Abstract
Oxidized low density lipoprotein (LDL) possesses several atherogenic properties. The mechanisms by which LDL becomes oxidized in vivo remain unknown, but previous studies have suggested that 15-lipoxygenase may be one of the factors involved in the initiation of LDL oxidation in the arterial wall. 3 wk after a retrovirus-mediated 15-lipoxygenase gene transfer into iliac arteries of normocholesterolemic rabbits there was a threefold increase in 15-lipoxygenase activity but no signs of LDL oxidation. However, when animals were made moderately hypercholesterolemic by feeding a 0.13% cholesterol diet for 2-3 wk starting from day 4 after the gene transfer, oxidation-specific lipid-protein adducts characteristic of oxidized LDL were detected in 15-lipoxygenase-transduced arteries. Control experiments in which contralateral iliac arteries were transduced with beta-galactosidase-containing retroviruses showed only occasional signs of the presence of oxidation-specific adducts. The results support the hypothesis that products derived from the 15-lipoxygenase activity are involved in the induction of LDL oxidation within the arterial wall, provided that sufficient concentrations of lipoproteins are present in the artery.
Full text
PDF


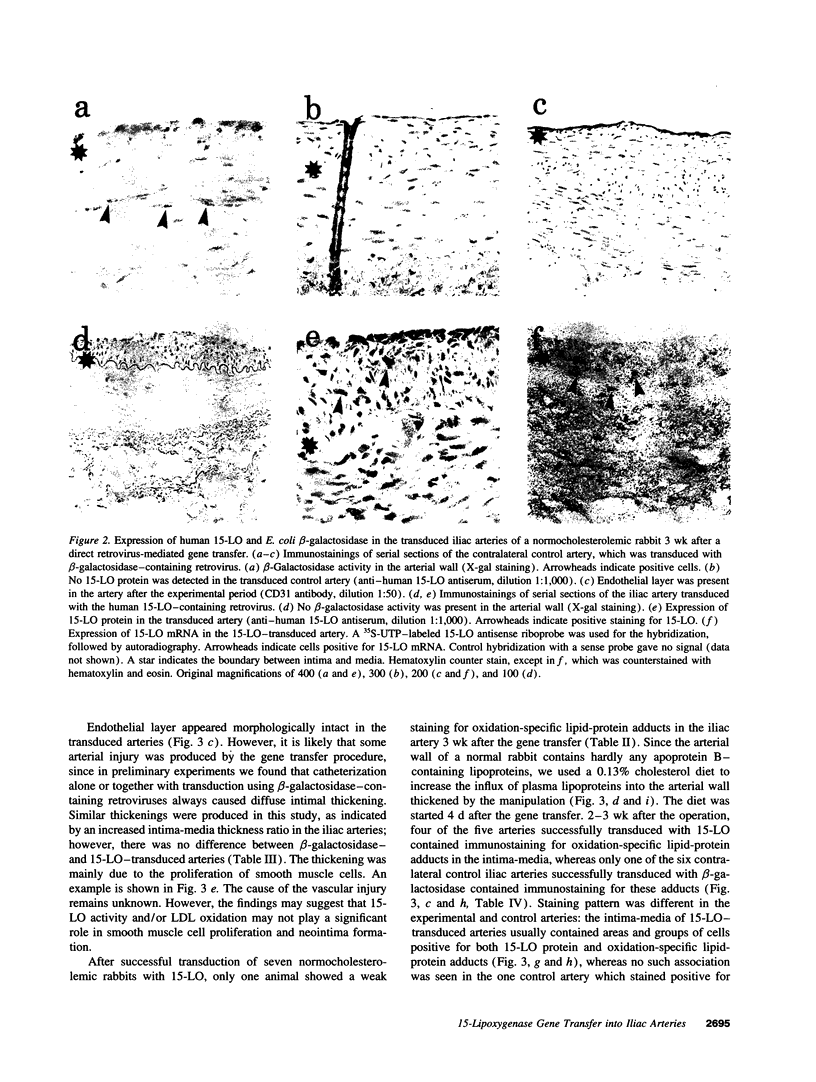

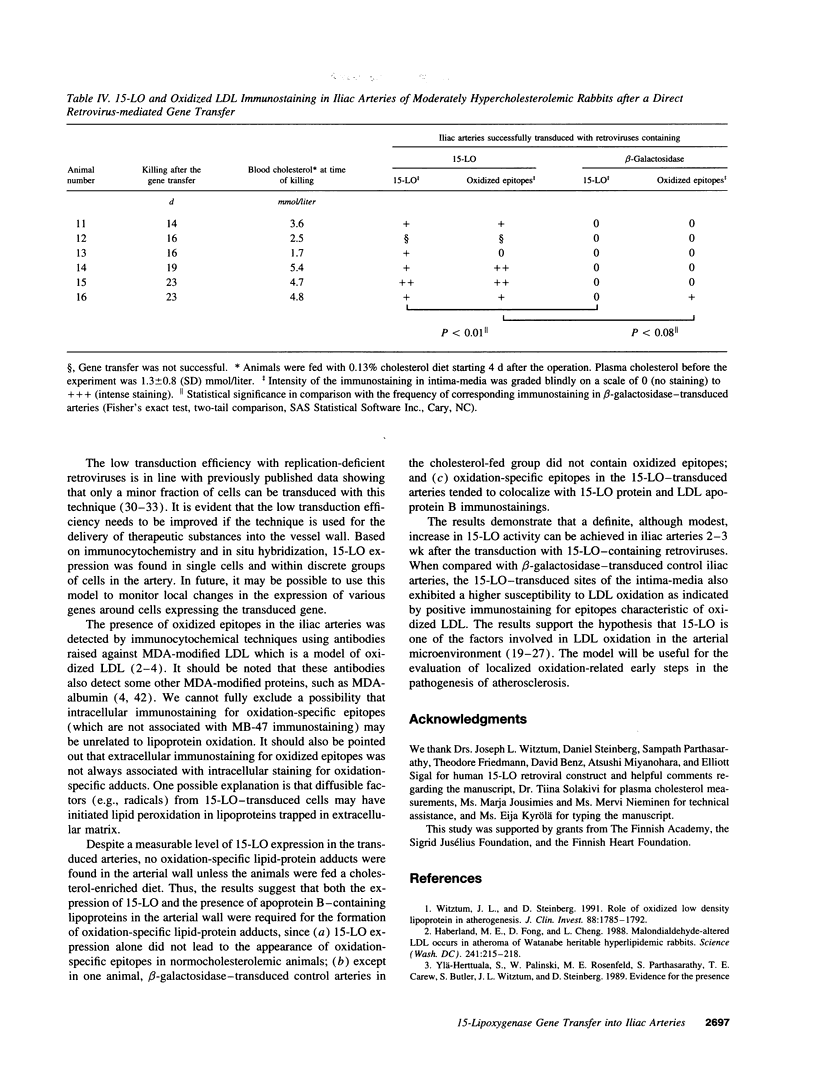

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belkner J., Wiesner R., Rathman J., Barnett J., Sigal E., Kühn H. Oxygenation of lipoproteins by mammalian lipoxygenases. Eur J Biochem. 1993 Apr 1;213(1):251–261. doi: 10.1111/j.1432-1033.1993.tb17755.x. [DOI] [PubMed] [Google Scholar]
- Boyd H. C., Gown A. M., Wolfbauer G., Chait A. Direct evidence for a protein recognized by a monoclonal antibody against oxidatively modified LDL in atherosclerotic lesions from a Watanabe heritable hyperlipidemic rabbit. Am J Pathol. 1989 Nov;135(5):815–825. [PMC free article] [PubMed] [Google Scholar]
- Carew T. E., Schwenke D. C., Steinberg D. Antiatherogenic effect of probucol unrelated to its hypocholesterolemic effect: evidence that antioxidants in vivo can selectively inhibit low density lipoprotein degradation in macrophage-rich fatty streaks and slow the progression of atherosclerosis in the Watanabe heritable hyperlipidemic rabbit. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7725–7729. doi: 10.1073/pnas.84.21.7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cathcart M. K., McNally A. K., Chisolm G. M. Lipoxygenase-mediated transformation of human low density lipoprotein to an oxidized and cytotoxic complex. J Lipid Res. 1991 Jan;32(1):63–70. [PubMed] [Google Scholar]
- Derian C. K., Lewis D. F. Activation of 15-lipoxygenase by low density lipoprotein in vascular endothelial cells. Relationship to the oxidative modification of low density lipoprotein. Prostaglandins Leukot Essent Fatty Acids. 1992 Jan;45(1):49–57. doi: 10.1016/0952-3278(92)90102-o. [DOI] [PubMed] [Google Scholar]
- Flugelman M. Y., Jaklitsch M. T., Newman K. D., Casscells W., Bratthauer G. L., Dichek D. A. Low level in vivo gene transfer into the arterial wall through a perforated balloon catheter. Circulation. 1992 Mar;85(3):1110–1117. doi: 10.1161/01.cir.85.3.1110. [DOI] [PubMed] [Google Scholar]
- Folcik V. A., Cathcart M. K. Assessment of 5-lipoxygenase involvement in human monocyte-mediated LDL oxidation. J Lipid Res. 1993 Jan;34(1):69–79. [PubMed] [Google Scholar]
- Haberland M. E., Fong D., Cheng L. Malondialdehyde-altered protein occurs in atheroma of Watanabe heritable hyperlipidemic rabbits. Science. 1988 Jul 8;241(4862):215–218. doi: 10.1126/science.2455346. [DOI] [PubMed] [Google Scholar]
- Itabe H., Takeshima E., Iwasaki H., Kimura J., Yoshida Y., Imanaka T., Takano T. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. Complex formation of oxidized phosphatidylcholines and polypeptides. J Biol Chem. 1994 May 27;269(21):15274–15279. [PubMed] [Google Scholar]
- Jessup W., Darley-Usmar V., O'Leary V., Bedwell S. 5-Lipoxygenase is not essential in macrophage-mediated oxidation of low-density lipoprotein. Biochem J. 1991 Aug 15;278(Pt 1):163–169. doi: 10.1042/bj2780163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens G., Chen Q., Esterbauer H., Mair S., Ledinski G., Dinges H. P. Immunostaining of human autopsy aortas with antibodies to modified apolipoprotein B and apoprotein(a). Arterioscler Thromb. 1993 Nov;13(11):1689–1699. doi: 10.1161/01.atv.13.11.1689. [DOI] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühn H., Belkner J., Zaiss S., Fährenklemper T., Wohlfeil S. Involvement of 15-lipoxygenase in early stages of atherogenesis. J Exp Med. 1994 Jun 1;179(6):1903–1911. doi: 10.1084/jem.179.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc G., Gal D., Takeshita S., Nikol S., Weir L., Isner J. M. Percutaneous arterial gene transfer in a rabbit model. Efficiency in normal and balloon-dilated atherosclerotic arteries. J Clin Invest. 1992 Sep;90(3):936–944. doi: 10.1172/JCI115970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim C. S., Chapman G. D., Gammon R. S., Muhlestein J. B., Bauman R. P., Stack R. S., Swain J. L. Direct in vivo gene transfer into the coronary and peripheral vasculatures of the intact dog. Circulation. 1991 Jun;83(6):2007–2011. doi: 10.1161/01.cir.83.6.2007. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyanohara A., Sharkey M. F., Witztum J. L., Steinberg D., Friedmann T. Efficient expression of retroviral vector-transduced human low density lipoprotein (LDL) receptor in LDL receptor-deficient rabbit fibroblasts in vitro. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6538–6542. doi: 10.1073/pnas.85.17.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G., Plautz G., Nabel G. J. Site-specific gene expression in vivo by direct gene transfer into the arterial wall. Science. 1990 Sep 14;249(4974):1285–1288. doi: 10.1126/science.2119055. [DOI] [PubMed] [Google Scholar]
- Nikkari T., Malo-Ranta U., Hiltunen T., Jaakkola O., Ylä-Herttuala S. Monitoring of lipoprotein oxidation by gas chromatographic analysis of hydroxy fatty acids. J Lipid Res. 1995 Jan;36(1):200–207. [PubMed] [Google Scholar]
- O'Leary V. J., Darley-Usmar V. M., Russell L. J., Stone D. Pro-oxidant effects of lipoxygenase-derived peroxides on the copper-initiated oxidation of low-density lipoprotein. Biochem J. 1992 Mar 15;282(Pt 3):631–634. doi: 10.1042/bj2820631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostareck-Lederer A., Ostareck D. H., Standart N., Thiele B. J. Translation of 15-lipoxygenase mRNA is inhibited by a protein that binds to a repeated sequence in the 3' untranslated region. EMBO J. 1994 Mar 15;13(6):1476–1481. doi: 10.1002/j.1460-2075.1994.tb06402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski W., Rosenfeld M. E., Ylä-Herttuala S., Gurtner G. C., Socher S. S., Butler S. W., Parthasarathy S., Carew T. E., Steinberg D., Witztum J. L. Low density lipoprotein undergoes oxidative modification in vivo. Proc Natl Acad Sci U S A. 1989 Feb;86(4):1372–1376. doi: 10.1073/pnas.86.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy S., Wieland E., Steinberg D. A role for endothelial cell lipoxygenase in the oxidative modification of low density lipoprotein. Proc Natl Acad Sci U S A. 1989 Feb;86(3):1046–1050. doi: 10.1073/pnas.86.3.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn M. T., Parthasarathy S., Fong L. G., Steinberg D. Oxidatively modified low density lipoproteins: a potential role in recruitment and retention of monocyte/macrophages during atherogenesis. Proc Natl Acad Sci U S A. 1987 May;84(9):2995–2998. doi: 10.1073/pnas.84.9.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin S. M., Parthasarathy S., Steinberg D. Evidence for a dominant role of lipoxygenase(s) in the oxidation of LDL by mouse peritoneal macrophages. J Lipid Res. 1991 Mar;32(3):449–456. [PubMed] [Google Scholar]
- Rapoport S. M., Schewe T. The maturational breakdown of mitochondria in reticulocytes. Biochim Biophys Acta. 1986 Dec 22;864(3-4):471–495. doi: 10.1016/0304-4157(86)90006-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Palinski W., Ylä-Herttuala S., Butler S., Witztum J. L. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis. 1990 May-Jun;10(3):336–349. doi: 10.1161/01.atv.10.3.336. [DOI] [PubMed] [Google Scholar]
- Salonen J. T., Ylä-Herttuala S., Yamamoto R., Butler S., Korpela H., Salonen R., Nyyssönen K., Palinski W., Witztum J. L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992 Apr 11;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Schwenke D. C., Carew T. E. Initiation of atherosclerotic lesions in cholesterol-fed rabbits. II. Selective retention of LDL vs. selective increases in LDL permeability in susceptible sites of arteries. Arteriosclerosis. 1989 Nov-Dec;9(6):908–918. doi: 10.1161/01.atv.9.6.908. [DOI] [PubMed] [Google Scholar]
- Sigal E., Craik C. S., Highland E., Grunberger D., Costello L. L., Dixon R. A., Nadel J. A. Molecular cloning and primary structure of human 15-lipoxygenase. Biochem Biophys Res Commun. 1988 Dec 15;157(2):457–464. doi: 10.1016/s0006-291x(88)80271-7. [DOI] [PubMed] [Google Scholar]
- Sigal E. The molecular biology of mammalian arachidonic acid metabolism. Am J Physiol. 1991 Feb;260(2 Pt 1):L13–L28. doi: 10.1152/ajplung.1991.260.2.L13. [DOI] [PubMed] [Google Scholar]
- Simon T. C., Makheja A. N., Bailey J. M. Formation of 15-hydroxyeicosatetraenoic acid (15-HETE) as the predominant eicosanoid in aortas from Watanabe Heritable Hyperlipidemic and cholesterol-fed rabbits. Atherosclerosis. 1989 Jan;75(1):31–38. doi: 10.1016/0021-9150(89)90204-9. [DOI] [PubMed] [Google Scholar]
- Sparrow C. P., Olszewski J. Cellular oxidative modification of low density lipoprotein does not require lipoxygenases. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):128–131. doi: 10.1073/pnas.89.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow C. P., Parthasarathy S., Steinberg D. Enzymatic modification of low density lipoprotein by purified lipoxygenase plus phospholipase A2 mimics cell-mediated oxidative modification. J Lipid Res. 1988 Jun;29(6):745–753. [PubMed] [Google Scholar]
- Steinbrecher U. P., Parthasarathy S., Leake D. S., Witztum J. L., Steinberg D. Modification of low density lipoprotein by endothelial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci U S A. 1984 Jun;81(12):3883–3887. doi: 10.1073/pnas.81.12.3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada T., Rosenfeld M., Ross R., Gown A. M. Immunocytochemical analysis of cellular components in atherosclerotic lesions. Use of monoclonal antibodies with the Watanabe and fat-fed rabbit. Arteriosclerosis. 1986 Nov-Dec;6(6):601–613. doi: 10.1161/01.atv.6.6.601. [DOI] [PubMed] [Google Scholar]
- Tsukada T., Tippens D., Gordon D., Ross R., Gown A. M. HHF35, a muscle-actin-specific monoclonal antibody. I. Immunocytochemical and biochemical characterization. Am J Pathol. 1987 Jan;126(1):51–60. [PMC free article] [PubMed] [Google Scholar]
- Witztum J. L., Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991 Dec;88(6):1785–1792. doi: 10.1172/JCI115499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S. Mammalian lipoxygenases: molecular structures and functions. Biochim Biophys Acta. 1992 Oct 30;1128(2-3):117–131. doi: 10.1016/0005-2760(92)90297-9. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Nikkari T., Hirvonen J., Laaksonen H., Möttönen M., Pesonen E., Raekallio J., Akerblom H. K. Biochemical composition of coronary arteries in Finnish children. Arteriosclerosis. 1986 Mar-Apr;6(2):230–236. doi: 10.1161/01.atv.6.2.230. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Butler S. W., Picard S., Steinberg D., Witztum J. L. Rabbit and human atherosclerotic lesions contain IgG that recognizes epitopes of oxidized LDL. Arterioscler Thromb. 1994 Jan;14(1):32–40. doi: 10.1161/01.atv.14.1.32. [DOI] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Palinski W., Rosenfeld M. E., Parthasarathy S., Carew T. E., Butler S., Witztum J. L., Steinberg D. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989 Oct;84(4):1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Glass C. K., Sigal E., Witztum J. L., Steinberg D. Colocalization of 15-lipoxygenase mRNA and protein with epitopes of oxidized low density lipoprotein in macrophage-rich areas of atherosclerotic lesions. Proc Natl Acad Sci U S A. 1990 Sep;87(18):6959–6963. doi: 10.1073/pnas.87.18.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylä-Herttuala S., Rosenfeld M. E., Parthasarathy S., Sigal E., Särkioja T., Witztum J. L., Steinberg D. Gene expression in macrophage-rich human atherosclerotic lesions. 15-lipoxygenase and acetyl low density lipoprotein receptor messenger RNA colocalize with oxidation specific lipid-protein adducts. J Clin Invest. 1991 Apr;87(4):1146–1152. doi: 10.1172/JCI115111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Witztum J. L., Casal D. C., Curtiss L. K., Bernstein S. Conservation of the low density lipoprotein receptor-binding domain of apoprotein B. Demonstration by a new monoclonal antibody, MB47. Arteriosclerosis. 1986 Mar-Apr;6(2):178–188. doi: 10.1161/01.atv.6.2.178. [DOI] [PubMed] [Google Scholar]





