Abstract
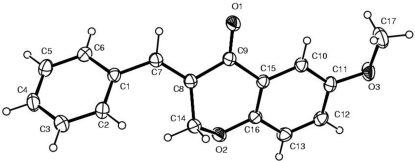
In the title compound, C17H14O3, the dihedral angle between the phenyl ring and the benzene ring of the chromanone moiety is 67.78 (3)°. The six-membered heterocyclic ring of the chromanone moiety adopts a half-chair conformation. The structure is stabilized by weak intermolecular C—H⋯O interactions that link the molecules into inversion dimers.
Related literature
For background literature, see: Finch & Tamm (1970 ▶); Geen et al.(1996 ▶); Tietze & Gerlitzer (1997 ▶); Cremer & Pople (1975 ▶). For a related structure, see: Suresh et al. (2007 ▶).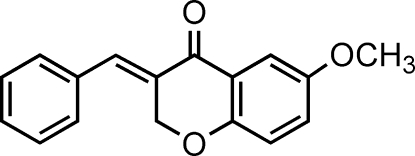
Experimental
Crystal data
C17H14O3
M r = 266.28
Triclinic,
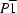
a = 7.2678 (2) Å
b = 8.3151 (2) Å
c = 11.7999 (4) Å
α = 95.964 (1)°
β = 103.828 (1)°
γ = 104.042 (1)°
V = 661.74 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.09 mm−1
T = 298 (2) K
0.45 × 0.42 × 0.38 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 1999 ▶) T min = 0.960, T max = 0.966
8868 measured reflections
3041 independent reflections
2404 reflections with I > 2σ(I)
R int = 0.018
Refinement
R[F 2 > 2σ(F 2)] = 0.039
wR(F 2) = 0.116
S = 1.04
3041 reflections
186 parameters
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.24 e Å−3
Δρmin = −0.23 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: APEX2 (Bruker, 2004 ▶); data reduction: SAINT-Plus (Bruker, 2004 ▶); program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 1997 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808031541/fl2222sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808031541/fl2222Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C6—H6⋯O1i | 0.93 | 2.53 | 3.4293 (18) | 163 |
Symmetry code: (i) 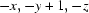 .
.
supplementary crystallographic information
Comment
The chroman-4-one (2,3-dihydro-4-oxo-4H-1-benzopyran) ring system occupies an important position among oxygen heterocyclics and features in a wide variety of compounds of biological and medicinal interest (Finch & Tamm, 1970). Many biologically active natural products containing a chroman ring system have been synthesized via 2-substituted chroman-4-one intermediates including alpha-tocopherol (vitamin E) (Geen et al., 1996). 3-arylidene-4-chromanones have also been isolated as natural products belonging to the class of compounds called homoisoflavonoids(Tietze & Gerlitzer, 1997).
The geometric parameters in the title compound agree with values reported for a similar structure (Suresh et al., 2007). The dihedral angle between the benzene ring of the chromanone moiety and the phenyl ring is 67.78 (3)°. The Chromanone moiety is fused with a six membered heterocyclic ring and the study of torsion angles, asymmetry parameters and least-square plane calculations shows that the chromanone adopts a half chair conformation with a deviation of C14 from the C8/C9/C15/C16/O2 plane by 0.616 (4) Å, Q2= 0.4053 (14) Å, Q3= -0.2052 (13)Å, and QT=0.4543 (14)Å (Cremer &Pople, (1975). The structure is stabilized by weak intermolecular C—H···O interaction that link the molecules into pairs around a center (Table 1). No other short intermolecular interactions were found.
Experimental
Methyl-(2Z)-2-bromo methyl-3-aryl prop-2-enoate (0.006 mole, 1.53 g) was treated with 4-methoxy phenol (0.006 mole, 0.9 ml) in the presence of potassium carbonate in acetone at reflux temperature for 3 hrs. The pure ester, 3-aryl-2-(4-methoxy)-phenoxymethylprop-2-enoate was obtained after purifying it using silica gel and column chromatography (3% ethyl acetate - hexane). Hydrolysis of this ester was carried out with KOH in aqueous 1,4–dioxane at room temperature. The reaction mixture was acidified and the precipitated acid was purified by recrystalization. Finally the acid was treated with triflouroacetic anhydride and the reaction mixture was refluxed in dichloro- methane for 1 hr. It was further purified by column chromatography (silica gel-3% ethyl acetate - hexane) and the crystals used for data collection were obtained by slow evaporation from methanol.
Refinement
H atoms were positioned geometrically and refined using riding model,with C—H = 0.93 Å and Uiso(H) = 1.2Ueq(C) for aromatic C—H, C—H = 0.97 Å and Uiso(H) = 1.2Ueq(C) for CH2, C—H = 0.96 Å and Uiso(H) = 1.5Uiso(C) for CH3.
Figures
Fig. 1.
ORTEP of the molecule with atoms represented as 30% probability ellipsoids.
Crystal data
| C17H14O3 | Z = 2 |
| Mr = 266.28 | F(000) = 280 |
| Triclinic, P1 | Dx = 1.336 Mg m−3 |
| Hall symbol: -p 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 7.2678 (2) Å | Cell parameters from 4851 reflections |
| b = 8.3151 (2) Å | θ = 2.6–28.3° |
| c = 11.7999 (4) Å | µ = 0.09 mm−1 |
| α = 95.964 (1)° | T = 298 K |
| β = 103.828 (1)° | Block, colourless |
| γ = 104.042 (1)° | 0.45 × 0.42 × 0.38 mm |
| V = 661.74 (3) Å3 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 3041 independent reflections |
| Radiation source: fine-focus sealed tube | 2404 reflections with I > 2σ(I) |
| graphite | Rint = 0.018 |
| φ and ω scans | θmax = 28.3°, θmin = 2.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 1999) | h = −8→9 |
| Tmin = 0.960, Tmax = 0.966 | k = −10→11 |
| 8868 measured reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.039 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.116 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.04 | w = 1/[σ2(Fo2) + (0.0554P)2 + 0.1403P] where P = (Fo2 + 2Fc2)/3 |
| 3041 reflections | (Δ/σ)max < 0.001 |
| 186 parameters | Δρmax = 0.25 e Å−3 |
| 0 restraints | Δρmin = −0.23 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes)are estimated using the full covariance matrix. The cell e.s.d.'s are takeninto account individually in the estimation of e.s.d.'s in distances, anglesand torsion angles; correlations between e.s.d.'s in cell parameters are onlyused when they are defined by crystal symmetry. An approximate (isotropic)treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR andgoodness of fit S are based on F2, conventional R-factors R are basedon F, with F set to zero for negative F2. The threshold expression ofF2 > σ(F2) is used only for calculating R-factors(gt) etc. and isnot relevant to the choice of reflections for refinement. R-factors basedon F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1 | −0.38800 (18) | 0.42099 (15) | 0.12980 (11) | 0.0357 (3) | |
| C2 | −0.49675 (19) | 0.45875 (16) | 0.20631 (12) | 0.0413 (3) | |
| H2 | −0.4480 | 0.5584 | 0.2610 | 0.050* | |
| C3 | −0.6764 (2) | 0.34969 (18) | 0.20189 (13) | 0.0469 (3) | |
| H3 | −0.7484 | 0.3771 | 0.2527 | 0.056* | |
| C4 | −0.7491 (2) | 0.20056 (18) | 0.12250 (14) | 0.0499 (3) | |
| H4 | −0.8689 | 0.1265 | 0.1205 | 0.060* | |
| C5 | −0.6433 (2) | 0.16181 (18) | 0.04615 (13) | 0.0526 (4) | |
| H5 | −0.6916 | 0.0606 | −0.0069 | 0.063* | |
| C6 | −0.4668 (2) | 0.27146 (17) | 0.04756 (12) | 0.0448 (3) | |
| H6 | −0.3995 | 0.2458 | −0.0066 | 0.054* | |
| C7 | −0.19833 (18) | 0.53387 (16) | 0.12988 (12) | 0.0384 (3) | |
| C8 | −0.04981 (18) | 0.62292 (15) | 0.22311 (11) | 0.0366 (3) | |
| C9 | 0.12813 (18) | 0.73207 (16) | 0.20106 (11) | 0.0375 (3) | |
| C10 | 0.47891 (18) | 0.89529 (15) | 0.29540 (11) | 0.0370 (3) | |
| H10 | 0.4781 | 0.9476 | 0.2294 | 0.044* | |
| C11 | 0.64996 (18) | 0.92856 (15) | 0.38626 (11) | 0.0390 (3) | |
| C12 | 0.65136 (19) | 0.84577 (17) | 0.48305 (12) | 0.0439 (3) | |
| H12 | 0.7679 | 0.8659 | 0.5429 | 0.053* | |
| C13 | 0.4833 (2) | 0.73495 (18) | 0.49135 (12) | 0.0439 (3) | |
| H13 | 0.4862 | 0.6800 | 0.5562 | 0.053* | |
| C14 | −0.03927 (19) | 0.62083 (18) | 0.35174 (11) | 0.0427 (3) | |
| H14A | −0.0509 | 0.7271 | 0.3874 | 0.051* | |
| H14B | −0.1486 | 0.5319 | 0.3584 | 0.051* | |
| C15 | 0.30664 (17) | 0.78222 (14) | 0.30322 (10) | 0.0336 (3) | |
| C16 | 0.30814 (18) | 0.70497 (15) | 0.40210 (11) | 0.0362 (3) | |
| C17 | 0.8268 (3) | 1.1358 (3) | 0.29888 (18) | 0.0764 (6) | |
| H17A | 0.8124 | 1.0644 | 0.2261 | 0.115* | |
| H17B | 0.9493 | 1.2225 | 0.3186 | 0.115* | |
| H17C | 0.7195 | 1.1861 | 0.2896 | 0.115* | |
| O1 | 0.13097 (14) | 0.77364 (14) | 0.10531 (9) | 0.0566 (3) | |
| O2 | 0.14409 (13) | 0.59397 (12) | 0.41471 (8) | 0.0454 (2) | |
| O3 | 0.82540 (14) | 1.03993 (13) | 0.38987 (9) | 0.0540 (3) | |
| H7 | −0.175 (2) | 0.5435 (19) | 0.0546 (15) | 0.051 (4)* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0311 (6) | 0.0373 (6) | 0.0357 (6) | 0.0073 (5) | 0.0046 (5) | 0.0092 (5) |
| C2 | 0.0344 (6) | 0.0382 (6) | 0.0475 (7) | 0.0079 (5) | 0.0088 (5) | 0.0016 (5) |
| C3 | 0.0365 (7) | 0.0516 (8) | 0.0538 (8) | 0.0108 (6) | 0.0158 (6) | 0.0100 (6) |
| C4 | 0.0349 (7) | 0.0470 (7) | 0.0587 (9) | −0.0018 (6) | 0.0082 (6) | 0.0122 (6) |
| C5 | 0.0497 (8) | 0.0431 (7) | 0.0504 (8) | −0.0009 (6) | 0.0048 (6) | −0.0030 (6) |
| C6 | 0.0426 (7) | 0.0480 (7) | 0.0384 (7) | 0.0072 (6) | 0.0091 (5) | 0.0016 (5) |
| C7 | 0.0339 (6) | 0.0423 (6) | 0.0378 (6) | 0.0081 (5) | 0.0092 (5) | 0.0087 (5) |
| C8 | 0.0310 (6) | 0.0403 (6) | 0.0380 (6) | 0.0084 (5) | 0.0097 (5) | 0.0074 (5) |
| C9 | 0.0321 (6) | 0.0423 (6) | 0.0353 (6) | 0.0069 (5) | 0.0071 (5) | 0.0079 (5) |
| C10 | 0.0331 (6) | 0.0381 (6) | 0.0375 (6) | 0.0086 (5) | 0.0064 (5) | 0.0066 (5) |
| C11 | 0.0297 (6) | 0.0386 (6) | 0.0444 (7) | 0.0081 (5) | 0.0059 (5) | 0.0025 (5) |
| C12 | 0.0347 (7) | 0.0539 (8) | 0.0385 (7) | 0.0150 (6) | 0.0006 (5) | 0.0039 (6) |
| C13 | 0.0413 (7) | 0.0551 (8) | 0.0352 (6) | 0.0154 (6) | 0.0067 (5) | 0.0111 (5) |
| C14 | 0.0321 (6) | 0.0548 (8) | 0.0392 (7) | 0.0076 (6) | 0.0114 (5) | 0.0059 (6) |
| C15 | 0.0298 (6) | 0.0357 (6) | 0.0334 (6) | 0.0087 (5) | 0.0067 (5) | 0.0032 (4) |
| C16 | 0.0334 (6) | 0.0401 (6) | 0.0349 (6) | 0.0101 (5) | 0.0100 (5) | 0.0047 (5) |
| C17 | 0.0437 (9) | 0.0871 (13) | 0.0821 (13) | −0.0091 (9) | 0.0049 (8) | 0.0372 (10) |
| O1 | 0.0392 (5) | 0.0764 (7) | 0.0416 (5) | −0.0049 (5) | 0.0035 (4) | 0.0223 (5) |
| O2 | 0.0369 (5) | 0.0580 (6) | 0.0403 (5) | 0.0077 (4) | 0.0104 (4) | 0.0173 (4) |
| O3 | 0.0305 (5) | 0.0574 (6) | 0.0614 (6) | −0.0006 (4) | −0.0003 (4) | 0.0152 (5) |
Geometric parameters (Å, °)
| C1—C2 | 1.3932 (18) | C10—C11 | 1.3805 (17) |
| C1—C6 | 1.3978 (17) | C10—C15 | 1.4007 (17) |
| C1—C7 | 1.4671 (17) | C10—H10 | 0.9300 |
| C2—C3 | 1.3830 (19) | C11—O3 | 1.3704 (15) |
| C2—H2 | 0.9300 | C11—C12 | 1.3927 (19) |
| C3—C4 | 1.378 (2) | C12—C13 | 1.371 (2) |
| C3—H3 | 0.9300 | C12—H12 | 0.9300 |
| C4—C5 | 1.377 (2) | C13—C16 | 1.3938 (17) |
| C4—H4 | 0.9300 | C13—H13 | 0.9300 |
| C5—C6 | 1.377 (2) | C14—O2 | 1.4434 (16) |
| C5—H5 | 0.9300 | C14—H14A | 0.9700 |
| C6—H6 | 0.9300 | C14—H14B | 0.9700 |
| C7—C8 | 1.3412 (17) | C15—C16 | 1.3883 (17) |
| C7—H7 | 0.950 (16) | C16—O2 | 1.3693 (15) |
| C8—C9 | 1.4846 (18) | C17—O3 | 1.403 (2) |
| C8—C14 | 1.5036 (18) | C17—H17A | 0.9600 |
| C9—O1 | 1.2187 (15) | C17—H17B | 0.9600 |
| C9—C15 | 1.4818 (16) | C17—H17C | 0.9600 |
| C2—C1—C6 | 118.20 (12) | O3—C11—C10 | 124.75 (12) |
| C2—C1—C7 | 122.70 (11) | O3—C11—C12 | 115.45 (11) |
| C6—C1—C7 | 119.07 (12) | C10—C11—C12 | 119.80 (12) |
| C3—C2—C1 | 120.72 (12) | C13—C12—C11 | 120.93 (12) |
| C3—C2—H2 | 119.6 | C13—C12—H12 | 119.5 |
| C1—C2—H2 | 119.6 | C11—C12—H12 | 119.5 |
| C4—C3—C2 | 120.26 (13) | C12—C13—C16 | 119.70 (12) |
| C4—C3—H3 | 119.9 | C12—C13—H13 | 120.1 |
| C2—C3—H3 | 119.9 | C16—C13—H13 | 120.1 |
| C5—C4—C3 | 119.63 (13) | O2—C14—C8 | 111.15 (10) |
| C5—C4—H4 | 120.2 | O2—C14—H14A | 109.4 |
| C3—C4—H4 | 120.2 | C8—C14—H14A | 109.4 |
| C6—C5—C4 | 120.64 (13) | O2—C14—H14B | 109.4 |
| C6—C5—H5 | 119.7 | C8—C14—H14B | 109.4 |
| C4—C5—H5 | 119.7 | H14A—C14—H14B | 108.0 |
| C5—C6—C1 | 120.49 (13) | C16—C15—C10 | 120.07 (11) |
| C5—C6—H6 | 119.8 | C16—C15—C9 | 119.69 (11) |
| C1—C6—H6 | 119.8 | C10—C15—C9 | 119.96 (11) |
| C8—C7—C1 | 128.33 (12) | O2—C16—C15 | 122.60 (11) |
| C8—C7—H7 | 115.3 (9) | O2—C16—C13 | 117.53 (11) |
| C1—C7—H7 | 116.4 (9) | C15—C16—C13 | 119.83 (12) |
| C7—C8—C9 | 118.66 (11) | O3—C17—H17A | 109.5 |
| C7—C8—C14 | 126.65 (12) | O3—C17—H17B | 109.5 |
| C9—C8—C14 | 114.65 (10) | H17A—C17—H17B | 109.5 |
| O1—C9—C15 | 121.81 (11) | O3—C17—H17C | 109.5 |
| O1—C9—C8 | 123.09 (11) | H17A—C17—H17C | 109.5 |
| C15—C9—C8 | 115.06 (11) | H17B—C17—H17C | 109.5 |
| C11—C10—C15 | 119.58 (12) | C16—O2—C14 | 113.85 (10) |
| C11—C10—H10 | 120.2 | C11—O3—C17 | 117.35 (11) |
| C15—C10—H10 | 120.2 | ||
| C6—C1—C2—C3 | 0.93 (19) | C11—C12—C13—C16 | −0.3 (2) |
| C7—C1—C2—C3 | 179.01 (12) | C7—C8—C14—O2 | 129.37 (13) |
| C1—C2—C3—C4 | 0.9 (2) | C9—C8—C14—O2 | −48.50 (15) |
| C2—C3—C4—C5 | −1.0 (2) | C11—C10—C15—C16 | 0.33 (18) |
| C3—C4—C5—C6 | −0.7 (2) | C11—C10—C15—C9 | −173.63 (11) |
| C4—C5—C6—C1 | 2.6 (2) | O1—C9—C15—C16 | −166.84 (12) |
| C2—C1—C6—C5 | −2.7 (2) | C8—C9—C15—C16 | 10.83 (17) |
| C7—C1—C6—C5 | 179.18 (12) | O1—C9—C15—C10 | 7.14 (19) |
| C2—C1—C7—C8 | 41.2 (2) | C8—C9—C15—C10 | −175.19 (10) |
| C6—C1—C7—C8 | −140.74 (14) | C10—C15—C16—O2 | 179.75 (11) |
| C1—C7—C8—C9 | −178.86 (12) | C9—C15—C16—O2 | −6.28 (18) |
| C1—C7—C8—C14 | 3.3 (2) | C10—C15—C16—C13 | −2.59 (18) |
| C7—C8—C9—O1 | 16.3 (2) | C9—C15—C16—C13 | 171.39 (11) |
| C14—C8—C9—O1 | −165.66 (13) | C12—C13—C16—O2 | −179.65 (11) |
| C7—C8—C9—C15 | −161.35 (11) | C12—C13—C16—C15 | 2.57 (19) |
| C14—C8—C9—C15 | 16.71 (16) | C15—C16—O2—C14 | −27.22 (16) |
| C15—C10—C11—O3 | −178.13 (11) | C13—C16—O2—C14 | 155.06 (11) |
| C15—C10—C11—C12 | 1.94 (18) | C8—C14—O2—C16 | 53.73 (14) |
| O3—C11—C12—C13 | 178.09 (12) | C10—C11—O3—C17 | 5.0 (2) |
| C10—C11—C12—C13 | −2.0 (2) | C12—C11—O3—C17 | −175.11 (15) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C6—H6···O1i | 0.93 | 2.53 | 3.4293 (18) | 163 |
Symmetry codes: (i) −x, −y+1, −z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FL2222).
References
- Bruker (1999). SADABS. Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (2004). APEX2 and SAINT-Plus Bruker AXS Inc., Madison, Wisconsin, USA.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Finch, R. E. & Tamm, C. (1970). Experientia, 26, 472–477. [DOI] [PubMed]
- Geen, G., Evans, J. M. & Vong, A. K. (1996). Comprehensive Heterocyclic Chemistry II, edited by A. Mckillop, Vol. 5, pp. 469–472. Oxford: Pergamon.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Suresh, R., Kanagam, C. C., Umarani, P. R., Manivannan, V. & Büyükgüngör, O. (2007). Acta Cryst. E63, o4387.
- Tietze, L. F. & Gerlitzer, J. (1997). Synthesis, pp.877–883.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808031541/fl2222sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808031541/fl2222Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report