Abstract
The crystal structure of the title compound, C11H16N2O2, contains two crystallographically independent molecules forming dimers by pairs of intermolecular N—H⋯N hydrogen bonds. The two molecules are related by a pseudo-twofold axis. The dihedral angle between the pyridine ring and the carbamate plane differs in the two molecules [12.1 (3) and 3.5 (3)°].
Related literature
For the preparation of the title compound, see: Laufer & Koch (2008 ▶); Koch et al. (2008 ▶). For applications of functionalized 2-aminopyridines, see, for example: Peifer et al. (2006 ▶); Kuo, DeAngelis et al. (2005 ▶); Kuo, Wang et al. (2005 ▶); Swahn et al. (2006 ▶).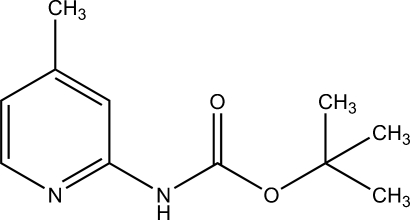
Experimental
Crystal data
C11H16N2O2
M r = 208.26
Orthorhombic,

a = 10.5850 (6) Å
b = 11.6854 (6) Å
c = 18.5568 (15) Å
V = 2295.3 (3) Å3
Z = 8
Cu Kα radiation
μ = 0.68 mm−1
T = 193 (2) K
0.51 × 0.16 × 0.06 mm
Data collection
Enraf–Nonius CAD-4 diffractometer
Absorption correction: none
4711 measured reflections
2471 independent reflections
1782 reflections with I > 2σ(I)
R int = 0.061
3 standard reflections frequency: 60 min intensity decay: 3%
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.149
S = 1.01
2471 reflections
280 parameters
H-atom parameters constrained
Δρmax = 0.25 e Å−3
Δρmin = −0.25 e Å−3
Data collection: CAD-4 Software (Enraf–Nonius, 1989 ▶); cell refinement: CAD-4 Software; data reduction: CORINC (Dräger & Gattow, 1971 ▶); program(s) used to solve structure: SIR97 (Altomare et al., 1999 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2003 ▶); software used to prepare material for publication: PLATON.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808032327/bt2808sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808032327/bt2808Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N8A—H8A⋯N2B | 0.94 | 2.05 | 2.980 (5) | 171 |
| N8B—H8B⋯N2A | 0.98 | 2.04 | 3.015 (5) | 173 |
Acknowledgments
The authors are thankful for financial support from the EU, part of the EU-Craft Programme, Framework Project 6 ‘MACROCEPT’.
supplementary crystallographic information
Comment
N-substituted 2-aminopyridin-4-yl derivatives can be found in different biological active compounds, like p38 MAP kinase inhibitors (Peifer et al., 2006), VEGFR-2 inhibitors (Kuo, Wang et al., 2005a), CDK inhibitors (Kuo, DeAngelis et al., 2005) or JNK3 inhibitors (Swahn et al., 2006). The title compound, tert-butyl 4-methylpyridin-2-ylcarbamate (I), was synthesized as an intermediate in the synthesis of 2-alkylsulfanyl-5-(2-aminopyridin-4-yl)-4-(4-fluorophenyl)imidazoles as potent p38 MAP kinase inhibitors (Laufer & Koch, 2008; Koch et al., 2008).
The crystal stucture of the title compound I, Fig. 1, contains two crystallographically independent molecules forming dimers by intermolecular N–H···N hydrogen bonds. The two molecules are related by a pseudo 2-fold axis.
As might be expected the pyridine ring as well as the carbamate fragment are planar. The dihedral angle between the pyridine ring and the carbamate plane of molecule A [12.1 (3)°] is bigger than in molecule B [3.5 (3)°].
The N8—C9 is shorter than a normal N—C-bond and longer than a N-C-bond (N8A—C9A: 1.373 (6) Å; N8B—C9B: 1.367 (5) Å), indicating the partially double bond character of the N8—C9-bond of the carbamate.
Experimental
To a solution of freshly distilled tert-butanol (450 ml) and di-tert-butyl dicarbonate (16.81 g, 77.0 mmol) was added slowly 2-amino-4-methylpyridine (7.57 g, 70.0 mmol). The mixture was stirred at room temperature for 3 d, the solvent was removed in vacuo and the residue was recrystallized from hot 2-propanol, affording 12.30 g (84%) of I as colourless crystals (Laufer & Koch, 2008).
Refinement
In the absence of significant anomalous dispersion effects, Friedel pairs were averaged. H-atom bonded to N were located from a difference Fourier map and constrained to this position. All hydrogen atoms bonded to C were placed at calculated positions with C—H = 0.95 Å (for aromatic C) or 0.98 Å (for sp3 C-atoms) and refined in the riding-model approximation with isotropic displacement parameters set to 1.2 (1.5 for methyl groups) times of the Ueq of the parent atom.
Figures
Fig. 1.
View of compound I. Displacement ellipsoids are drawn at the 50% probability level. H atoms are depicted as circles of arbitrary size. Hydrogen bonds are drawn as dashed lines.
Crystal data
| C11H16N2O2 | F(000) = 896 |
| Mr = 208.26 | Dx = 1.205 Mg m−3 |
| Orthorhombic, P212121 | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 25 reflections |
| a = 10.5850 (6) Å | θ = 21–26° |
| b = 11.6854 (6) Å | µ = 0.68 mm−1 |
| c = 18.5568 (15) Å | T = 193 K |
| V = 2295.3 (3) Å3 | Plate, colourless |
| Z = 8 | 0.51 × 0.16 × 0.06 mm |
Data collection
| Enraf–Nonius CAD-4 diffractometer | Rint = 0.061 |
| Radiation source: rotating anode | θmax = 70.0°, θmin = 4.5° |
| graphite | h = −12→12 |
| ω/2θ scans | k = −13→14 |
| 4711 measured reflections | l = −22→22 |
| 2471 independent reflections | 3 standard reflections every 60 min |
| 1782 reflections with I > 2σ(I) | intensity decay: 3% |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.057 | H-atom parameters constrained |
| wR(F2) = 0.149 | w = 1/[σ2(Fo2) + (0.0707P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max = 0.002 |
| 2471 reflections | Δρmax = 0.25 e Å−3 |
| 280 parameters | Δρmin = −0.25 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0021 (4) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Friedel pairs merged. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C1A | 0.0792 (4) | 0.7774 (3) | 0.3417 (2) | 0.0335 (10) | |
| N2A | 0.1189 (4) | 0.7180 (3) | 0.28496 (19) | 0.0364 (8) | |
| C3A | 0.0776 (5) | 0.6104 (4) | 0.2792 (3) | 0.0451 (12) | |
| H3A | 0.1052 | 0.5663 | 0.2392 | 0.054* | |
| C4A | −0.0032 (5) | 0.5596 (4) | 0.3282 (3) | 0.0462 (12) | |
| H4A | −0.0309 | 0.4830 | 0.3215 | 0.055* | |
| C5A | −0.0430 (4) | 0.6223 (4) | 0.3872 (3) | 0.0407 (11) | |
| C6A | 0.0026 (4) | 0.7332 (4) | 0.3947 (2) | 0.0393 (11) | |
| H6A | −0.0189 | 0.7777 | 0.4357 | 0.047* | |
| C7A | −0.1333 (6) | 0.5727 (5) | 0.4407 (3) | 0.0613 (15) | |
| H7A | −0.1060 | 0.4952 | 0.4536 | 0.092* | |
| H7B | −0.2180 | 0.5696 | 0.4195 | 0.092* | |
| H7C | −0.1349 | 0.6207 | 0.4840 | 0.092* | |
| N8A | 0.1233 (4) | 0.8913 (3) | 0.3411 (2) | 0.0382 (9) | |
| H8A | 0.1733 | 0.9180 | 0.3026 | 0.046* | |
| C9A | 0.0976 (4) | 0.9746 (4) | 0.3912 (2) | 0.0379 (11) | |
| O10A | 0.0460 (4) | 0.9595 (3) | 0.44786 (18) | 0.0545 (10) | |
| O11A | 0.1393 (3) | 1.0749 (2) | 0.36505 (16) | 0.0373 (7) | |
| C12A | 0.1276 (4) | 1.1805 (4) | 0.4084 (2) | 0.0370 (10) | |
| C13A | 0.2094 (5) | 1.1704 (5) | 0.4741 (3) | 0.0490 (12) | |
| H13A | 0.2964 | 1.1536 | 0.4595 | 0.073* | |
| H13B | 0.1778 | 1.1084 | 0.5048 | 0.073* | |
| H13C | 0.2074 | 1.2426 | 0.5009 | 0.073* | |
| C14A | −0.0098 (5) | 1.2060 (4) | 0.4244 (3) | 0.0497 (13) | |
| H14A | −0.0440 | 1.1466 | 0.4562 | 0.075* | |
| H14B | −0.0578 | 1.2074 | 0.3793 | 0.075* | |
| H14C | −0.0165 | 1.2807 | 0.4482 | 0.075* | |
| C15A | 0.1797 (5) | 1.2711 (4) | 0.3565 (3) | 0.0512 (13) | |
| H15A | 0.1313 | 1.2694 | 0.3115 | 0.077* | |
| H15B | 0.2687 | 1.2548 | 0.3463 | 0.077* | |
| H15C | 0.1724 | 1.3469 | 0.3786 | 0.077* | |
| C1B | 0.3724 (4) | 0.8850 (3) | 0.1817 (2) | 0.0294 (9) | |
| N2B | 0.2977 (4) | 0.9520 (3) | 0.2209 (2) | 0.0382 (9) | |
| C3B | 0.3289 (5) | 1.0633 (4) | 0.2247 (3) | 0.0447 (12) | |
| H3B | 0.2760 | 1.1129 | 0.2518 | 0.054* | |
| C4B | 0.4328 (5) | 1.1091 (4) | 0.1916 (2) | 0.0413 (11) | |
| H4B | 0.4524 | 1.1879 | 0.1972 | 0.050* | |
| C5B | 0.5093 (4) | 1.0392 (4) | 0.1496 (2) | 0.0357 (10) | |
| C6B | 0.4761 (4) | 0.9242 (4) | 0.1449 (2) | 0.0348 (10) | |
| H6B | 0.5250 | 0.8732 | 0.1163 | 0.042* | |
| C7B | 0.6208 (5) | 1.0861 (4) | 0.1101 (3) | 0.0491 (12) | |
| H7D | 0.5975 | 1.1007 | 0.0598 | 0.074* | |
| H7E | 0.6900 | 1.0305 | 0.1117 | 0.074* | |
| H7F | 0.6479 | 1.1577 | 0.1328 | 0.074* | |
| N8B | 0.3330 (4) | 0.7691 (3) | 0.18270 (19) | 0.0336 (8) | |
| H8B | 0.2592 | 0.7567 | 0.2135 | 0.040* | |
| C9B | 0.3881 (4) | 0.6812 (4) | 0.1456 (2) | 0.0313 (9) | |
| O10B | 0.4763 (3) | 0.6874 (3) | 0.10641 (17) | 0.0434 (8) | |
| O11B | 0.3214 (3) | 0.5851 (2) | 0.16178 (16) | 0.0377 (7) | |
| C12B | 0.3526 (4) | 0.4770 (4) | 0.1243 (3) | 0.0406 (11) | |
| C13B | 0.3232 (6) | 0.4884 (5) | 0.0454 (3) | 0.0589 (15) | |
| H13D | 0.3829 | 0.5418 | 0.0231 | 0.088* | |
| H13E | 0.2369 | 0.5175 | 0.0395 | 0.088* | |
| H13F | 0.3304 | 0.4134 | 0.0221 | 0.088* | |
| C14B | 0.4870 (5) | 0.4394 (4) | 0.1387 (3) | 0.0481 (12) | |
| H14D | 0.5003 | 0.4327 | 0.1908 | 0.072* | |
| H14E | 0.5457 | 0.4962 | 0.1189 | 0.072* | |
| H14F | 0.5021 | 0.3651 | 0.1158 | 0.072* | |
| C15B | 0.2625 (5) | 0.3941 (4) | 0.1610 (4) | 0.0652 (17) | |
| H15D | 0.1752 | 0.4186 | 0.1524 | 0.098* | |
| H15E | 0.2793 | 0.3932 | 0.2129 | 0.098* | |
| H15F | 0.2749 | 0.3171 | 0.1412 | 0.098* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1A | 0.036 (2) | 0.024 (2) | 0.040 (2) | 0.0047 (18) | 0.0011 (19) | 0.0019 (19) |
| N2A | 0.0373 (19) | 0.0282 (18) | 0.0438 (19) | −0.0015 (16) | 0.0037 (17) | −0.0048 (16) |
| C3A | 0.045 (3) | 0.026 (2) | 0.064 (3) | −0.003 (2) | −0.006 (2) | −0.004 (2) |
| C4A | 0.042 (3) | 0.030 (2) | 0.067 (3) | −0.007 (2) | −0.003 (2) | 0.008 (2) |
| C5A | 0.038 (2) | 0.034 (2) | 0.050 (3) | −0.005 (2) | −0.005 (2) | 0.013 (2) |
| C6A | 0.042 (3) | 0.034 (2) | 0.042 (2) | 0.002 (2) | 0.005 (2) | 0.005 (2) |
| C7A | 0.066 (4) | 0.052 (3) | 0.066 (3) | −0.021 (3) | 0.001 (3) | 0.017 (3) |
| N8A | 0.046 (2) | 0.0258 (18) | 0.043 (2) | −0.0051 (17) | 0.0153 (19) | −0.0017 (16) |
| C9A | 0.041 (3) | 0.033 (2) | 0.039 (2) | −0.006 (2) | 0.008 (2) | −0.0046 (19) |
| O10A | 0.076 (3) | 0.0417 (19) | 0.0456 (18) | −0.0087 (19) | 0.0239 (19) | −0.0056 (16) |
| O11A | 0.0443 (18) | 0.0245 (15) | 0.0431 (16) | −0.0019 (14) | 0.0098 (14) | −0.0037 (13) |
| C12A | 0.041 (2) | 0.025 (2) | 0.045 (2) | 0.001 (2) | 0.006 (2) | −0.011 (2) |
| C13A | 0.048 (3) | 0.048 (3) | 0.051 (3) | 0.004 (2) | −0.003 (2) | −0.008 (3) |
| C14A | 0.040 (3) | 0.046 (3) | 0.063 (3) | 0.007 (2) | 0.003 (2) | −0.016 (2) |
| C15A | 0.066 (3) | 0.024 (2) | 0.063 (3) | −0.004 (2) | 0.012 (3) | −0.005 (2) |
| C1B | 0.033 (2) | 0.0230 (19) | 0.032 (2) | 0.0001 (18) | 0.0006 (18) | −0.0032 (16) |
| N2B | 0.041 (2) | 0.0272 (18) | 0.046 (2) | −0.0003 (16) | 0.0107 (17) | −0.0017 (17) |
| C3B | 0.055 (3) | 0.022 (2) | 0.057 (3) | 0.003 (2) | 0.017 (3) | −0.002 (2) |
| C4B | 0.049 (3) | 0.029 (2) | 0.046 (3) | −0.001 (2) | 0.006 (2) | 0.001 (2) |
| C5B | 0.034 (2) | 0.036 (2) | 0.037 (2) | −0.0042 (19) | 0.0002 (19) | 0.0039 (19) |
| C6B | 0.038 (2) | 0.030 (2) | 0.036 (2) | 0.004 (2) | 0.002 (2) | −0.0007 (18) |
| C7B | 0.041 (3) | 0.045 (3) | 0.061 (3) | −0.011 (2) | 0.010 (2) | −0.002 (2) |
| N8B | 0.0341 (19) | 0.0244 (17) | 0.0422 (19) | −0.0048 (16) | 0.0077 (16) | −0.0066 (15) |
| C9B | 0.034 (2) | 0.026 (2) | 0.033 (2) | 0.0006 (19) | 0.0024 (19) | −0.0018 (18) |
| O10B | 0.0500 (19) | 0.0297 (16) | 0.0503 (18) | 0.0016 (15) | 0.0171 (17) | −0.0035 (14) |
| O11B | 0.0369 (16) | 0.0251 (15) | 0.0513 (18) | −0.0044 (13) | 0.0061 (15) | −0.0104 (14) |
| C12B | 0.039 (3) | 0.028 (2) | 0.055 (3) | 0.005 (2) | −0.003 (2) | −0.011 (2) |
| C13B | 0.070 (4) | 0.047 (3) | 0.060 (3) | 0.022 (3) | −0.016 (3) | −0.020 (3) |
| C14B | 0.047 (3) | 0.041 (3) | 0.056 (3) | 0.001 (2) | −0.005 (2) | −0.002 (2) |
| C15B | 0.056 (3) | 0.031 (3) | 0.108 (5) | −0.004 (3) | 0.015 (3) | −0.016 (3) |
Geometric parameters (Å, °)
| C1A—N2A | 1.330 (5) | C1B—N2B | 1.330 (5) |
| C1A—C6A | 1.375 (6) | C1B—C6B | 1.372 (6) |
| C1A—N8A | 1.411 (5) | C1B—N8B | 1.418 (5) |
| N2A—C3A | 1.336 (5) | N2B—C3B | 1.344 (5) |
| C3A—C4A | 1.382 (7) | C3B—C4B | 1.369 (6) |
| C3A—H3A | 0.9500 | C3B—H3B | 0.9500 |
| C4A—C5A | 1.383 (7) | C4B—C5B | 1.388 (6) |
| C4A—H4A | 0.9500 | C4B—H4B | 0.9500 |
| C5A—C6A | 1.389 (6) | C5B—C6B | 1.392 (6) |
| C5A—C7A | 1.494 (7) | C5B—C7B | 1.494 (6) |
| C6A—H6A | 0.9500 | C6B—H6B | 0.9500 |
| C7A—H7A | 0.9800 | C7B—H7D | 0.9800 |
| C7A—H7B | 0.9800 | C7B—H7E | 0.9800 |
| C7A—H7C | 0.9800 | C7B—H7F | 0.9800 |
| N8A—C9A | 1.373 (5) | N8B—C9B | 1.367 (5) |
| N8A—H8A | 0.9418 | N8B—H8B | 0.9790 |
| C9A—O10A | 1.199 (5) | C9B—O10B | 1.184 (5) |
| C9A—O11A | 1.343 (5) | C9B—O11B | 1.361 (5) |
| O11A—C12A | 1.477 (5) | O11B—C12B | 1.479 (5) |
| C12A—C13A | 1.500 (6) | C12B—C13B | 1.503 (7) |
| C12A—C14A | 1.514 (7) | C12B—C14B | 1.512 (7) |
| C12A—C15A | 1.533 (6) | C12B—C15B | 1.520 (7) |
| C13A—H13A | 0.9800 | C13B—H13D | 0.9800 |
| C13A—H13B | 0.9800 | C13B—H13E | 0.9800 |
| C13A—H13C | 0.9800 | C13B—H13F | 0.9800 |
| C14A—H14A | 0.9800 | C14B—H14D | 0.9800 |
| C14A—H14B | 0.9800 | C14B—H14E | 0.9800 |
| C14A—H14C | 0.9800 | C14B—H14F | 0.9800 |
| C15A—H15A | 0.9800 | C15B—H15D | 0.9800 |
| C15A—H15B | 0.9800 | C15B—H15E | 0.9800 |
| C15A—H15C | 0.9800 | C15B—H15F | 0.9800 |
| N2A—C1A—C6A | 123.8 (4) | N2B—C1B—C6B | 123.6 (4) |
| N2A—C1A—N8A | 112.4 (4) | N2B—C1B—N8B | 112.3 (4) |
| C6A—C1A—N8A | 123.8 (4) | C6B—C1B—N8B | 124.1 (4) |
| C1A—N2A—C3A | 116.8 (4) | C1B—N2B—C3B | 116.8 (4) |
| N2A—C3A—C4A | 123.6 (5) | N2B—C3B—C4B | 123.5 (4) |
| N2A—C3A—H3A | 118.2 | N2B—C3B—H3B | 118.2 |
| C4A—C3A—H3A | 118.2 | C4B—C3B—H3B | 118.2 |
| C3A—C4A—C5A | 118.8 (4) | C3B—C4B—C5B | 119.3 (4) |
| C3A—C4A—H4A | 120.6 | C3B—C4B—H4B | 120.3 |
| C5A—C4A—H4A | 120.6 | C5B—C4B—H4B | 120.3 |
| C4A—C5A—C6A | 117.9 (4) | C4B—C5B—C6B | 117.2 (4) |
| C4A—C5A—C7A | 121.0 (4) | C4B—C5B—C7B | 121.4 (4) |
| C6A—C5A—C7A | 121.2 (5) | C6B—C5B—C7B | 121.4 (4) |
| C1A—C6A—C5A | 119.0 (4) | C1B—C6B—C5B | 119.5 (4) |
| C1A—C6A—H6A | 120.5 | C1B—C6B—H6B | 120.3 |
| C5A—C6A—H6A | 120.5 | C5B—C6B—H6B | 120.3 |
| C5A—C7A—H7A | 109.5 | C5B—C7B—H7D | 109.5 |
| C5A—C7A—H7B | 109.5 | C5B—C7B—H7E | 109.5 |
| H7A—C7A—H7B | 109.5 | H7D—C7B—H7E | 109.5 |
| C5A—C7A—H7C | 109.5 | C5B—C7B—H7F | 109.5 |
| H7A—C7A—H7C | 109.5 | H7D—C7B—H7F | 109.5 |
| H7B—C7A—H7C | 109.5 | H7E—C7B—H7F | 109.5 |
| C9A—N8A—C1A | 126.7 (4) | C9B—N8B—C1B | 125.8 (4) |
| C9A—N8A—H8A | 113.0 | C9B—N8B—H8B | 121.6 |
| C1A—N8A—H8A | 120.3 | C1B—N8B—H8B | 112.5 |
| O10A—C9A—O11A | 126.5 (4) | O10B—C9B—O11B | 126.5 (4) |
| O10A—C9A—N8A | 125.5 (4) | O10B—C9B—N8B | 126.9 (4) |
| O11A—C9A—N8A | 108.0 (3) | O11B—C9B—N8B | 106.7 (3) |
| C9A—O11A—C12A | 120.3 (3) | C9B—O11B—C12B | 119.0 (3) |
| O11A—C12A—C13A | 109.2 (4) | O11B—C12B—C13B | 109.6 (4) |
| O11A—C12A—C14A | 110.6 (4) | O11B—C12B—C14B | 112.0 (4) |
| C13A—C12A—C14A | 114.2 (4) | C13B—C12B—C14B | 113.2 (4) |
| O11A—C12A—C15A | 101.8 (3) | O11B—C12B—C15B | 101.1 (3) |
| C13A—C12A—C15A | 110.9 (4) | C13B—C12B—C15B | 111.3 (5) |
| C14A—C12A—C15A | 109.4 (4) | C14B—C12B—C15B | 109.0 (4) |
| C12A—C13A—H13A | 109.5 | C12B—C13B—H13D | 109.5 |
| C12A—C13A—H13B | 109.5 | C12B—C13B—H13E | 109.5 |
| H13A—C13A—H13B | 109.5 | H13D—C13B—H13E | 109.5 |
| C12A—C13A—H13C | 109.5 | C12B—C13B—H13F | 109.5 |
| H13A—C13A—H13C | 109.5 | H13D—C13B—H13F | 109.5 |
| H13B—C13A—H13C | 109.5 | H13E—C13B—H13F | 109.5 |
| C12A—C14A—H14A | 109.5 | C12B—C14B—H14D | 109.5 |
| C12A—C14A—H14B | 109.5 | C12B—C14B—H14E | 109.5 |
| H14A—C14A—H14B | 109.5 | H14D—C14B—H14E | 109.5 |
| C12A—C14A—H14C | 109.5 | C12B—C14B—H14F | 109.5 |
| H14A—C14A—H14C | 109.5 | H14D—C14B—H14F | 109.5 |
| H14B—C14A—H14C | 109.5 | H14E—C14B—H14F | 109.5 |
| C12A—C15A—H15A | 109.5 | C12B—C15B—H15D | 109.5 |
| C12A—C15A—H15B | 109.5 | C12B—C15B—H15E | 109.5 |
| H15A—C15A—H15B | 109.5 | H15D—C15B—H15E | 109.5 |
| C12A—C15A—H15C | 109.5 | C12B—C15B—H15F | 109.5 |
| H15A—C15A—H15C | 109.5 | H15D—C15B—H15F | 109.5 |
| H15B—C15A—H15C | 109.5 | H15E—C15B—H15F | 109.5 |
| C6A—C1A—N2A—C3A | 2.1 (6) | C6B—C1B—N2B—C3B | −1.2 (7) |
| N8A—C1A—N2A—C3A | −177.2 (4) | N8B—C1B—N2B—C3B | 178.6 (4) |
| C1A—N2A—C3A—C4A | 0.3 (7) | C1B—N2B—C3B—C4B | −1.0 (8) |
| N2A—C3A—C4A—C5A | −0.7 (7) | N2B—C3B—C4B—C5B | 2.2 (8) |
| C3A—C4A—C5A—C6A | −1.2 (7) | C3B—C4B—C5B—C6B | −1.2 (7) |
| C3A—C4A—C5A—C7A | 178.2 (5) | C3B—C4B—C5B—C7B | 177.6 (5) |
| N2A—C1A—C6A—C5A | −4.0 (7) | N2B—C1B—C6B—C5B | 2.0 (7) |
| N8A—C1A—C6A—C5A | 175.3 (4) | N8B—C1B—C6B—C5B | −177.7 (4) |
| C4A—C5A—C6A—C1A | 3.4 (7) | C4B—C5B—C6B—C1B | −0.8 (6) |
| C7A—C5A—C6A—C1A | −176.0 (5) | C7B—C5B—C6B—C1B | −179.5 (4) |
| N2A—C1A—N8A—C9A | 178.9 (4) | N2B—C1B—N8B—C9B | 177.5 (4) |
| C6A—C1A—N8A—C9A | −0.4 (7) | C6B—C1B—N8B—C9B | −2.7 (7) |
| C1A—N8A—C9A—O10A | 9.6 (8) | C1B—N8B—C9B—O10B | −0.7 (7) |
| C1A—N8A—C9A—O11A | −169.8 (4) | C1B—N8B—C9B—O11B | 179.9 (4) |
| O10A—C9A—O11A—C12A | 2.3 (7) | O10B—C9B—O11B—C12B | −4.7 (6) |
| N8A—C9A—O11A—C12A | −178.3 (4) | N8B—C9B—O11B—C12B | 174.8 (3) |
| C9A—O11A—C12A—C13A | 65.5 (5) | C9B—O11B—C12B—C13B | −65.3 (5) |
| C9A—O11A—C12A—C14A | −61.0 (5) | C9B—O11B—C12B—C14B | 61.2 (5) |
| C9A—O11A—C12A—C15A | −177.2 (4) | C9B—O11B—C12B—C15B | 177.1 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N8A—H8A···N2B | 0.94 | 2.05 | 2.980 (5) | 171 |
| N8B—H8B···N2A | 0.98 | 2.04 | 3.015 (5) | 173 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BT2808).
References
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
- Dräger, M. & Gattow, G. (1971). Acta Chem. Scand.25, 761–762.
- Enraf–Nonius (1989). CAD-4 Software Enraf–Nonius, Delft, The Netherlands.
- Koch, P., Bäuerlein, C., Jank, H. & Laufer, S. (2008). J. Med. Chem.51, 5630–5640.
- Kuo, G.-H., DeAngelis, A., Emanuel, S., Wang, A., Zhang, Y., Connolly, P. J., Chen, X., Gruninger, R. H., Rugg, C., Fuentes-Pesquera, A., Middleton, S. A., Jolliffe, L. & Murray, W. V. (2005). J. Med. Chem.48, 4535–4546. [DOI] [PubMed]
- Kuo, G.-H., Wang, A., Emanuel, S., DeAngelis, A., Zhang, R., Connolly, P. J., Murray, W. V., Gruninger, R. H., Rugg, C., Sechler, J., Fuentes-Pesquera, A., Johnson, D., Middleton, S. A., Jolliffe, L. & Chen, X. (2005). J. Med. Chem.48, 1886–1900. [DOI] [PubMed]
- Laufer, S. & Koch, P. (2008). Org. Biomol. Chem.6, 437–439. [DOI] [PubMed]
- Peifer, C., Wagner, G. & Laufer, S. (2006). Curr. Top. Med. Chem.6, 113–149. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Swahn, B.-M., Xue, Y., Arzel, E., Kallin, E., Magnus, A., Plobeck, N. & Viklund, J. (2006). Bioorg. Med. Chem. Lett.16, 1396–1401. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808032327/bt2808sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808032327/bt2808Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



