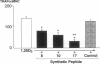Abstract
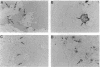
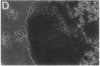
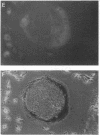
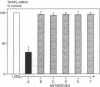
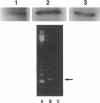
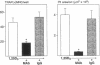
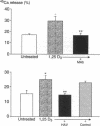
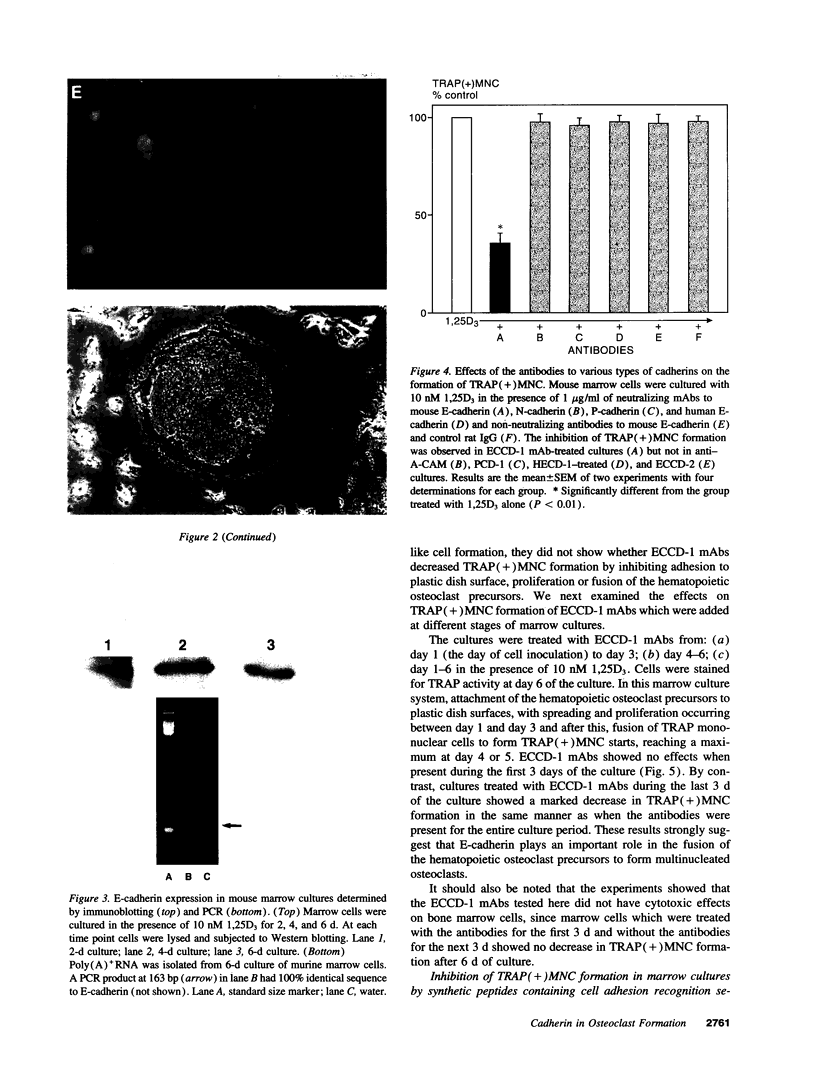
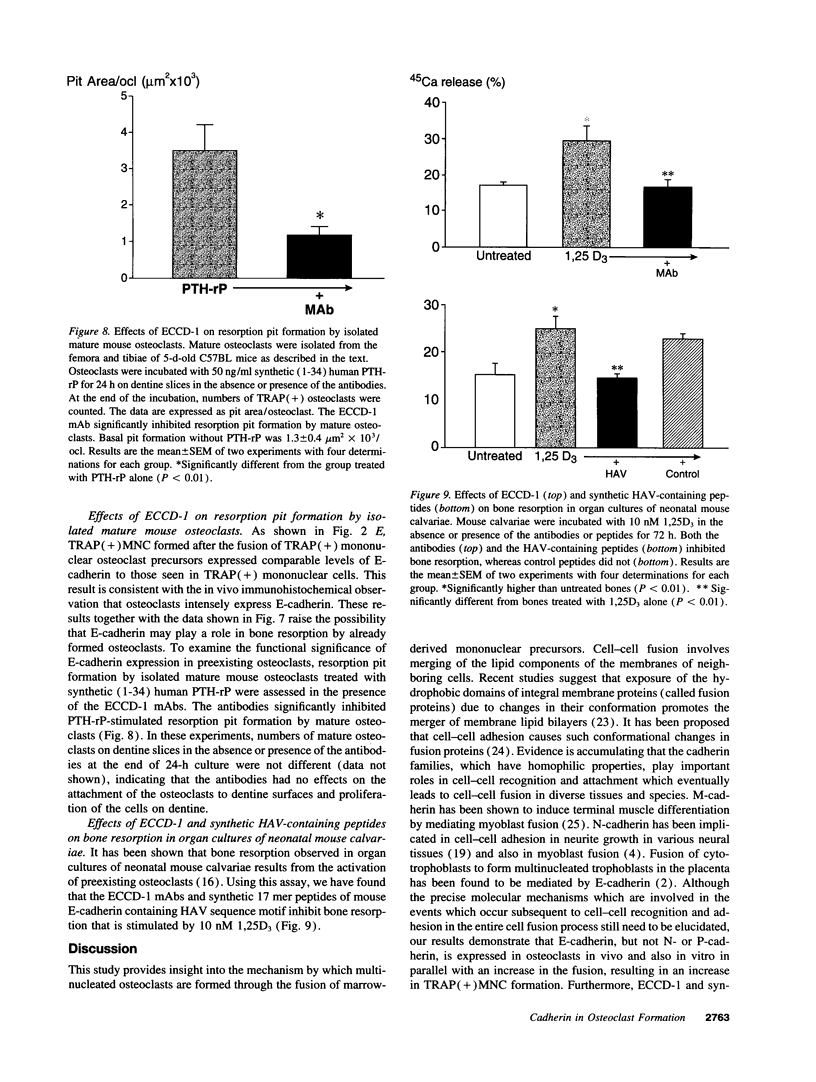
A critical step in bone resorption is the fusion of mononuclear osteoclast precursors to form multinucleated osteoclasts. However, little is known of the molecular mechanisms that are responsible for this important process. Since the expression of proteins in the cadherin family of homophilic calcium-dependent cell adhesion molecules is involved in the fusion process for certain other cells, we examined their role in osteoclast formation. Immunohistochemical examination of human and mouse bone using monoclonal antibodies to human and mouse E-cadherin clearly demonstrated positive staining in osteoclasts. N- and P-cadherin were not detected. In cultures of murine marrow mononuclear cells in which osteoclasts form by cell fusion, E-cadherin expression determined by Western blotting reached the highest levels as fusion was taking place. Expression of E-cadherin gene fragment was also detected in the marrow cultures by polymerase chain reaction. To study the functional role of E-cadherin expression in osteoclastic differentiation, neutralizing monoclonal antibodies were examined for their effects on osteoclast formation. The antibodies decreased the number of tartrate-resistant acid phosphatase (a marker of murine osteoclast)-positive multinucleated cell (TRAP-positive MNC) by inhibiting the fusion of mononuclear osteoclast precursors, but not proliferation of these cells or their attachment to plastic dish surfaces. This inhibitory effect was reversible. Furthermore, synthetic peptides containing the cell adhesion recognition sequence of cadherins also decreased TRAP-positive MNC formation. The antibodies and peptides inhibited not only osteoclast formation but also bone resorption. Antibodies to other types of cadherins and control rat IgG had no effects in these culture systems. Our findings suggest that E-cadherin expression may be involved in fusion (differentiation) of hemopoietic osteoclast precursors into mature multinucleated osteoclasts.
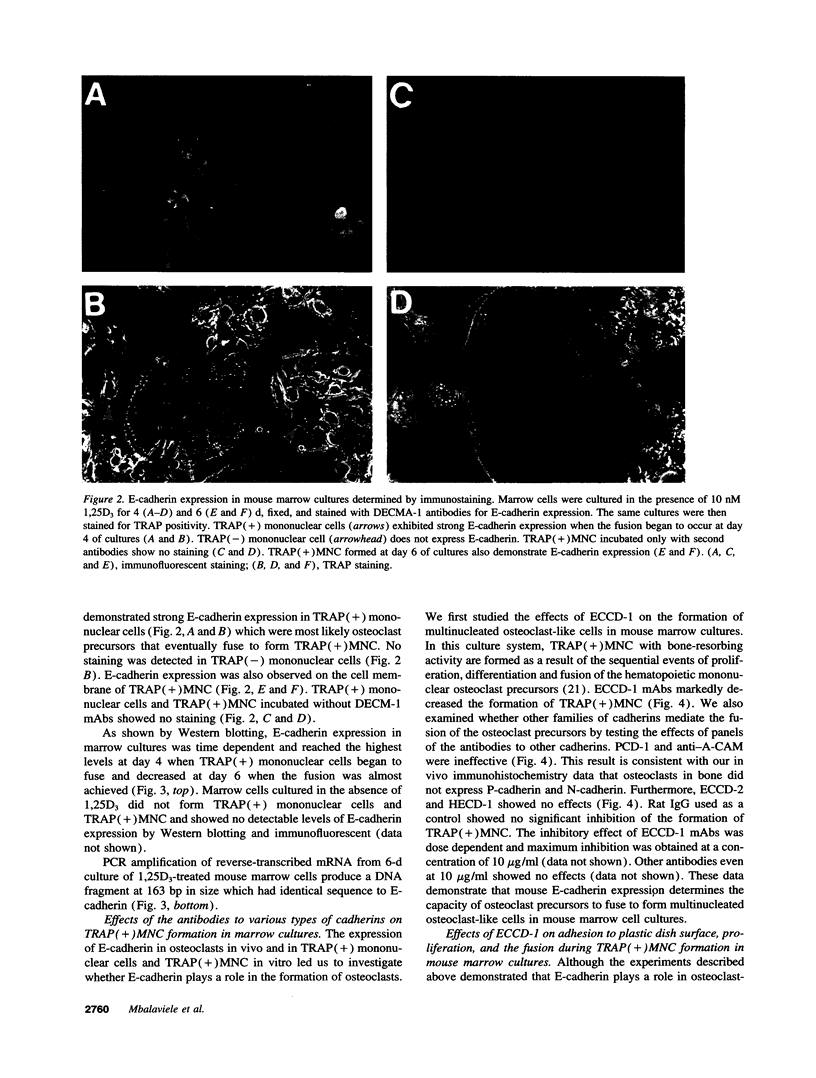
Full text
PDF


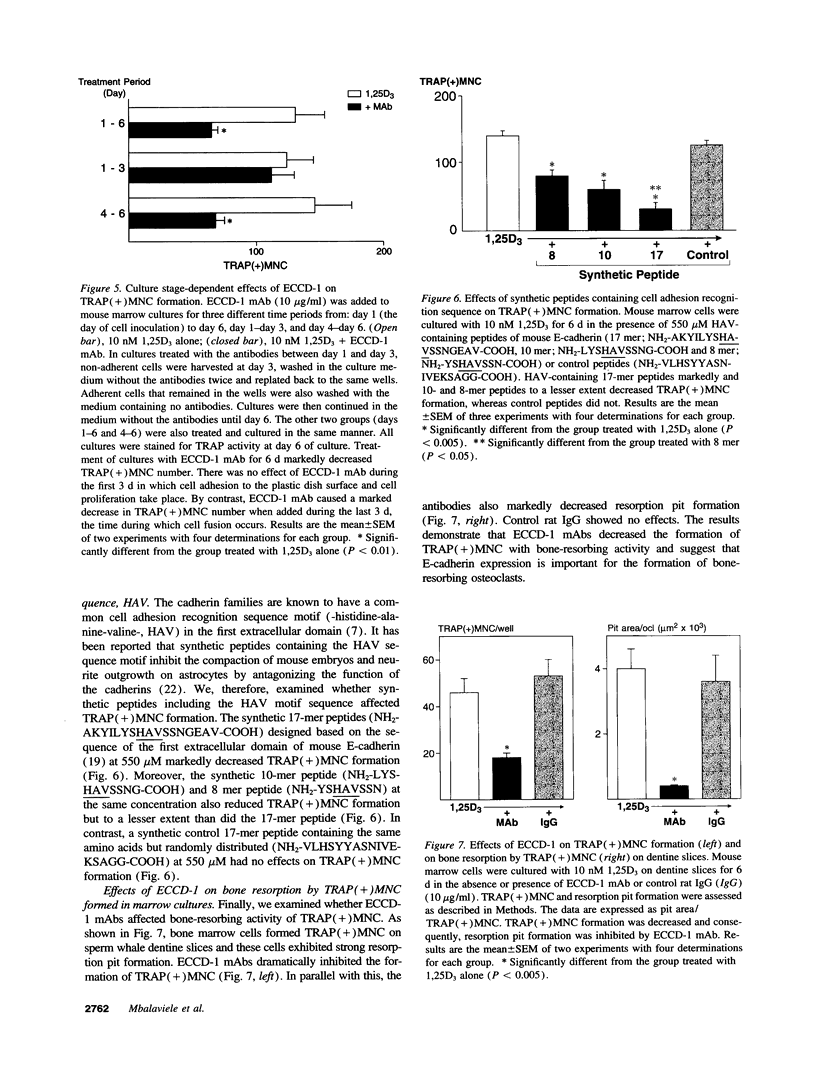


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blaschuk O. W., Pouliot Y., Holland P. C. Identification of a conserved region common to cadherins and influenza strain A hemagglutinins. J Mol Biol. 1990 Feb 20;211(4):679–682. doi: 10.1016/0022-2836(90)90065-T. [DOI] [PubMed] [Google Scholar]
- Blaschuk O. W., Sullivan R., David S., Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990 May;139(1):227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- Blobel C. P., Wolfsberg T. G., Turck C. W., Myles D. G., Primakoff P., White J. M. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature. 1992 Mar 19;356(6366):248–252. doi: 10.1038/356248a0. [DOI] [PubMed] [Google Scholar]
- Boyce B. F., Chen H., Soriano P., Mundy G. R. Histomorphometric and immunocytochemical studies of src-related osteopetrosis. Bone. 1993 May-Jun;14(3):335–340. doi: 10.1016/8756-3282(93)90161-3. [DOI] [PubMed] [Google Scholar]
- Boyde A., Ali N. N., Jones S. J. Resorption of dentine by isolated osteoclasts in vitro. Br Dent J. 1984 Mar 24;156(6):216–220. doi: 10.1038/sj.bdj.4805313. [DOI] [PubMed] [Google Scholar]
- Byers S., Amaya E., Munro S., Blaschuk O. Fibroblast growth factor receptors contain a conserved HAV region common to cadherins and influenza strain A hemagglutinins: a role in protein-protein interactions? Dev Biol. 1992 Aug;152(2):411–414. doi: 10.1016/0012-1606(92)90149-b. [DOI] [PubMed] [Google Scholar]
- Cepek K. L., Shaw S. K., Parker C. M., Russell G. J., Morrow J. S., Rimm D. L., Brenner M. B. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the alpha E beta 7 integrin. Nature. 1994 Nov 10;372(6502):190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Coutifaris C., Kao L. C., Sehdev H. M., Chin U., Babalola G. O., Blaschuk O. W., Strauss J. F., 3rd E-cadherin expression during the differentiation of human trophoblasts. Development. 1991 Nov;113(3):767–777. doi: 10.1242/dev.113.3.767. [DOI] [PubMed] [Google Scholar]
- Cowin P. Unraveling the cytoplasmic interactions of the cadherin superfamily. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):10759–10761. doi: 10.1073/pnas.91.23.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donalies M., Cramer M., Ringwald M., Starzinski-Powitz A. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8024–8028. doi: 10.1073/pnas.88.18.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett I. R., Mundy G. R. Relationship between interleukin-1 and prostaglandins in resorbing neonatal calvaria. J Bone Miner Res. 1989 Oct;4(5):789–794. doi: 10.1002/jbmr.5650040520. [DOI] [PubMed] [Google Scholar]
- Grunwald G. B. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993 Oct;5(5):797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Hasty P., Bradley A., Morris J. H., Edmondson D. G., Venuti J. M., Olson E. N., Klein W. H. Muscle deficiency and neonatal death in mice with a targeted mutation in the myogenin gene. Nature. 1993 Aug 5;364(6437):501–506. doi: 10.1038/364501a0. [DOI] [PubMed] [Google Scholar]
- Kliman H. J., Nestler J. E., Sermasi E., Sanger J. M., Strauss J. F., 3rd Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986 Apr;118(4):1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Koch P. J., Franke W. W. Desmosomal cadherins: another growing multigene family of adhesion molecules. Curr Opin Cell Biol. 1994 Oct;6(5):682–687. doi: 10.1016/0955-0674(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Mege R. M., Goudou D., Diaz C., Nicolet M., Garcia L., Geraud G., Rieger F. N-cadherin and N-CAM in myoblast fusion: compared localisation and effect of blockade by peptides and antibodies. J Cell Sci. 1992 Dec;103(Pt 4):897–906. doi: 10.1242/jcs.103.4.897. [DOI] [PubMed] [Google Scholar]
- Miyatani S., Shimamura K., Hatta M., Nagafuchi A., Nose A., Matsunaga M., Hatta K., Takeichi M. Neural cadherin: role in selective cell-cell adhesion. Science. 1989 Aug 11;245(4918):631–635. doi: 10.1126/science.2762814. [DOI] [PubMed] [Google Scholar]
- Nose A., Tsuji K., Takeichi M. Localization of specificity determining sites in cadherin cell adhesion molecules. Cell. 1990 Apr 6;61(1):147–155. doi: 10.1016/0092-8674(90)90222-z. [DOI] [PubMed] [Google Scholar]
- Okazaki M., Takeshita S., Kawai S., Kikuno R., Tsujimura A., Kudo A., Amann E. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J Biol Chem. 1994 Apr 22;269(16):12092–12098. [PubMed] [Google Scholar]
- Oreffo R. O., Bonewald L., Kukita A., Garrett I. R., Seyedin S. M., Rosen D., Mundy G. R. Inhibitory effects of the bone-derived growth factors osteoinductive factor and transforming growth factor-beta on isolated osteoclasts. Endocrinology. 1990 Jun;126(6):3069–3075. doi: 10.1210/endo-126-6-3069. [DOI] [PubMed] [Google Scholar]
- Oreffo R. O., Mundy G. R., Seyedin S. M., Bonewald L. F. Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem Biophys Res Commun. 1989 Feb 15;158(3):817–823. doi: 10.1016/0006-291x(89)92795-2. [DOI] [PubMed] [Google Scholar]
- Overduin M., Harvey T. S., Bagby S., Tong K. I., Yau P., Takeichi M., Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995 Jan 20;267(5196):386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano K., Tanihara H., Heimark R. L., Obata S., Davidson M., St John T., Taketani S., Suzuki S. Protocadherins: a large family of cadherin-related molecules in central nervous system. EMBO J. 1993 Jun;12(6):2249–2256. doi: 10.1002/j.1460-2075.1993.tb05878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura K., Takahashi T., Takeichi M. E-cadherin expression in a particular subset of sensory neurons. Dev Biol. 1992 Aug;152(2):242–254. doi: 10.1016/0012-1606(92)90132-z. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Sano K., Tanihara H. Diversity of the cadherin family: evidence for eight new cadherins in nervous tissue. Cell Regul. 1991 Apr;2(4):261–270. doi: 10.1091/mbc.2.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Akatsu T., Udagawa N., Sasaki T., Yamaguchi A., Moseley J. M., Martin T. J., Suda T. Osteoblastic cells are involved in osteoclast formation. Endocrinology. 1988 Nov;123(5):2600–2602. doi: 10.1210/endo-123-5-2600. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Yamana H., Yoshiki S., Roodman G. D., Mundy G. R., Jones S. J., Boyde A., Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology. 1988 Apr;122(4):1373–1382. doi: 10.1210/endo-122-4-1373. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991 Mar 22;251(5000):1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Tang A., Amagai M., Granger L. G., Stanley J. R., Udey M. C. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993 Jan 7;361(6407):82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- Vestweber D., Kemler R. Identification of a putative cell adhesion domain of uvomorulin. EMBO J. 1985 Dec 16;4(13A):3393–3398. doi: 10.1002/j.1460-2075.1985.tb04095.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. M. Membrane fusion. Science. 1992 Nov 6;258(5084):917–924. doi: 10.1126/science.1439803. [DOI] [PubMed] [Google Scholar]
- Yoneda T., Lowe C., Lee C. H., Gutierrez G., Niewolna M., Williams P. J., Izbicka E., Uehara Y., Mundy G. R. Herbimycin A, a pp60c-src tyrosine kinase inhibitor, inhibits osteoclastic bone resorption in vitro and hypercalcemia in vivo. J Clin Invest. 1993 Jun;91(6):2791–2795. doi: 10.1172/JCI116521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda T., Niewolna M., Lowe C., Izbicka E., Mundy G. R. Hormonal regulation of pp60c-src expression during osteoclast formation in vitro. Mol Endocrinol. 1993 Oct;7(10):1313–1318. doi: 10.1210/mend.7.10.7505394. [DOI] [PubMed] [Google Scholar]
- van de Wijngaert F. P., Burger E. H. Demonstration of tartrate-resistant acid phosphatase in un-decalcified, glycolmethacrylate-embedded mouse bone: a possible marker for (pre)osteoclast identification. J Histochem Cytochem. 1986 Oct;34(10):1317–1323. doi: 10.1177/34.10.3745910. [DOI] [PubMed] [Google Scholar]