Abstract
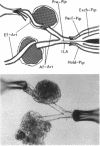
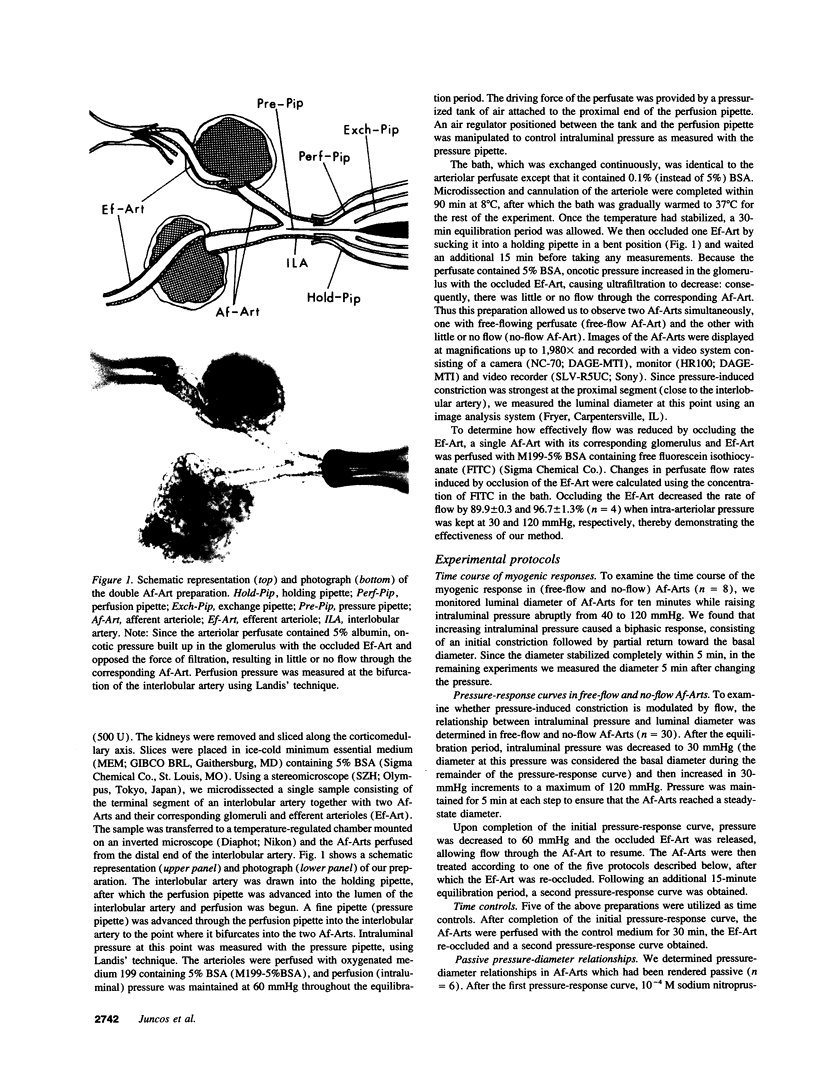
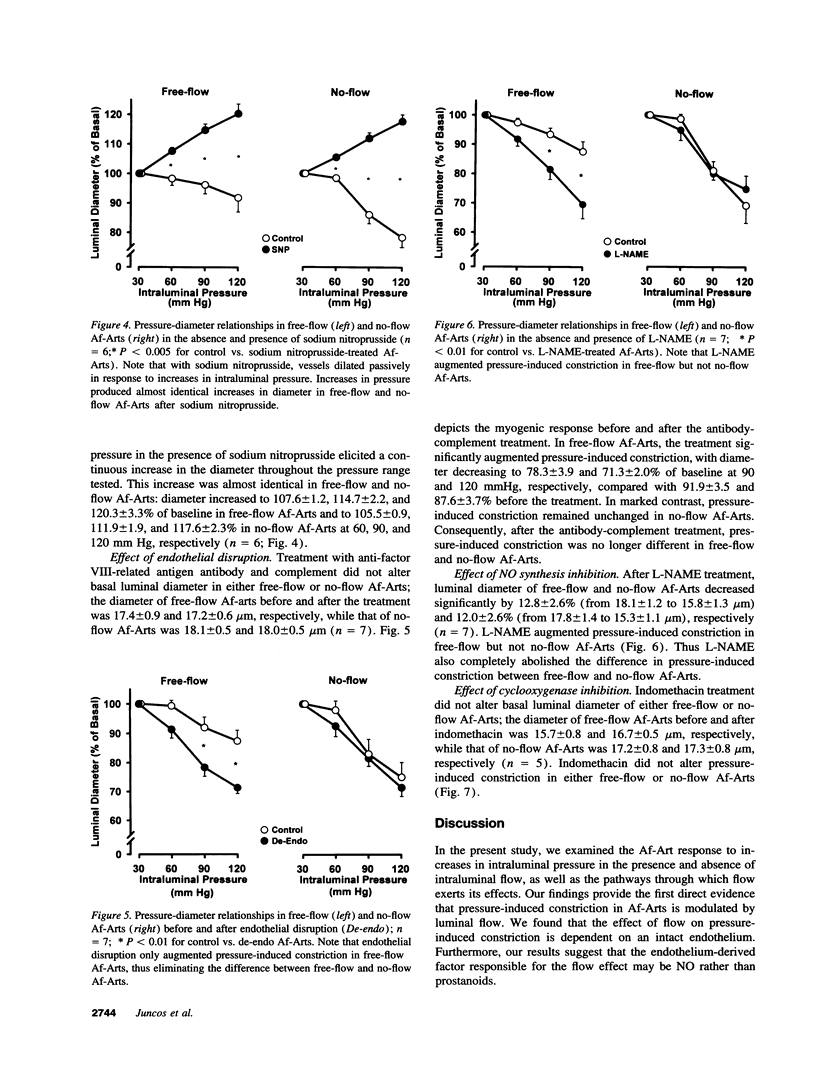
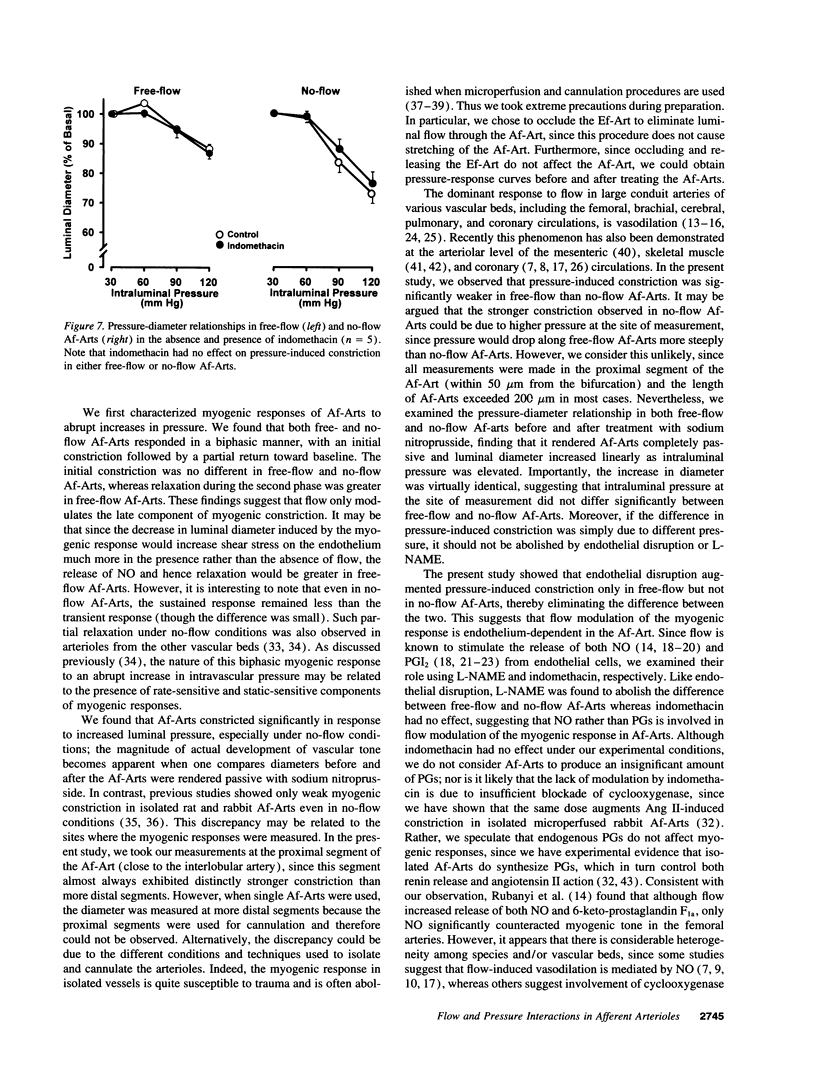
Flow may be a physiological stimulus of the endothelial release of nitric oxide (NO) and prostaglandins (PGs). We tested the hypothesis that pressure-induced constriction of the glomerular afferent arteriole (Af-Art) is modulated by luminal flow via endothelial production of NO. We microdissected the terminal segment of an interlobular artery together with two Af-Arts, their glomeruli (GL) and efferent arterioles (Ef-Art). The two Af-Arts were perfused simultaneously from the interlobular artery, while one Ef-Art was occluded. Since the arteriolar perfusate contained 5% albumin, oncotic pressure built up in the glomerulus with the occluded Ef-Art and opposed the force of filtration, resulting in little or no flow through the corresponding Af-Art. Thus this preparation allowed us to observe free-flow and no-flow Af-Arts simultaneously during stepwise 30-mmHg increases in intraluminal pressure (from 30 to 120 mmHg). Pressure-induced constriction was weaker in free-flow than no-flow Af-Arts, with the luminal diameter decreasing by 11.1 +/- 1.7 and 25.6 +/- 2.3% (n = 30), respectively, at 120 mmHg. To examine whether flow modulates myogenic constriction through endothelium-derived NO and/or PGs, we examined pressure-induced constriction before and after (a) disruption of the endothelium, (b) inhibition of NO synthesis with NW-nitro-L-arginine methyl ester (L-NAME), or (c) inhibition of cyclooxygenase with indomethacin. Both endothelial disruption and L-NAME augmented pressure-induced constriction in free-flow but not no-flow Af-Arts, abolishing the differences between the two. However, indomethacin had no effect in either free-flow or no-flow Af-Arts. These results suggest that intraluminal flow attenuates pressure-induced constriction in Af-Arts via endothelium-derived NO. Thus flow-stimulated NO release may be important in the fine control of glomerular hemodynamics.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima S., Ren Y., Juncos L. A., Carretero O. A., Ito S. Glomerular prostaglandins modulate vascular reactivity of the downstream efferent arterioles. Kidney Int. 1994 Mar;45(3):650–658. doi: 10.1038/ki.1994.87. [DOI] [PubMed] [Google Scholar]
- Aukland K., Oien A. H. Renal autoregulation: models combining tubuloglomerular feedback and myogenic response. Am J Physiol. 1987 Apr;252(4 Pt 2):F768–F783. doi: 10.1152/ajprenal.1987.252.4.F768. [DOI] [PubMed] [Google Scholar]
- Baumann J. E., Persson P. B., Ehmke H., Nafz B., Kirchheim H. R. Role of endothelium-derived relaxing factor in renal autoregulation in conscious dogs. Am J Physiol. 1992 Aug;263(2 Pt 2):F208–F213. doi: 10.1152/ajprenal.1992.263.2.F208. [DOI] [PubMed] [Google Scholar]
- Beierwaltes W. H., Sigmon D. H., Carretero O. A. Endothelium modulates renal blood flow but not autoregulation. Am J Physiol. 1992 Jun;262(6 Pt 2):F943–F949. doi: 10.1152/ajprenal.1992.262.6.F943. [DOI] [PubMed] [Google Scholar]
- Bevan J. A., Laher I. Pressure and flow-dependent vascular tone. FASEB J. 1991 Jun;5(9):2267–2273. doi: 10.1096/fasebj.5.9.1860618. [DOI] [PubMed] [Google Scholar]
- Bhagyalakshmi A., Frangos J. A. Mechanism of shear-induced prostacyclin production in endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):31–37. doi: 10.1016/s0006-291x(89)80172-x. [DOI] [PubMed] [Google Scholar]
- Buga G. M., Gold M. E., Fukuto J. M., Ignarro L. J. Shear stress-induced release of nitric oxide from endothelial cells grown on beads. Hypertension. 1991 Feb;17(2):187–193. doi: 10.1161/01.hyp.17.2.187. [DOI] [PubMed] [Google Scholar]
- DE WARDENER H. E., McSWINEY R. R., MILES B. E. Renal haemodynamics in primary polycythaemia. Lancet. 1951 Aug 4;2(6675):204–205. doi: 10.1016/s0140-6736(51)91442-0. [DOI] [PubMed] [Google Scholar]
- Dacey R. G., Jr, Duling B. R. A study of rat intracerebral arterioles: methods, morphology, and reactivity. Am J Physiol. 1982 Oct;243(4):H598–H606. doi: 10.1152/ajpheart.1982.243.4.H598. [DOI] [PubMed] [Google Scholar]
- Davis M. J., Sikes P. J. Myogenic responses of isolated arterioles: test for a rate-sensitive mechanism. Am J Physiol. 1990 Dec;259(6 Pt 2):H1890–H1900. doi: 10.1152/ajpheart.1990.259.6.H1890. [DOI] [PubMed] [Google Scholar]
- Duling B. R., Gore R. W., Dacey R. G., Jr, Damon D. N. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol. 1981 Jul;241(1):H108–H116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- Edwards R. M. Segmental effects of norepinephrine and angiotensin II on isolated renal microvessels. Am J Physiol. 1983 May;244(5):F526–F534. doi: 10.1152/ajprenal.1983.244.5.F526. [DOI] [PubMed] [Google Scholar]
- Eskinder H., Harder D. R., Lombard J. H. Role of the vascular endothelium in regulating the response of small arteries of the dog kidney to transmural pressure elevation and reduced PO2. Circ Res. 1990 May;66(5):1427–1435. doi: 10.1161/01.res.66.5.1427. [DOI] [PubMed] [Google Scholar]
- Falcone J. C., Davis M. J., Meininger G. A. Endothelial independence of myogenic response in isolated skeletal muscle arterioles. Am J Physiol. 1991 Jan;260(1 Pt 2):H130–H135. doi: 10.1152/ajpheart.1991.260.1.H130. [DOI] [PubMed] [Google Scholar]
- Frangos J. A., Eskin S. G., McIntire L. V., Ives C. L. Flow effects on prostacyclin production by cultured human endothelial cells. Science. 1985 Mar 22;227(4693):1477–1479. doi: 10.1126/science.3883488. [DOI] [PubMed] [Google Scholar]
- Garcia-Roldan J. L., Bevan J. A. Flow-induced constriction and dilation of cerebral resistance arteries. Circ Res. 1990 May;66(5):1445–1448. doi: 10.1161/01.res.66.5.1445. [DOI] [PubMed] [Google Scholar]
- Griffith T. M., Edwards D. H. Myogenic autoregulation of flow may be inversely related to endothelium-derived relaxing factor activity. Am J Physiol. 1990 Apr;258(4 Pt 2):H1171–H1180. doi: 10.1152/ajpheart.1990.258.4.H1171. [DOI] [PubMed] [Google Scholar]
- Hutcheson I. R., Griffith T. M. Release of endothelium-derived relaxing factor is modulated both by frequency and amplitude of pulsatile flow. Am J Physiol. 1991 Jul;261(1 Pt 2):H257–H262. doi: 10.1152/ajpheart.1991.261.1.H257. [DOI] [PubMed] [Google Scholar]
- Hwa J. J., Bevan J. A. Stretch-dependent (myogenic) tone in rabbit ear resistance arteries. Am J Physiol. 1986 Jan;250(1 Pt 2):H87–H95. doi: 10.1152/ajpheart.1986.250.1.H87. [DOI] [PubMed] [Google Scholar]
- Imig J. D., Gebremedhin D., Harder D. R., Roman R. J. Modulation of vascular tone in renal microcirculation by erythrocytes: role of EDRF. Am J Physiol. 1993 Jan;264(1 Pt 2):H190–H195. doi: 10.1152/ajpheart.1993.264.1.H190. [DOI] [PubMed] [Google Scholar]
- Ito S., Arima S., Ren Y. L., Juncos L. A., Carretero O. A. Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest. 1993 May;91(5):2012–2019. doi: 10.1172/JCI116423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Carretero O. A., Abe K., Beierwaltes W. H., Yoshinaga K. Effect of prostanoids on renin release from rabbit afferent arterioles with and without macula densa. Kidney Int. 1989 May;35(5):1138–1144. doi: 10.1038/ki.1989.102. [DOI] [PubMed] [Google Scholar]
- Ito S., Carretero O. A. An in vitro approach to the study of macula densa-mediated glomerular hemodynamics. Kidney Int. 1990 Dec;38(6):1206–1210. doi: 10.1038/ki.1990.335. [DOI] [PubMed] [Google Scholar]
- Ito S., Johnson C. S., Carretero O. A. Modulation of angiotensin II-induced vasoconstriction by endothelium-derived relaxing factor in the isolated microperfused rabbit afferent arteriole. J Clin Invest. 1991 May;87(5):1656–1663. doi: 10.1172/JCI115181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S., Ren Y. Evidence for the role of nitric oxide in macula densa control of glomerular hemodynamics. J Clin Invest. 1993 Aug;92(2):1093–1098. doi: 10.1172/JCI116615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh S., Carretero O. A. Role of the macula densa in renin release. Hypertension. 1985 May-Jun;7(3 Pt 2):I49–I54. doi: 10.1161/01.hyp.7.3_pt_2.i49. [DOI] [PubMed] [Google Scholar]
- Kaiser L., Hull S. S., Jr, Sparks H. V., Jr Methylene blue and ETYA block flow-dependent dilation in canine femoral artery. Am J Physiol. 1986 Jun;250(6 Pt 2):H974–H981. doi: 10.1152/ajpheart.1986.250.6.H974. [DOI] [PubMed] [Google Scholar]
- Katusić Z. S. Endothelium-independent contractions to NG-monomethyl-L-arginine in canine basilar artery. Stroke. 1991 Nov;22(11):1399–1404. doi: 10.1161/01.str.22.11.1399. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Endothelium regulates skeletal muscle microcirculation by a blood flow velocity-sensing mechanism. Am J Physiol. 1990 Mar;258(3 Pt 2):H916–H920. doi: 10.1152/ajpheart.1990.258.3.H916. [DOI] [PubMed] [Google Scholar]
- Koller A., Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. Circ Res. 1990 Aug;67(2):529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- Kuo L., Arko F., Chilian W. M., Davis M. J. Coronary venular responses to flow and pressure. Circ Res. 1993 Mar;72(3):607–615. doi: 10.1161/01.res.72.3.607. [DOI] [PubMed] [Google Scholar]
- Kuo L., Chilian W. M., Davis M. J. Coronary arteriolar myogenic response is independent of endothelium. Circ Res. 1990 Mar;66(3):860–866. doi: 10.1161/01.res.66.3.860. [DOI] [PubMed] [Google Scholar]
- Kuo L., Chilian W. M., Davis M. J. Interaction of pressure- and flow-induced responses in porcine coronary resistance vessels. Am J Physiol. 1991 Dec;261(6 Pt 2):H1706–H1715. doi: 10.1152/ajpheart.1991.261.6.H1706. [DOI] [PubMed] [Google Scholar]
- Kuo L., Davis M. J., Chilian W. M. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. Am J Physiol. 1990 Oct;259(4 Pt 2):H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- Liu Y., Harder D. R., Lombard J. H. Myogenic activation of canine small renal arteries after nonchemical removal of the endothelium. Am J Physiol. 1994 Jul;267(1 Pt 2):H302–H307. doi: 10.1152/ajpheart.1994.267.1.H302. [DOI] [PubMed] [Google Scholar]
- Majid D. S., Navar L. G. Suppression of blood flow autoregulation plateau during nitric oxide blockade in canine kidney. Am J Physiol. 1992 Jan;262(1 Pt 2):F40–F46. doi: 10.1152/ajprenal.1992.262.1.F40. [DOI] [PubMed] [Google Scholar]
- McCarron J. G., Osol G., Halpern W. Myogenic responses are independent of the endothelium in rat pressurized posterior cerebral arteries. Blood Vessels. 1989;26(5):315–319. doi: 10.1159/000158780. [DOI] [PubMed] [Google Scholar]
- Meininger G. A., Davis M. J. Cellular mechanisms involved in the vascular myogenic response. Am J Physiol. 1992 Sep;263(3 Pt 2):H647–H659. doi: 10.1152/ajpheart.1992.263.3.H647. [DOI] [PubMed] [Google Scholar]
- Meininger G. A., Faber J. E. Adrenergic facilitation of myogenic response in skeletal muscle arterioles. Am J Physiol. 1991 May;260(5 Pt 2):H1424–H1432. doi: 10.1152/ajpheart.1991.260.5.H1424. [DOI] [PubMed] [Google Scholar]
- Moore L. C., Casellas D. Tubuloglomerular feedback dependence of autoregulation in rat juxtamedullary afferent arterioles. Kidney Int. 1990 Jun;37(6):1402–1408. doi: 10.1038/ki.1990.129. [DOI] [PubMed] [Google Scholar]
- Nashat F. S., Scholefield F. R., Tappin J. W., Wilcox C. S. The effects of changes in haematocrit on the intrarenal distribution of blood flow in the dog's kidney. J Physiol. 1969 May;201(3):639–655. doi: 10.1113/jphysiol.1969.sp008777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navar L. G. Renal autoregulation: perspectives from whole kidney and single nephron studies. Am J Physiol. 1978 May;234(5):F357–F370. doi: 10.1152/ajprenal.1978.234.5.F357. [DOI] [PubMed] [Google Scholar]
- Pohl U., Herlan K., Huang A., Bassenge E. EDRF-mediated shear-induced dilation opposes myogenic vasoconstriction in small rabbit arteries. Am J Physiol. 1991 Dec;261(6 Pt 2):H2016–H2023. doi: 10.1152/ajpheart.1991.261.6.H2016. [DOI] [PubMed] [Google Scholar]
- Pohl U., Holtz J., Busse R., Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986 Jan;8(1):37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Robertson C. R., Deen W. M., Troy J. L., Brenner B. M. Dynamics of glomerular ultrafiltration in the rat. 3. Hemodynamics and autoregulation. Am J Physiol. 1972 Nov;223(5):1191–1200. doi: 10.1152/ajplegacy.1972.223.5.1191. [DOI] [PubMed] [Google Scholar]
- Rosenblum W. I., Nishimura H., Nelson G. H. L-NMMA in brain microcirculation of mice is inhibited by blockade of cyclooxygenase and by superoxide dismutase. Am J Physiol. 1992 May;262(5 Pt 2):H1343–H1349. doi: 10.1152/ajpheart.1992.262.5.H1343. [DOI] [PubMed] [Google Scholar]
- Sigurdsson S. B., Johansson B., Mellander S. Rate-dependent myogenic response of vascular smooth muscle during imposed changes in length and force. Acta Physiol Scand. 1977 Feb;99(2):183–189. doi: 10.1111/j.1748-1716.1977.tb10369.x. [DOI] [PubMed] [Google Scholar]
- Smiesko V., Lang D. J., Johnson P. C. Dilator response of rat mesenteric arcading arterioles to increased blood flow velocity. Am J Physiol. 1989 Dec;257(6 Pt 2):H1958–H1965. doi: 10.1152/ajpheart.1989.257.6.H1958. [DOI] [PubMed] [Google Scholar]
- Thomas G., Ramwell P. W. Interaction of non-arginine compounds with the endothelium-derived relaxing factor inhibitor, NG-monomethyl L-arginine. J Pharmacol Exp Ther. 1992 Feb;260(2):676–679. [PubMed] [Google Scholar]
- Wilcox C. S., Deng X., Doll A. H., Snellen H., Welch W. J. Nitric oxide mediates renal vasodilation during erythropoietin-induced polycythemia. Kidney Int. 1993 Aug;44(2):430–435. doi: 10.1038/ki.1993.261. [DOI] [PubMed] [Google Scholar]
- Wilcox C. S., Payne J., Harrison B. D. Renal function in patients with chronic hypoxaemia and cor pulmonale following reversal of polycythaemia. Nephron. 1982;30(2):173–177. doi: 10.1159/000182456. [DOI] [PubMed] [Google Scholar]
- Wilcox C. S., Welch W. J., Murad F., Gross S. S., Taylor G., Levi R., Schmidt H. H. Nitric oxide synthase in macula densa regulates glomerular capillary pressure. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11993–11997. doi: 10.1073/pnas.89.24.11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan B. H., Robinette J. B., Conger J. D. Effect of angiotensin II and norepinephrine on isolated rat afferent and efferent arterioles. Am J Physiol. 1990 Mar;258(3 Pt 2):F741–F750. doi: 10.1152/ajprenal.1990.258.3.F741. [DOI] [PubMed] [Google Scholar]
- van Grondelle A., Worthen G. S., Ellis D., Mathias M. M., Murphy R. C., Strife R. J., Reeves J. T., Voelkel N. F. Altering hydrodynamic variables influences PGI2 production by isolated lungs and endothelial cells. J Appl Physiol Respir Environ Exerc Physiol. 1984 Aug;57(2):388–395. doi: 10.1152/jappl.1984.57.2.388. [DOI] [PubMed] [Google Scholar]