8 | 106.3 |
| O5—K2—O5v | 130.0 (2) | H7—C5—H8 | 106.4 |
| O1—K2—K1vii | 132.72 (12) | N1—C6—N2 | 120.6 (4) |
| O1v—K2—K1vii | 47.16 (12) | N1—C6—N3 | 125.2 (4) |
| O2vi—K2—K1vii | 47.57 (13) | N2—C6—N3 | 112.1 (3) |
| O1v—K2—O1—C1 | −49.9 (4) | O2ii—K1—O5—K2 | −53.73 (14) |
| O2vi—K2—O1—C1 | 77.6 (4) | O2iii—K1—O5—K2 | −101.4 (5) |
| O2ii—K2—O1—C1 | 178.5 (4) | O5i—K1—O5—K2 | 114.12 (11) |
| O5—K2—O1—C1 | −115.5 (4) | C1ii—K1—O5—K2 | −73.12 (16) |
| O5v—K2—O1—C1 | 15.6 (4) | C1iii—K1—O5—K2 | −38.4 (6) |
| K1vii—K2—O1—C1 | 27.7 (5) | K2iv—K1—O5—K2 | −179.55 (14) |
| K1—K2—O1—C1 | −151.7 (4) | C6—N3—N4—O3 | −2.1 (16) |
| O1v—K2—O1—K1 | 101.82 (8) | C6—N3—N4—O4 | 175.9 (10) |
| O2vi—K2—O1—K1 | −130.65 (16) | K2viii—O2—C1—O1 | −49.8 (12) |
| O2ii—K2—O1—K1 | −29.78 (10) | K1viii—O2—C1—O1 | 57.6 (12) |
| O5—K2—O1—K1 | 36.21 (12) | K2viii—O2—C1—C2 | 135.8 (5) |
| O5v—K2—O1—K1 | 167.33 (12) | K1viii—O2—C1—C2 | −116.8 (5) |
| K1vii—K2—O1—K1 | 179.47 (16) | K2viii—O2—C1—K1viii | −107.4 (3) |
| O1i—K1—O1—C1 | 49.8 (4) | K2—O1—C1—O2 | −114.3 (9) |
| O2ii—K1—O1—C1 | −178.2 (4) | K1—O1—C1—O2 | 105.4 (9) |
| O2iii—K1—O1—C1 | −77.9 (4) | K2—O1—C1—C2 | 60.2 (8) |
| O5i—K1—O1—C1 | −15.8 (4) | K1—O1—C1—C2 | −80.1 (8) |
| O5—K1—O1—C1 | 116.3 (4) | K2—O1—C1—K1viii | −79.4 (3) |
| C1ii—K1—O1—C1 | 175.0 (4) | K1—O1—C1—K1viii | 140.3 (2) |
| C1iii—K1—O1—C1 | −76.9 (5) | C6—N1—C2—C3 | −67.0 (11) |
| K2—K1—O1—C1 | 152.2 (4) | C6—N1—C2—C1 | 167.2 (9) |
| K2iv—K1—O1—C1 | −28.3 (5) | O2—C1—C2—C3 | −146.7 (9) |
| O1i—K1—O1—K2 | −102.45 (8) | O1—C1—C2—C3 | 38.4 (11) |
| O2ii—K1—O1—K2 | 29.60 (10) | K1viii—C1—C2—C3 | 164.0 (6) |
| O2iii—K1—O1—K2 | 129.84 (15) | O2—C1—C2—N1 | −22.9 (10) |
| O5i—K1—O1—K2 | −168.04 (12) | O1—C1—C2—N1 | 162.2 (8) |
| O5—K1—O1—K2 | −35.89 (12) | K1viii—C1—C2—N1 | −72.2 (6) |
| C1ii—K1—O1—K2 | 22.82 (11) | N1—C2—C3—C4 | 72.2 (10) |
| C1iii—K1—O1—K2 | 130.87 (14) | C1—C2—C3—C4 | −168.9 (7) |
| K2iv—K1—O1—K2 | 179.46 (17) | C2—C3—C4—C5 | −39.8 (16) |
| O1—K2—O5—K1 | −34.97 (11) | C3—C4—C5—N2 | −19.3 (15) |
| O1v—K2—O5—K1 | 168.20 (12) | C6—N2—C5—C4 | 52.9 (12) |
| O2vi—K2—O5—K1 | 104.1 (5) | C2—N1—C6—N2 | 42.7 (16) |
| O2ii—K2—O5—K1 | 53.89 (13) | C2—N1—C6—N3 | −154.9 (9) |
| O5v—K2—O5—K1 | −113.49 (10) | C5—N2—C6—N1 | −43.0 (15) |
| K1vii—K2—O5—K1 | −179.56 (14) | C5—N2—C6—N3 | 152.5 (8) |
| O1—K1—O5—K2 | 34.09 (11) | N4—N3—C6—N1 | 12.3 (17) |
| O1i—K1—O5—K2 | −168.08 (12) | N4—N3—C6—N2 | 176.0 (9) |
Symmetry codes: (i) −x+1, −y+1, z; (ii) −x+1/2, y−1/2, −z+1/2; (iii) x+1/2, −y+3/2, −z+1/2; (iv) x+1, y, z; (v) −x, −y+1, z; (vi) x−1/2, −y+3/2, −z+1/2; (vii) x−1, y, z; (viii) −x+1/2, y+1/2, −z+1/2.
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O5—H11···O1ii | 0.87 (2) | 2.09 (5) | 2.885 (5) | 153 (10) |
| O5—H10···O3ix | 0.86 (2) | 2.03 (4) | 2.793 (4) | 148 (6) |
| N2—H9···N3x | 0.86 | 2.39 | 3.080 (4) | 138 |
| N1—H2···O3 | 0.86 | 2.09 | 2.561 (5) | 114 |
Symmetry codes: (ii) −x+1/2, y−1/2, −z+1/2; (ix) x, y−1, z; (x) x, −y+2, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG2410).
References
- Apreyan, R. A., Karapetyan, H. A. & Petrosyan, A. M. (2008a). J. Mol. Struct.874, 187–193.
- Apreyan, R. A., Karapetyan, H. A. & Petrosyan, A. M. (2008b). J. Mol. Struct.875, 272–281.
- Apreyan, R. A. & Petrosyan, A. M. (2008). In preparation.
- Enraf–Nonius (1988). CAD-4 Manual. Enraf–Nonius, Delft, The Netherlands.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Karapetyan, H. A. (2008a). Acta Cryst. E64, o943. [DOI] [PMC free article] [PubMed]
- Karapetyan, H. A. (2008b). Acta Cryst. E64, o1222. [DOI] [PMC free article] [PubMed]
- Karapetyan, H. A., Antipin, M. Yu., Sukiasyan, R. P. & Petrosyan, A. M. (2007). J. Mol. Struct.831, 90–96.
- Kurtz, S. K. & Perry, T. T. (1968). J. Appl. Phys.39, 3798–3812.
- Paul, R., Anderson, G. W. & Callahan, F. M. (1961). J. Org. Chem.26, 3347–3350.
- Petrosyan, A. M., Sukiasyan, R. P., Karapetyan, H. A., Antipin, M. Yu. & Apreyan, R. A. (2005). J. Cryst. Growth, 275, e1927–e1933.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (1997). HELENA University of Utrecht, The Netherlands.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808023817/hg2410sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808023817/hg2410Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




