Abstract
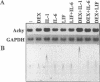
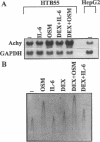
Although it is a well known fact that hepatocytes are the primary source of plasma proteinase inhibitors, including alpha 1-antichymotrypsin, this protein has also been detected in lung epithelial cells, which may suggest its local production. We have demonstrated that lung-derived epithelial cells are capable of synthesizing high levels of alpha 1-antichymotrypsin. In normal bronchial epithelial cells, as well as in the HTB55 human adenocarcinoma cell line, alpha 1-antichymotrypsin synthesis was under the control of inflammatory cytokines, of which oncostatin M was the most potent stimulator. This finding is consistent with a role for this inhibitor in protecting the lung epithelium from damage by chymotrypsin-like enzymes released from phagocytes such as neutrophils following pathogen invasion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann H., Gauldie J. The acute phase response. Immunol Today. 1994 Feb;15(2):74–80. doi: 10.1016/0167-5699(94)90137-6. [DOI] [PubMed] [Google Scholar]
- Baumann H., Richards C., Gauldie J. Interaction among hepatocyte-stimulating factors, interleukin 1, and glucocorticoids for regulation of acute phase plasma proteins in human hepatoma (HepG2) cells. J Immunol. 1987 Dec 15;139(12):4122–4128. [PubMed] [Google Scholar]
- Baumann H., Ziegler S. F., Mosley B., Morella K. K., Pajovic S., Gearing D. P. Reconstitution of the response to leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor in hepatoma cells. J Biol Chem. 1993 Apr 25;268(12):8414–8417. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Burnett D., McGillivray D. H., Stockley R. A. Evidence that alveolar macrophages can synthesize and secrete alpha 1-antichymotrypsin. Am Rev Respir Dis. 1984 Mar;129(3):473–476. doi: 10.1164/arrd.1984.129.3.473. [DOI] [PubMed] [Google Scholar]
- Finlay T. H., Tamir S., Kadner S. S., Cruz M. R., Yavelow J., Levitz M. alpha 1-Antitrypsin- and anchorage-independent growth of MCF-7 breast cancer cells. Endocrinology. 1993 Sep;133(3):996–1002. doi: 10.1210/endo.133.3.8365378. [DOI] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Northemann W., Fey G., Baumann H. IFN beta 2/BSF2/IL-6 is the monocyte-derived HSF that regulates receptor-specific acute phase gene regulation in hepatocytes. Ann N Y Acad Sci. 1989;557:46–59. [PubMed] [Google Scholar]
- Hurlimann J., van Melle G. Prognostic value of serum proteins synthesized by breast carcinoma cells. Am J Clin Pathol. 1991 Jun;95(6):835–843. doi: 10.1093/ajcp/95.6.835. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Taga T., Akira S. Cytokine signal transduction. Cell. 1994 Jan 28;76(2):253–262. doi: 10.1016/0092-8674(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Koj A., Magielska-Zero D., Bereta J., Kurdowska A., Rokita H., Gauldie J. The cascade of inflammatory cytokines regulating synthesis of acute phase proteins. Tokai J Exp Clin Med. 1988 Dec;13(6):255–264. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laine A., Hachulla E., Strecker G., Michalski J. C., Wieruszeski J. M. Structure determination of the glycans of human-serum alpha 1-antichymotrypsin using 1H-NMR spectroscopy and deglycosylation by N-glycanase. Eur J Biochem. 1991 Apr 10;197(1):209–215. doi: 10.1111/j.1432-1033.1991.tb15901.x. [DOI] [PubMed] [Google Scholar]
- Laursen I., Lykkesfeldt A. E. Purification and characterization of an alpha 1-antichymotrypsin-like 66 kDa protein from the human breast cancer cell line, MCF-7. Biochim Biophys Acta. 1992 May 22;1121(1-2):119–129. doi: 10.1016/0167-4838(92)90345-e. [DOI] [PubMed] [Google Scholar]
- Molmenti E. P., Ziambaras T., Perlmutter D. H. Evidence for an acute phase response in human intestinal epithelial cells. J Biol Chem. 1993 Jul 5;268(19):14116–14124. [PubMed] [Google Scholar]
- Morii M., Travis J. Structural alterations in alpha 1-antichymotrypsin from normal and acute phase human plasma. Biochem Biophys Res Commun. 1983 Mar 16;111(2):438–443. doi: 10.1016/0006-291x(83)90325-x. [DOI] [PubMed] [Google Scholar]
- Permanetter W., Meister P. Distribution of lysozyme (muramidase) and alpha 1-antichymotrypsin in normal and neoplastic epithelial tissues: a survey. Acta Histochem. 1984;74(2):173–179. doi: 10.1016/s0065-1281(84)80005-7. [DOI] [PubMed] [Google Scholar]
- Potempa J., Korzus E., Travis J. The serpin superfamily of proteinase inhibitors: structure, function, and regulation. J Biol Chem. 1994 Jun 10;269(23):15957–15960. [PubMed] [Google Scholar]
- Richards C. D., Brown T. J., Shoyab M., Baumann H., Gauldie J. Recombinant oncostatin M stimulates the production of acute phase proteins in HepG2 cells and rat primary hepatocytes in vitro. J Immunol. 1992 Mar 15;148(6):1731–1736. [PubMed] [Google Scholar]
- Rose-John S., Dietrich A., Marks F. Molecular cloning of mouse protein kinase C (PKC) cDNA from Swiss 3T3 fibroblasts. Gene. 1988 Dec 30;74(2):465–471. doi: 10.1016/0378-1119(88)90179-5. [DOI] [PubMed] [Google Scholar]
- Rubin H., Wang Z. M., Nickbarg E. B., McLarney S., Naidoo N., Schoenberger O. L., Johnson J. L., Cooperman B. S. Cloning, expression, purification, and biological activity of recombinant native and variant human alpha 1-antichymotrypsins. J Biol Chem. 1990 Jan 15;265(2):1199–1207. [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Schoester M., Heinrich P. C., Graeve L. Regulation of interleukin-6 receptor expression by interleukin-6 in human monocytes--a re-examination. FEBS Lett. 1994 May 30;345(2-3):131–134. doi: 10.1016/0014-5793(94)00416-1. [DOI] [PubMed] [Google Scholar]
- Stockley R. A., Burnett D. Alpha 1-antichymotrypsin in infected and noninfected sputum. Am Rev Respir Dis. 1980 Jul;122(1):81–88. doi: 10.1164/arrd.1980.122.1.81. [DOI] [PubMed] [Google Scholar]
- Takizawa H., Ohtoshi T., Ohta K., Yamashita N., Hirohata S., Hirai K., Hiramatsu K., Ito K. Growth inhibition of human lung cancer cell lines by interleukin 6 in vitro: a possible role in tumor growth via an autocrine mechanism. Cancer Res. 1993 Sep 15;53(18):4175–4181. [PubMed] [Google Scholar]
- Tamir S., Kadner S. S., Katz J., Finlay T. H. Regulation of antitrypsin and antichymotrypsin synthesis by MCF-7 breast cancer cell sublines. Endocrinology. 1990 Sep;127(3):1319–1328. doi: 10.1210/endo-127-3-1319. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]