Abstract
In the title compound, [Co(C28H22N2O2)(C5H5N)2]ClO4·0.5CH4O·0.5H2O, each CoIII ion is coordinated by the tetradentate N,N′-bis(2-oxidobenzylidene)-1,2-diphenylethane-1,2-diamine ligand [Co—N = 1.900 (3) and 1.903 (3) Å; Co—O = 1.885 (3) and 1.891 (3) Å] and two pyridine ligands [Co—N = 1.967 (4) and 1.977 (3) Å] in a distorted octahedral geometry. The packing of the cations and anions forms voids of 258 Å3, which are filled by methanol and solvent water molecules with half occupancies. O—H⋯O hydrogen bonds between solvent molecules, perchlorate anions and water molecules, and between water molecules and O atoms of the ligand, help to consolidate the crystal packing.
Related literature
For related crystal structures, see: Korendovych & Rybak-Akimova (2003 ▶); Shi et al. (1995 ▶). For general background, see: Amirnasr et al. (2001 ▶); Botteher et al., 1997 ▶; Cmi et al. (1998 ▶); Henson et al. (1999 ▶); Polson et al. (1997 ▶); Yamada (1999 ▶); Zhang et al. (1990 ▶).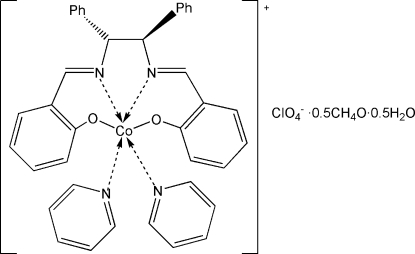
Experimental
Crystal data
[Co(C28H22N2O2)(C5H5N)2]ClO4·0.5CH4O·0.5H2O
M r = 760.09
Orthorhombic,

a = 10.8900 (3) Å
b = 18.6219 (5) Å
c = 18.6557 (6) Å
V = 3783.24 (19) Å3
Z = 4
Mo Kα radiation
μ = 0.58 mm−1
T = 273 (2) K
0.18 × 0.16 × 0.14 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2003 ▶) T min = 0.903, T max = 0.924
42911 measured reflections
7407 independent reflections
5476 reflections with I > 2σ(I)
R int = 0.064
Refinement
R[F 2 > 2σ(F 2)] = 0.050
wR(F 2) = 0.145
S = 1.02
7407 reflections
463 parameters
13 restraints
H-atom parameters constrained
Δρmax = 0.48 e Å−3
Δρmin = −0.37 e Å−3
Absolute structure: Flack (1983 ▶), with 3248 Friedel pairs
Flack parameter: 0.03 (2)
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: SAINT-Plus (Bruker, 2001 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: XP (Sheldrick, 1998 ▶); software used to prepare material for publication: XP.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808031887/cv2457sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808031887/cv2457Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O8—H8D⋯O6i | 0.85 | 1.98 | 2.831 (14) | 178 |
| O8—H8C⋯O7 | 0.85 | 1.96 | 2.807 (19) | 177 |
| O7—H7⋯O2 | 0.82 | 2.08 | 2.897 (11) | 171 |
Symmetry code: (i) 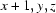 .
.
Acknowledgments
This work was supported by the Natural Science Foundation of China.
supplementary crystallographic information
Comment
The cobalt complexes with tetradentate Schiff base ligands have been extensively studied due to their important utilities in mimic cobalamin (B12) coenzymes (Amirnasr et al., 2001; Cmi et al., 1998; Polson et al., 1997), and as dioxygen carriers and oxygen activators (Yamada, 1999; Henson et al., 1999) . In addition, CoIII Schiff base complexes have also been used as antimicrobial agents when their two axial positions are occupied by two amine ligands (Botteher et al., 1997). Herein, we report the new CoIII complex based on the chiral tetradentate Schiff base ligand (-)-(1S,2S)-N,N'-Bis(salicylidene)-1,2- diphenyl-1,2-ethanediamine (L), whose structure has been reported recently (Korendovych & Rybak-Akimova, 2003).
In the cation (Fig. 1), the coordination sphere of CoIII ion is a distorted octahedron, in which four equational positions come from two N atoms and two O atoms of the tetradentate Schiff base ligand and the apical positions are occupied by N atoms of two pyridine molecules. The bond lengths of Co—O(L) and Co—N(L) are 1.885 (3), 1.891 (3)A% and 1.900 (3), 1.903 (3)A%, respectively, which are in agreement with the corresponding bond lengths in the similar CoIII Schiff base complex trans-[Co(salen)(py)2][BPh4] (Shi et al., 1995)). The distances of Co—Npy 1.967 (4) and 1.977 (3)A% are also consistent with those distances in the same complex, but slightly longer than the distances of Co—NSchiff base.
Experimental
The free Schiff base ligand L was synthesized according to the literature (Zhang et al., 1990). The synthsis of the title complex was carried out by reacting CoClO4.6H2O, pyridine and L (molar ratio 1:2:1 in methanol. After the stirring process was continued for about 30 min at room temperature, the mixture was filtered and the filtrate was allowed to partial evaporate in air for several days to produce crystals suitable for X-ray diffraction. Anal. Calcd for C38.5H35ClCoN4O7: C, 60.84; H, 4.64; N, 7.37. Found: C, 60.64; H, 4.65; N, 7.39.
Refinement
The occupancies of methanol (O7, C39) and crystalline water (O8) molecules were set to 0.5 and not refined. The common Uiso was refined for O7 and C39 atoms (methanol). Atom O8 was also refined isotropically. All H atoms were placed in idealized positions (C—H 0.93-0.98 Å; O-H 0.82-0.85 Å), and refined as riding with Uiso(H) = 1.2-1.5Ueq of the parent atom.
Figures
Fig. 1.
A view of the cation of the title compound with the atom-labelling scheme. Displacement ellipsoids are drawn at the 30% probability level. H atoms omited for clarity.
Crystal data
| [Co(C28H22N2O2)(C5H5N)2]ClO4·0.5CH4O·0.5H2O | Dx = 1.334 Mg m−3Dm = 1.334 Mg m−3Dm measured by not measured |
| Mr = 760.09 | Mo Kα radiation, λ = 0.71073 Å |
| Orthorhombic, P212121 | Cell parameters from 8558 reflections |
| a = 10.8900 (3) Å | θ = 2.4–20.8° |
| b = 18.6219 (5) Å | µ = 0.58 mm−1 |
| c = 18.6557 (6) Å | T = 273 K |
| V = 3783.24 (19) Å3 | Block, red-brown |
| Z = 4 | 0.18 × 0.16 × 0.14 mm |
| F(000) = 1576 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 7407 independent reflections |
| Radiation source: fine-focus sealed tube | 5476 reflections with I > 2σ(I) |
| graphite | Rint = 0.064 |
| φ and ω scans | θmax = 26.0°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 2003) | h = −13→13 |
| Tmin = 0.903, Tmax = 0.924 | k = −22→22 |
| 42911 measured reflections | l = −23→23 |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.050 | w = 1/[σ2(Fo2) + (0.0885P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.145 | (Δ/σ)max = 0.001 |
| S = 1.02 | Δρmax = 0.48 e Å−3 |
| 7407 reflections | Δρmin = −0.37 e Å−3 |
| 463 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 13 restraints | Extinction coefficient: 0.0014 (5) |
| Primary atom site location: structure-invariant direct methods | Absolute structure: Flack (1983), 3248 Friedel pairs |
| Secondary atom site location: difference Fourier map | Flack parameter: 0.03 (2) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| Co1 | 0.50476 (4) | 0.57650 (2) | 0.75640 (2) | 0.04751 (16) | |
| Cl1 | 0.10093 (14) | 0.50610 (10) | 0.55336 (8) | 0.1022 (5) | |
| O1 | 0.6076 (3) | 0.65694 (14) | 0.76901 (15) | 0.0568 (7) | |
| O2 | 0.5983 (3) | 0.55521 (14) | 0.67367 (15) | 0.0619 (7) | |
| O3 | 0.1244 (5) | 0.5541 (3) | 0.4915 (2) | 0.1324 (18) | |
| O4 | 0.2142 (4) | 0.4859 (3) | 0.5844 (3) | 0.1202 (15) | |
| O5 | 0.0367 (5) | 0.4459 (4) | 0.5318 (4) | 0.175 (3) | |
| O6 | 0.0303 (5) | 0.5444 (3) | 0.6048 (3) | 0.1398 (18) | |
| O7 | 0.6798 (12) | 0.6643 (6) | 0.5737 (6) | 0.153 (4)* | 0.50 |
| H7 | 0.6495 | 0.6359 | 0.6022 | 0.230* | 0.50 |
| O8 | 0.9347 (13) | 0.6770 (6) | 0.5559 (7) | 0.163 (4)* | 0.50 |
| H8C | 0.8572 | 0.6746 | 0.5606 | 0.195* | 0.50 |
| H8D | 0.9657 | 0.6377 | 0.5703 | 0.195* | 0.50 |
| N1 | 0.4061 (3) | 0.59406 (15) | 0.83873 (17) | 0.0460 (7) | |
| N2 | 0.3989 (3) | 0.49659 (15) | 0.74280 (16) | 0.0462 (7) | |
| N3 | 0.6234 (3) | 0.52046 (17) | 0.81394 (17) | 0.0506 (8) | |
| N4 | 0.4010 (4) | 0.63945 (18) | 0.6973 (2) | 0.0601 (9) | |
| C1 | 0.5455 (4) | 0.68095 (18) | 0.8912 (2) | 0.0498 (9) | |
| C2 | 0.6202 (4) | 0.69134 (19) | 0.8297 (2) | 0.0497 (9) | |
| C3 | 0.7149 (4) | 0.7448 (2) | 0.8337 (3) | 0.0571 (10) | |
| H3 | 0.7637 | 0.7540 | 0.7938 | 0.069* | |
| C4 | 0.7341 (4) | 0.7824 (2) | 0.8957 (3) | 0.0607 (11) | |
| H4 | 0.7955 | 0.8171 | 0.8973 | 0.073* | |
| C5 | 0.6637 (4) | 0.7697 (2) | 0.9561 (3) | 0.0632 (11) | |
| H5 | 0.6799 | 0.7947 | 0.9982 | 0.076* | |
| C6 | 0.5700 (4) | 0.7204 (2) | 0.9542 (2) | 0.0578 (10) | |
| H6 | 0.5222 | 0.7129 | 0.9948 | 0.069* | |
| C7 | 0.4382 (4) | 0.63562 (19) | 0.8909 (2) | 0.0477 (9) | |
| H7A | 0.3883 | 0.6364 | 0.9313 | 0.057* | |
| C8 | 0.5417 (3) | 0.4304 (2) | 0.6702 (2) | 0.0482 (8) | |
| C9 | 0.5718 (4) | 0.3609 (2) | 0.6450 (2) | 0.0578 (10) | |
| H9 | 0.5178 | 0.3231 | 0.6536 | 0.069* | |
| C10 | 0.6775 (4) | 0.3479 (2) | 0.6087 (3) | 0.0666 (12) | |
| H10 | 0.6975 | 0.3016 | 0.5941 | 0.080* | |
| C11 | 0.7546 (4) | 0.4046 (3) | 0.5938 (3) | 0.0729 (13) | |
| H11 | 0.8273 | 0.3959 | 0.5692 | 0.087* | |
| C12 | 0.7280 (4) | 0.4727 (3) | 0.6140 (3) | 0.0688 (12) | |
| H12 | 0.7809 | 0.5098 | 0.6013 | 0.083* | |
| C13 | 0.6209 (4) | 0.4882 (2) | 0.6542 (2) | 0.0539 (10) | |
| C14 | 0.4274 (3) | 0.4402 (2) | 0.7069 (2) | 0.0493 (9) | |
| H14 | 0.3701 | 0.4033 | 0.7045 | 0.059* | |
| C15 | 0.2981 (3) | 0.5456 (2) | 0.8473 (2) | 0.0473 (9) | |
| H15 | 0.3202 | 0.5088 | 0.8826 | 0.057* | |
| C16 | 0.2763 (3) | 0.5075 (2) | 0.7757 (2) | 0.0486 (9) | |
| H16 | 0.2305 | 0.5406 | 0.7448 | 0.058* | |
| C17 | 0.1814 (3) | 0.5823 (2) | 0.8738 (2) | 0.0510 (9) | |
| C18 | 0.1280 (4) | 0.6393 (3) | 0.8389 (3) | 0.0713 (12) | |
| H18 | 0.1664 | 0.6588 | 0.7989 | 0.086* | |
| C19 | 0.0192 (4) | 0.6679 (3) | 0.8621 (3) | 0.0758 (13) | |
| H19 | −0.0159 | 0.7057 | 0.8367 | 0.091* | |
| C20 | −0.0378 (4) | 0.6424 (2) | 0.9208 (3) | 0.0686 (12) | |
| H20 | −0.1112 | 0.6629 | 0.9361 | 0.082* | |
| C21 | 0.0128 (4) | 0.5855 (2) | 0.9584 (2) | 0.0649 (11) | |
| H21 | −0.0263 | 0.5672 | 0.9988 | 0.078* | |
| C22 | 0.1243 (4) | 0.5560 (2) | 0.9345 (2) | 0.0536 (9) | |
| H22 | 0.1601 | 0.5183 | 0.9598 | 0.064* | |
| C23 | 0.1990 (4) | 0.4396 (2) | 0.7842 (2) | 0.0571 (10) | |
| C24 | 0.2292 (6) | 0.3877 (3) | 0.8359 (3) | 0.0832 (15) | |
| H24 | 0.2987 | 0.3929 | 0.8644 | 0.100* | |
| C25 | 0.1518 (7) | 0.3278 (3) | 0.8433 (4) | 0.105 (2) | |
| H25 | 0.1708 | 0.2934 | 0.8776 | 0.126* | |
| C26 | 0.0507 (6) | 0.3185 (4) | 0.8023 (4) | 0.1008 (18) | |
| H26 | 0.0012 | 0.2783 | 0.8083 | 0.121* | |
| C27 | 0.0227 (5) | 0.3690 (4) | 0.7522 (4) | 0.1023 (19) | |
| H27 | −0.0467 | 0.3632 | 0.7237 | 0.123* | |
| C28 | 0.0970 (4) | 0.4298 (3) | 0.7429 (3) | 0.0739 (12) | |
| H28 | 0.0765 | 0.4637 | 0.7084 | 0.089* | |
| C29 | 0.5918 (4) | 0.4749 (2) | 0.8660 (3) | 0.0630 (11) | |
| H29 | 0.5086 | 0.4681 | 0.8749 | 0.076* | |
| C30 | 0.6749 (5) | 0.4372 (3) | 0.9074 (3) | 0.0778 (13) | |
| H30 | 0.6486 | 0.4067 | 0.9437 | 0.093* | |
| C31 | 0.7970 (5) | 0.4464 (4) | 0.8932 (4) | 0.0970 (19) | |
| H31 | 0.8562 | 0.4212 | 0.9188 | 0.116* | |
| C32 | 0.8306 (4) | 0.4940 (3) | 0.8399 (4) | 0.0849 (16) | |
| H32 | 0.9133 | 0.5017 | 0.8301 | 0.102* | |
| C33 | 0.7431 (4) | 0.5298 (2) | 0.8013 (3) | 0.0631 (11) | |
| H33 | 0.7675 | 0.5615 | 0.7654 | 0.076* | |
| C34 | 0.3626 (6) | 0.6221 (3) | 0.6330 (3) | 0.0879 (17) | |
| H34 | 0.3810 | 0.5764 | 0.6160 | 0.105* | |
| C35 | 0.2964 (7) | 0.6674 (3) | 0.5888 (4) | 0.114 (3) | |
| H35 | 0.2703 | 0.6521 | 0.5438 | 0.137* | |
| C36 | 0.2701 (7) | 0.7351 (4) | 0.6125 (4) | 0.117 (2) | |
| H36 | 0.2287 | 0.7672 | 0.5829 | 0.140* | |
| C37 | 0.3057 (6) | 0.7558 (3) | 0.6813 (4) | 0.0915 (17) | |
| H37 | 0.2856 | 0.8008 | 0.6996 | 0.110* | |
| C38 | 0.3721 (5) | 0.7068 (2) | 0.7213 (3) | 0.0691 (12) | |
| H38 | 0.3985 | 0.7203 | 0.7668 | 0.083* | |
| C39 | 0.5940 (19) | 0.7285 (10) | 0.5644 (10) | 0.153 (4)* | 0.50 |
| H39A | 0.5563 | 0.7398 | 0.6095 | 0.230* | 0.50 |
| H39B | 0.5316 | 0.7170 | 0.5299 | 0.230* | 0.50 |
| H39C | 0.6403 | 0.7692 | 0.5479 | 0.230* | 0.50 |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Co1 | 0.0420 (3) | 0.0414 (3) | 0.0591 (3) | −0.0055 (2) | 0.0071 (3) | −0.0055 (2) |
| Cl1 | 0.0737 (9) | 0.1434 (14) | 0.0895 (9) | −0.0270 (9) | 0.0163 (7) | −0.0380 (9) |
| O1 | 0.0533 (16) | 0.0459 (14) | 0.0712 (18) | −0.0099 (12) | 0.0084 (14) | −0.0100 (12) |
| O2 | 0.0624 (17) | 0.0527 (16) | 0.0705 (17) | −0.0156 (14) | 0.0165 (15) | −0.0143 (13) |
| O3 | 0.125 (4) | 0.198 (5) | 0.073 (2) | −0.016 (4) | 0.013 (2) | −0.008 (3) |
| O4 | 0.073 (3) | 0.155 (4) | 0.132 (3) | −0.013 (3) | 0.003 (3) | −0.009 (3) |
| O5 | 0.109 (4) | 0.213 (6) | 0.204 (6) | −0.068 (4) | 0.018 (4) | −0.090 (5) |
| O6 | 0.141 (4) | 0.157 (4) | 0.121 (3) | 0.013 (4) | 0.047 (3) | −0.035 (3) |
| N1 | 0.0350 (15) | 0.0403 (16) | 0.0626 (18) | 0.0018 (13) | 0.0020 (14) | −0.0051 (14) |
| N2 | 0.0385 (15) | 0.0437 (16) | 0.0564 (17) | −0.0024 (13) | 0.0045 (15) | −0.0035 (14) |
| N3 | 0.0392 (17) | 0.0451 (17) | 0.068 (2) | −0.0015 (14) | −0.0004 (15) | −0.0106 (15) |
| N4 | 0.060 (2) | 0.0524 (19) | 0.068 (2) | −0.0124 (18) | −0.0020 (19) | 0.0029 (16) |
| C1 | 0.044 (2) | 0.0363 (19) | 0.070 (2) | 0.0064 (16) | −0.0002 (18) | −0.0026 (17) |
| C2 | 0.041 (2) | 0.0355 (19) | 0.073 (3) | 0.0044 (16) | −0.0035 (19) | −0.0068 (18) |
| C3 | 0.044 (2) | 0.047 (2) | 0.081 (3) | 0.0023 (18) | −0.003 (2) | −0.003 (2) |
| C4 | 0.047 (2) | 0.040 (2) | 0.096 (3) | 0.0008 (18) | −0.018 (2) | −0.010 (2) |
| C5 | 0.054 (3) | 0.053 (2) | 0.083 (3) | 0.002 (2) | −0.016 (2) | −0.014 (2) |
| C6 | 0.057 (3) | 0.046 (2) | 0.070 (3) | 0.0029 (19) | −0.009 (2) | −0.0085 (19) |
| C7 | 0.046 (2) | 0.0396 (18) | 0.058 (2) | 0.0036 (17) | 0.0047 (18) | −0.0028 (17) |
| C8 | 0.0429 (19) | 0.0465 (19) | 0.0552 (19) | −0.0043 (17) | 0.0000 (15) | −0.0079 (18) |
| C9 | 0.050 (2) | 0.052 (2) | 0.071 (3) | −0.0026 (19) | 0.002 (2) | −0.0113 (19) |
| C10 | 0.052 (2) | 0.062 (3) | 0.086 (3) | 0.003 (2) | 0.004 (2) | −0.017 (2) |
| C11 | 0.046 (3) | 0.082 (3) | 0.091 (3) | −0.001 (2) | 0.014 (2) | −0.027 (3) |
| C12 | 0.050 (2) | 0.074 (3) | 0.082 (3) | −0.015 (2) | 0.018 (2) | −0.015 (2) |
| C13 | 0.049 (2) | 0.055 (2) | 0.057 (2) | −0.0091 (19) | 0.0057 (18) | −0.0100 (18) |
| C14 | 0.045 (2) | 0.043 (2) | 0.060 (2) | −0.0073 (17) | 0.0032 (18) | −0.0049 (17) |
| C15 | 0.039 (2) | 0.045 (2) | 0.058 (2) | 0.0004 (16) | 0.0063 (17) | 0.0015 (17) |
| C16 | 0.0388 (18) | 0.045 (2) | 0.062 (2) | −0.0017 (17) | 0.0045 (16) | −0.0038 (17) |
| C17 | 0.0359 (18) | 0.052 (2) | 0.065 (2) | 0.0015 (17) | 0.0010 (17) | −0.0017 (19) |
| C18 | 0.059 (3) | 0.070 (3) | 0.085 (3) | 0.015 (2) | 0.010 (2) | 0.009 (2) |
| C19 | 0.054 (3) | 0.075 (3) | 0.098 (3) | 0.027 (2) | 0.008 (3) | 0.006 (2) |
| C20 | 0.049 (2) | 0.068 (3) | 0.089 (3) | 0.011 (2) | 0.004 (2) | −0.014 (2) |
| C21 | 0.047 (2) | 0.081 (3) | 0.066 (2) | −0.004 (2) | 0.009 (2) | −0.013 (2) |
| C22 | 0.047 (2) | 0.056 (2) | 0.059 (2) | 0.0006 (18) | 0.0067 (18) | −0.0029 (17) |
| C23 | 0.049 (2) | 0.056 (2) | 0.066 (2) | −0.0120 (19) | 0.0161 (19) | −0.009 (2) |
| C24 | 0.095 (4) | 0.062 (3) | 0.092 (3) | −0.030 (3) | −0.003 (3) | 0.005 (3) |
| C25 | 0.130 (5) | 0.074 (3) | 0.112 (4) | −0.036 (4) | 0.028 (4) | 0.002 (3) |
| C26 | 0.089 (4) | 0.098 (4) | 0.116 (4) | −0.045 (3) | 0.030 (3) | −0.027 (3) |
| C27 | 0.052 (3) | 0.113 (4) | 0.141 (5) | −0.031 (3) | 0.016 (4) | −0.054 (4) |
| C28 | 0.049 (2) | 0.081 (3) | 0.092 (3) | −0.012 (2) | 0.004 (2) | −0.021 (3) |
| C29 | 0.046 (2) | 0.058 (2) | 0.086 (3) | 0.007 (2) | −0.001 (2) | −0.003 (2) |
| C30 | 0.070 (3) | 0.064 (3) | 0.099 (3) | 0.008 (3) | −0.015 (3) | 0.008 (3) |
| C31 | 0.068 (4) | 0.096 (4) | 0.127 (5) | 0.030 (3) | −0.036 (4) | −0.020 (4) |
| C32 | 0.045 (2) | 0.087 (4) | 0.123 (4) | 0.010 (3) | −0.011 (3) | −0.034 (4) |
| C33 | 0.040 (2) | 0.067 (3) | 0.082 (3) | −0.003 (2) | 0.003 (2) | −0.022 (2) |
| C34 | 0.111 (5) | 0.067 (3) | 0.085 (4) | −0.016 (3) | −0.021 (3) | 0.007 (3) |
| C35 | 0.154 (7) | 0.075 (4) | 0.114 (5) | −0.020 (4) | −0.063 (5) | 0.025 (3) |
| C36 | 0.137 (6) | 0.082 (4) | 0.131 (6) | −0.004 (4) | −0.045 (5) | 0.036 (4) |
| C37 | 0.103 (4) | 0.058 (3) | 0.113 (4) | 0.004 (3) | −0.013 (4) | 0.015 (3) |
| C38 | 0.069 (3) | 0.059 (3) | 0.079 (3) | −0.002 (2) | −0.002 (2) | 0.006 (2) |
Geometric parameters (Å, °)
| Co1—O1 | 1.885 (3) | C15—C17 | 1.525 (5) |
| Co1—O2 | 1.891 (3) | C15—C16 | 1.530 (5) |
| Co1—N2 | 1.900 (3) | C15—H15 | 0.9800 |
| Co1—N1 | 1.903 (3) | C16—C23 | 1.528 (5) |
| Co1—N4 | 1.967 (4) | C16—H16 | 0.9800 |
| Co1—N3 | 1.977 (3) | C17—C18 | 1.374 (6) |
| Cl1—O5 | 1.381 (5) | C17—C22 | 1.383 (5) |
| Cl1—O4 | 1.414 (5) | C18—C19 | 1.368 (6) |
| Cl1—O6 | 1.421 (5) | C18—H18 | 0.9300 |
| Cl1—O3 | 1.482 (5) | C19—C20 | 1.347 (7) |
| O1—C2 | 1.309 (5) | C19—H19 | 0.9300 |
| O2—C13 | 1.322 (5) | C20—C21 | 1.385 (6) |
| O7—C39 | 1.53 (2) | C20—H20 | 0.9300 |
| O7—H7 | 0.8200 | C21—C22 | 1.407 (5) |
| O8—H8C | 0.8501 | C21—H21 | 0.9300 |
| O8—H8D | 0.8501 | C22—H22 | 0.9300 |
| N1—C7 | 1.291 (5) | C23—C28 | 1.364 (6) |
| N1—C15 | 1.492 (5) | C23—C24 | 1.403 (7) |
| N2—C14 | 1.284 (4) | C24—C25 | 1.405 (7) |
| N2—C16 | 1.483 (5) | C24—H24 | 0.9300 |
| N3—C29 | 1.334 (6) | C25—C26 | 1.351 (9) |
| N3—C33 | 1.337 (5) | C25—H25 | 0.9300 |
| N4—C34 | 1.310 (6) | C26—C27 | 1.362 (10) |
| N4—C38 | 1.369 (6) | C26—H26 | 0.9300 |
| C1—C6 | 1.411 (6) | C27—C28 | 1.401 (7) |
| C1—C2 | 1.420 (6) | C27—H27 | 0.9300 |
| C1—C7 | 1.441 (5) | C28—H28 | 0.9300 |
| C2—C3 | 1.436 (6) | C29—C30 | 1.381 (6) |
| C3—C4 | 1.367 (6) | C29—H29 | 0.9300 |
| C3—H3 | 0.9300 | C30—C31 | 1.367 (8) |
| C4—C5 | 1.383 (7) | C30—H30 | 0.9300 |
| C4—H4 | 0.9300 | C31—C32 | 1.381 (8) |
| C5—C6 | 1.373 (6) | C31—H31 | 0.9300 |
| C5—H5 | 0.9300 | C32—C33 | 1.368 (7) |
| C6—H6 | 0.9300 | C32—H32 | 0.9300 |
| C7—H7A | 0.9300 | C33—H33 | 0.9300 |
| C8—C13 | 1.412 (5) | C34—C35 | 1.382 (8) |
| C8—C9 | 1.416 (6) | C34—H34 | 0.9300 |
| C8—C14 | 1.431 (5) | C35—C36 | 1.366 (10) |
| C9—C10 | 1.357 (6) | C35—H35 | 0.9300 |
| C9—H9 | 0.9300 | C36—C37 | 1.396 (10) |
| C10—C11 | 1.378 (7) | C36—H36 | 0.9300 |
| C10—H10 | 0.9300 | C37—C38 | 1.382 (7) |
| C11—C12 | 1.354 (7) | C37—H37 | 0.9300 |
| C11—H11 | 0.9300 | C38—H38 | 0.9300 |
| C12—C13 | 1.416 (6) | C39—H39A | 0.9600 |
| C12—H12 | 0.9300 | C39—H39B | 0.9600 |
| C14—H14 | 0.9300 | C39—H39C | 0.9600 |
| O1—Co1—O2 | 87.05 (11) | N1—C15—H15 | 107.5 |
| O1—Co1—N2 | 178.89 (13) | C17—C15—H15 | 107.5 |
| O2—Co1—N2 | 93.08 (12) | C16—C15—H15 | 107.5 |
| O1—Co1—N1 | 95.62 (12) | N2—C16—C23 | 115.1 (3) |
| O2—Co1—N1 | 177.32 (12) | N2—C16—C15 | 106.6 (3) |
| N2—Co1—N1 | 84.24 (13) | C23—C16—C15 | 112.3 (3) |
| O1—Co1—N4 | 86.43 (14) | N2—C16—H16 | 107.5 |
| O2—Co1—N4 | 88.66 (15) | C23—C16—H16 | 107.5 |
| N2—Co1—N4 | 92.48 (14) | C15—C16—H16 | 107.5 |
| N1—Co1—N4 | 91.49 (14) | C18—C17—C22 | 118.1 (4) |
| O1—Co1—N3 | 87.91 (13) | C18—C17—C15 | 123.1 (4) |
| O2—Co1—N3 | 88.89 (14) | C22—C17—C15 | 118.7 (3) |
| N2—Co1—N3 | 93.19 (12) | C19—C18—C17 | 121.2 (5) |
| N1—Co1—N3 | 91.22 (13) | C19—C18—H18 | 119.4 |
| N4—Co1—N3 | 173.94 (14) | C17—C18—H18 | 119.4 |
| O5—Cl1—O4 | 110.2 (4) | C20—C19—C18 | 121.2 (5) |
| O5—Cl1—O6 | 109.3 (3) | C20—C19—H19 | 119.4 |
| O4—Cl1—O6 | 109.2 (3) | C18—C19—H19 | 119.4 |
| O5—Cl1—O3 | 110.5 (4) | C19—C20—C21 | 119.9 (4) |
| O4—Cl1—O3 | 109.2 (3) | C19—C20—H20 | 120.0 |
| O6—Cl1—O3 | 108.4 (3) | C21—C20—H20 | 120.0 |
| C2—O1—Co1 | 124.0 (3) | C20—C21—C22 | 118.8 (4) |
| C13—O2—Co1 | 121.5 (2) | C20—C21—H21 | 120.6 |
| C39—O7—H7 | 109.5 | C22—C21—H21 | 120.6 |
| H8C—O8—H8D | 108.3 | C17—C22—C21 | 120.6 (4) |
| C7—N1—C15 | 119.7 (3) | C17—C22—H22 | 119.7 |
| C7—N1—Co1 | 123.9 (3) | C21—C22—H22 | 119.7 |
| C15—N1—Co1 | 115.3 (2) | C28—C23—C24 | 119.1 (4) |
| C14—N2—C16 | 123.1 (3) | C28—C23—C16 | 120.1 (4) |
| C14—N2—Co1 | 124.3 (2) | C24—C23—C16 | 120.7 (4) |
| C16—N2—Co1 | 112.6 (2) | C23—C24—C25 | 118.2 (5) |
| C29—N3—C33 | 117.6 (4) | C23—C24—H24 | 120.9 |
| C29—N3—Co1 | 124.2 (3) | C25—C24—H24 | 120.9 |
| C33—N3—Co1 | 118.2 (3) | C26—C25—C24 | 122.4 (6) |
| C34—N4—C38 | 116.9 (4) | C26—C25—H25 | 118.8 |
| C34—N4—Co1 | 123.4 (3) | C24—C25—H25 | 118.8 |
| C38—N4—Co1 | 119.6 (3) | C25—C26—C27 | 118.8 (6) |
| C6—C1—C2 | 119.6 (4) | C25—C26—H26 | 120.6 |
| C6—C1—C7 | 117.5 (4) | C27—C26—H26 | 120.6 |
| C2—C1—C7 | 122.7 (3) | C26—C27—C28 | 120.9 (6) |
| O1—C2—C1 | 124.9 (3) | C26—C27—H27 | 119.5 |
| O1—C2—C3 | 117.4 (4) | C28—C27—H27 | 119.5 |
| C1—C2—C3 | 117.6 (4) | C23—C28—C27 | 120.5 (5) |
| C4—C3—C2 | 120.6 (4) | C23—C28—H28 | 119.7 |
| C4—C3—H3 | 119.7 | C27—C28—H28 | 119.7 |
| C2—C3—H3 | 119.7 | N3—C29—C30 | 124.1 (4) |
| C3—C4—C5 | 121.1 (4) | N3—C29—H29 | 117.9 |
| C3—C4—H4 | 119.5 | C30—C29—H29 | 117.9 |
| C5—C4—H4 | 119.5 | C31—C30—C29 | 117.7 (5) |
| C6—C5—C4 | 120.4 (4) | C31—C30—H30 | 121.1 |
| C6—C5—H5 | 119.8 | C29—C30—H30 | 121.1 |
| C4—C5—H5 | 119.8 | C30—C31—C32 | 118.6 (5) |
| C5—C6—C1 | 120.7 (4) | C30—C31—H31 | 120.7 |
| C5—C6—H6 | 119.7 | C32—C31—H31 | 120.7 |
| C1—C6—H6 | 119.7 | C33—C32—C31 | 120.5 (5) |
| N1—C7—C1 | 125.0 (4) | C33—C32—H32 | 119.8 |
| N1—C7—H7A | 117.5 | C31—C32—H32 | 119.8 |
| C1—C7—H7A | 117.5 | N3—C33—C32 | 121.5 (5) |
| C13—C8—C9 | 119.0 (3) | N3—C33—H33 | 119.2 |
| C13—C8—C14 | 122.4 (3) | C32—C33—H33 | 119.2 |
| C9—C8—C14 | 118.5 (3) | N4—C34—C35 | 124.3 (6) |
| C10—C9—C8 | 121.7 (4) | N4—C34—H34 | 117.9 |
| C10—C9—H9 | 119.2 | C35—C34—H34 | 117.9 |
| C8—C9—H9 | 119.2 | C36—C35—C34 | 118.6 (6) |
| C9—C10—C11 | 118.7 (4) | C36—C35—H35 | 120.7 |
| C9—C10—H10 | 120.6 | C34—C35—H35 | 120.7 |
| C11—C10—H10 | 120.6 | C35—C36—C37 | 119.6 (6) |
| C12—C11—C10 | 122.1 (4) | C35—C36—H36 | 120.2 |
| C12—C11—H11 | 118.9 | C37—C36—H36 | 120.2 |
| C10—C11—H11 | 118.9 | C38—C37—C36 | 117.4 (6) |
| C11—C12—C13 | 121.0 (4) | C38—C37—H37 | 121.3 |
| C11—C12—H12 | 119.5 | C36—C37—H37 | 121.3 |
| C13—C12—H12 | 119.5 | N4—C38—C37 | 123.2 (5) |
| O2—C13—C8 | 123.2 (3) | N4—C38—H38 | 118.4 |
| O2—C13—C12 | 119.4 (4) | C37—C38—H38 | 118.4 |
| C8—C13—C12 | 117.4 (4) | O7—C39—H39A | 109.5 |
| N2—C14—C8 | 124.4 (3) | O7—C39—H39B | 109.5 |
| N2—C14—H14 | 117.8 | H39A—C39—H39B | 109.5 |
| C8—C14—H14 | 117.8 | O7—C39—H39C | 109.5 |
| N1—C15—C17 | 114.8 (3) | H39A—C39—H39C | 109.5 |
| N1—C15—C16 | 108.0 (3) | H39B—C39—H39C | 109.5 |
| C17—C15—C16 | 111.2 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O8—H8D···O6i | 0.85 | 1.98 | 2.831 (14) | 178 |
| O8—H8C···O7 | 0.85 | 1.96 | 2.807 (19) | 177 |
| O7—H7···O2 | 0.82 | 2.08 | 2.897 (11) | 171 |
Symmetry codes: (i) x+1, y, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: CV2457).
References
- Amirnasr, M., Schenk, K. J., Gorji, A. & Vafazadef, R. (2001). Polyhedron, 20, 695–702.
- Botteher, A., Takeuchi, T., Hardcastle, K. I., Meade, T. J. & Gray, H. B. (1997). Inorg. Chem.36, 2498–2504.
- Bruker (2001). SAINT-Plus Bruker AXS Inc., Madison,Wisconsin, USA.
- Bruker (2004). APEX2 Bruker AXS Inc., Madison, Wisconsin, USA.
- Cmi, R., Moore, S. J. & Marzilli, L. G. (1998). Inorg. Chem.37, 6890–6897. [DOI] [PubMed]
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Henson, N. J., Hay, P. J. & Redondo, A. (1999). Inorg. Chem.38, 1618–1626.
- Korendovych, I. V. & Rybak-Akimova, E. V. (2003). Acta Cryst. E59, o1498–o1500.
- Polson, S. M., Cini, R., Pifferi, C. & Marzilli, L. G. (1997). Inorg. Chem.36, 314–322. [DOI] [PubMed]
- Sheldrick, G. M. (1998). XP Bruker AXS Inc., Madison, Wisconsin, USA.
- Sheldrick, G. M. (2003). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Shi, X.-H., You, X.-Z., Li, C., Song, B.-L., Li, T.-H. & Huang, X.-Y. (1995). Acta Cryst. C51, 206–207.
- Yamada, S. (1999). Coord. Chem. Rev.191–192, 537–555.
- Zhang, W., Loebach, J. L., Wilson, S. R. & Jacobsen, E. N. (1990). J. Am. Chem. Soc.112, 2801–2803.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808031887/cv2457sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808031887/cv2457Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



