Abstract
The title compound, C11H12N4O4, was synthesized by the reaction of (2,4-dinitrophenyl)hydrazine with cyclopentanone. The cyclopentyl fragment is disordered over two sites with occupancies of 0.63 (1) and 0.37 (1). An intramolecular N—H⋯O hydrogen bond helps to establish the conformation. Pairs of molecules are held together by π–π interactions between adjacent benzene rings [centroid-to-centroid distance 3.589 (2) Å].
Related literature
For background literature on Schiff bases, see: Liang (2007 ▶). For information on the properties of dinitrophenylhydrazones, see: Baughman et al. (2004 ▶); Zare et al. (2005 ▶); El-Seify & El-Dossoki (2006 ▶); Kim & Yoon (1998 ▶). For bond-length data, see: Allen et al. (1987 ▶); Allen (2002 ▶).
Experimental
Crystal data
C11H12N4O4
M r = 264.25
Monoclinic,

a = 6.962 (3) Å
b = 21.840 (10) Å
c = 8.162 (4) Å
β = 98.528 (9)°
V = 1227.3 (10) Å3
Z = 4
Mo Kα radiation
μ = 0.11 mm−1
T = 295 (2) K
0.15 × 0.10 × 0.06 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.984, T max = 0.993
6423 measured reflections
2168 independent reflections
1353 reflections with I > 2σ(I)
R int = 0.029
Refinement
R[F 2 > 2σ(F 2)] = 0.042
wR(F 2) = 0.129
S = 1.02
2168 reflections
192 parameters
H-atom parameters constrained
Δρmax = 0.13 e Å−3
Δρmin = −0.14 e Å−3
Data collection: SMART (Bruker, 2003 ▶); cell refinement: SAINT (Bruker, 2003 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808033345/fb2114sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808033345/fb2114Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N3—H3⋯O2 | 0.86 | 1.99 | 2.605 (2) | 128 |
Acknowledgments
This project was supported by the Postgraduate Foundation of Taishan University (grant No. Y06-2-08).
supplementary crystallographic information
Comment
Schiff bases and their complexes are widely used in the fields of biology, catalysis etc. (Liang, 2007). Especially, the dinitrophenylhydrazones exhibit good nonlinear optical (NLO) and crystalline properties (Baughman et al., 2004). The benzophenone-2,4-dinitrophenylhydrazone derivatives are important because of their significant molecular nonlinearities and remarkable ability to crystallize in non-centrosymmetric crystal systems (Zare et al., 2005; El-Seify & El-Dossoki, 2006; Kim & Yoon, 1998). In order to search for new dinitrophenylhydrazones, the title compound was synthesized and its crystal structure is reported here (Fig. 1). The obtained unrestrained bond lengths and angles are in good agreement with the expected values (Allen et al., 1987) in the non-disordered region. In the crystal structure (Fig. 2), the molecules are stabilized by N—H···O hydrogen bonds (Table 1), C—H···N interactions (C6—H6···N4: 0.93, 2.39, 2.722 (3) Å and 101.1°) and by π–π electron interactions between the benzene rings. The distances between the centroids of the stacked benzene rings are 3.589 (2) Å though the molecules are situated in rather equidistant planes.
Experimental
The title compound was synthesized by the reaction of (2,4-dinitro-phenyl)-hydrazine (1 mmol, 198.1 mg) with cyclopentanone (1 mmol, 84.1 mg) in ethanol (30 ml) under reflux conditions (348 K) for 3 h. The solvent was removed and the solid product was recrystallized from tetrahydrofuran. Brown crystals that were suitable for X-ray diffraction study were grown in the course of three days. Yield, 227.2 mg, 86%; m. p. 318–320 K.
Analysis calculated for C11H12N4O4: C 50.00, H 4.58, N 21.20%; found: C 49.97, H 4.52, N 21.15%.
Refinement
All the H atoms except those attached to the disordered atoms C9, C9', C10 and C10' could have been distinguished in the difference electron density maps. During the refinement the H atoms were situated into idealized positions, constrained and refined as riding atoms. The constraints: Caryl—H = 0.93; Cmethylene—H 0.97 Å, N—H = 0.86 Å; Uiso(H) = 1.2Ueq(carrier atom). The disorder was treated with the following restraints: The distances C9—C10 and C9'—C10' were restrained to 1.485 (10) Å, the distances C8—C9, C8—C9', C10—C11 and C10'—C11 to 1.520 (10) Å and the distances C7—C8, C7—C11 to 1.503 (10) Å. The values of these distances were retrieved from the Cambridge Crystal Structure Database (version 5.29 plus updates to January 2008; Allen, 2002) for the structures that contained the fragment —NH—N ═cyclopentyl that is present in the title structure. The retrieved structures HULJON, KERWUA, NAQSAZ and RAKHUH are without disorder, errors and with the R-factor < 0.05. The displacement parameters of the atoms C9', C10', C9' and C10' were restrained by the command SIMU with the default parameters (0.04, 0.08, 1.7) of the refinement program SHELXL97 (Sheldrick, 2008).
Figures
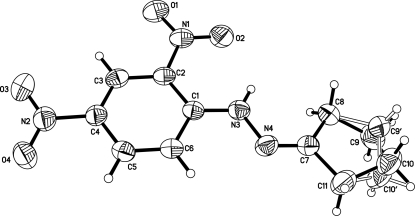
Fig. 1.
The title molecular with displacement ellipsoids drawn at the 50% probability level.
Fig. 2.
The view of the structure. The dashed lines indicate the N—H···O hydrogen bonds and C—H···N interactions as well as π–π ring electron interactions.
Crystal data
| C11H12N4O4 | F(000) = 552 |
| Mr = 264.25 | Dx = 1.430 Mg m−3 |
| Monoclinic, P21/n | Melting point = 318–320 K |
| Hall symbol: -P 2yn | Mo Kα radiation, λ = 0.71073 Å |
| a = 6.962 (3) Å | Cell parameters from 1213 reflections |
| b = 21.84 (1) Å | θ = 3.1–21.3° |
| c = 8.162 (4) Å | µ = 0.11 mm−1 |
| β = 98.528 (9)° | T = 295 K |
| V = 1227.3 (10) Å3 | Block, brown |
| Z = 4 | 0.15 × 0.10 × 0.06 mm |
Data collection
| Bruker SMART CCD diffractometer | 2168 independent reflections |
| Radiation source: fine-focus sealed tube | 1353 reflections with I > 2σ(I) |
| graphite | Rint = 0.029 |
| φ and ω scans | θmax = 25.0°, θmin = 1.9° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −7→8 |
| Tmin = 0.984, Tmax = 0.993 | k = −20→26 |
| 6423 measured reflections | l = −9→9 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.042 | H-atom parameters constrained |
| wR(F2) = 0.129 | w = 1/[σ2(Fo2) + (0.0582P)2 + 0.1369P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.02 | (Δ/σ)max < 0.001 |
| 2168 reflections | Δρmax = 0.13 e Å−3 |
| 192 parameters | Δρmin = −0.14 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| 64 constraints | Extinction coefficient: 0.008 (2) |
| Primary atom site location: structure-invariant direct methods |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| O1 | 0.7332 (3) | −0.13258 (8) | 0.3023 (2) | 0.0970 (6) | |
| O2 | 0.6598 (2) | −0.04170 (8) | 0.3749 (2) | 0.0883 (6) | |
| O3 | 1.2739 (3) | −0.17276 (10) | 0.0323 (3) | 0.1106 (7) | |
| O4 | 1.4641 (3) | −0.09968 (8) | −0.0180 (2) | 0.0979 (6) | |
| N1 | 0.7661 (3) | −0.07782 (10) | 0.3142 (2) | 0.0715 (5) | |
| N2 | 1.3231 (3) | −0.11920 (11) | 0.0400 (2) | 0.0802 (6) | |
| N3 | 0.8813 (2) | 0.05130 (8) | 0.3322 (2) | 0.0643 (5) | |
| H3 | 0.7731 | 0.0416 | 0.3643 | 0.077* | |
| N4 | 0.9487 (3) | 0.11105 (8) | 0.3468 (2) | 0.0716 (5) | |
| C1 | 0.9877 (3) | 0.00874 (9) | 0.2673 (2) | 0.0563 (5) | |
| C2 | 0.9367 (3) | −0.05376 (9) | 0.2535 (2) | 0.0583 (5) | |
| C3 | 1.0473 (3) | −0.09546 (10) | 0.1801 (2) | 0.0641 (6) | |
| H3A | 1.0114 | −0.1365 | 0.1717 | 0.077* | |
| C4 | 1.2096 (3) | −0.07577 (10) | 0.1204 (2) | 0.0634 (6) | |
| C5 | 1.2658 (3) | −0.01508 (10) | 0.1343 (3) | 0.0653 (6) | |
| H5 | 1.3777 | −0.0024 | 0.0944 | 0.078* | |
| C6 | 1.1586 (3) | 0.02600 (10) | 0.2058 (3) | 0.0631 (6) | |
| H6 | 1.1986 | 0.0666 | 0.2145 | 0.076* | |
| C7 | 0.8305 (3) | 0.15004 (10) | 0.3923 (3) | 0.0690 (6) | |
| C8 | 0.6290 (3) | 0.14145 (10) | 0.4287 (3) | 0.0785 (7) | |
| H8A | 0.6292 | 0.1186 | 0.5306 | 0.094* | |
| H8B | 0.5503 | 0.1197 | 0.3391 | 0.094* | |
| C9 | 0.5515 (8) | 0.2067 (3) | 0.4458 (10) | 0.0954 (16) | 0.631 (10) |
| H9A | 0.4572 | 0.2078 | 0.5221 | 0.114* | 0.631 (10) |
| H9B | 0.4921 | 0.2227 | 0.3393 | 0.114* | 0.631 (10) |
| C10 | 0.7337 (8) | 0.2425 (3) | 0.5134 (10) | 0.0972 (15) | 0.631 (10) |
| H10A | 0.7157 | 0.2860 | 0.4924 | 0.117* | 0.631 (10) |
| H10B | 0.7690 | 0.2360 | 0.6316 | 0.117* | 0.631 (10) |
| C10' | 0.7036 (14) | 0.2469 (4) | 0.4218 (16) | 0.090 (2) | 0.369 (10) |
| H10C | 0.6345 | 0.2536 | 0.3111 | 0.108* | 0.369 (10) |
| H10D | 0.7224 | 0.2859 | 0.4788 | 0.108* | 0.369 (10) |
| C9' | 0.5961 (19) | 0.2020 (4) | 0.5176 (14) | 0.087 (2) | 0.369 (10) |
| H9'1 | 0.4591 | 0.2119 | 0.5080 | 0.105* | 0.369 (10) |
| H9'2 | 0.6518 | 0.2006 | 0.6337 | 0.105* | 0.369 (10) |
| C11 | 0.8888 (4) | 0.21600 (11) | 0.4170 (4) | 0.0979 (8) | |
| H11A | 0.8868 | 0.2367 | 0.3116 | 0.117* | |
| H11B | 1.0176 | 0.2196 | 0.4805 | 0.117* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0998 (14) | 0.0748 (12) | 0.1191 (15) | −0.0291 (10) | 0.0247 (11) | 0.0028 (10) |
| O2 | 0.0678 (11) | 0.0882 (12) | 0.1131 (14) | −0.0079 (9) | 0.0276 (10) | 0.0071 (10) |
| O3 | 0.1313 (17) | 0.0738 (12) | 0.1310 (17) | 0.0164 (11) | 0.0340 (13) | −0.0039 (11) |
| O4 | 0.0988 (14) | 0.1102 (14) | 0.0911 (13) | 0.0162 (11) | 0.0353 (11) | 0.0015 (11) |
| N1 | 0.0660 (13) | 0.0744 (14) | 0.0725 (13) | −0.0132 (11) | 0.0047 (10) | 0.0104 (10) |
| N2 | 0.0869 (15) | 0.0837 (16) | 0.0694 (13) | 0.0160 (13) | 0.0095 (11) | 0.0062 (11) |
| N3 | 0.0584 (11) | 0.0658 (11) | 0.0695 (12) | −0.0059 (9) | 0.0124 (9) | 0.0045 (9) |
| N4 | 0.0675 (12) | 0.0604 (11) | 0.0879 (14) | −0.0060 (10) | 0.0151 (10) | 0.0050 (10) |
| C1 | 0.0544 (12) | 0.0634 (12) | 0.0494 (11) | −0.0002 (10) | 0.0019 (9) | 0.0087 (9) |
| C2 | 0.0562 (12) | 0.0630 (12) | 0.0535 (11) | −0.0059 (10) | 0.0011 (9) | 0.0108 (10) |
| C3 | 0.0697 (14) | 0.0613 (13) | 0.0575 (12) | −0.0040 (11) | −0.0025 (11) | 0.0099 (10) |
| C4 | 0.0685 (14) | 0.0646 (13) | 0.0555 (12) | 0.0088 (11) | 0.0032 (10) | 0.0091 (10) |
| C5 | 0.0593 (12) | 0.0761 (14) | 0.0610 (13) | −0.0003 (11) | 0.0104 (10) | 0.0106 (11) |
| C6 | 0.0625 (13) | 0.0638 (13) | 0.0626 (13) | −0.0067 (10) | 0.0080 (11) | 0.0077 (10) |
| C7 | 0.0733 (14) | 0.0660 (13) | 0.0688 (14) | −0.0015 (12) | 0.0145 (11) | 0.0070 (11) |
| C8 | 0.0805 (16) | 0.0807 (15) | 0.0784 (15) | −0.0003 (12) | 0.0254 (12) | 0.0018 (12) |
| C9 | 0.104 (3) | 0.095 (3) | 0.093 (3) | 0.023 (2) | 0.030 (3) | 0.011 (3) |
| C10 | 0.118 (3) | 0.076 (3) | 0.098 (3) | 0.011 (2) | 0.016 (3) | 0.002 (3) |
| C10' | 0.110 (4) | 0.066 (3) | 0.094 (4) | 0.012 (3) | 0.016 (4) | 0.007 (4) |
| C9' | 0.095 (4) | 0.080 (3) | 0.090 (4) | 0.010 (3) | 0.026 (4) | 0.007 (4) |
| C11 | 0.1034 (19) | 0.0699 (15) | 0.123 (2) | −0.0041 (14) | 0.0257 (16) | 0.0030 (14) |
Geometric parameters (Å, °)
| O1—N1 | 1.219 (2) | C7—C11 | 1.502 (3) |
| O2—N1 | 1.234 (2) | C8—C9 | 1.538 (6) |
| O3—N2 | 1.218 (2) | C8—C9' | 1.542 (10) |
| O4—N2 | 1.227 (3) | C8—H8A | 0.9700 |
| N1—C2 | 1.452 (3) | C8—H8B | 0.9700 |
| N2—C4 | 1.453 (3) | C9—C10 | 1.522 (7) |
| N3—C1 | 1.345 (3) | C9—H9A | 0.9700 |
| N3—N4 | 1.386 (2) | C9—H9B | 0.9700 |
| N3—H3 | 0.8600 | C10—C11 | 1.540 (6) |
| N4—C7 | 1.277 (3) | C10—H10A | 0.9700 |
| C1—C6 | 1.409 (3) | C10—H10B | 0.9700 |
| C1—C2 | 1.411 (3) | C10'—C11 | 1.461 (9) |
| C2—C3 | 1.385 (3) | C10'—C9' | 1.519 (2) |
| C3—C4 | 1.364 (3) | C10'—H10C | 0.9700 |
| C3—H3A | 0.9300 | C10'—H10D | 0.9700 |
| C4—C5 | 1.382 (3) | C9'—H9'1 | 0.9700 |
| C5—C6 | 1.354 (3) | C9'—H9'2 | 0.9700 |
| C5—H5 | 0.9300 | C11—H11A | 0.9700 |
| C6—H6 | 0.9300 | C11—H11B | 0.9700 |
| C7—C8 | 1.488 (3) | ||
| O1—N1—O2 | 122.8 (2) | C9—C8—H8B | 110.8 |
| O1—N1—C2 | 118.8 (2) | C9'—C8—H8B | 131.9 |
| O2—N1—C2 | 118.38 (19) | H8A—C8—H8B | 108.9 |
| O3—N2—O4 | 123.3 (2) | C8—C9—C10 | 102.9 (5) |
| O3—N2—C4 | 118.9 (2) | C8—C9—H9A | 111.2 |
| O4—N2—C4 | 117.9 (2) | C10—C9—H9A | 111.2 |
| C1—N3—N4 | 119.05 (18) | C8—C9—H9B | 111.2 |
| C1—N3—H3 | 120.5 | C10—C9—H9B | 111.2 |
| N4—N3—H3 | 120.5 | H9A—C9—H9B | 109.1 |
| C7—N4—N3 | 115.38 (19) | C11—C10—C9 | 103.4 (5) |
| N3—C1—C6 | 119.90 (19) | C11—C10—H10A | 111.1 |
| N3—C1—C2 | 123.60 (19) | C9—C10—H10A | 111.1 |
| C6—C1—C2 | 116.5 (2) | C11—C10—H10B | 111.1 |
| C3—C2—C1 | 121.43 (19) | C9—C10—H10B | 111.1 |
| C3—C2—N1 | 116.4 (2) | H10A—C10—H10B | 109.0 |
| C1—C2—N1 | 122.1 (2) | C11—C10'—C9' | 102.7 (7) |
| C4—C3—C2 | 119.3 (2) | C11—C10'—H10C | 111.2 |
| C4—C3—H3A | 120.3 | C9'—C10'—H10C | 111.2 |
| C2—C3—H3A | 120.3 | C11—C10'—H10D | 111.2 |
| C3—C4—C5 | 120.8 (2) | C9'—C10'—H10D | 111.2 |
| C3—C4—N2 | 119.4 (2) | H10C—C10'—H10D | 109.1 |
| C5—C4—N2 | 119.8 (2) | C10'—C9'—C8 | 101.1 (7) |
| C6—C5—C4 | 120.2 (2) | C10'—C9'—H9'1 | 111.5 |
| C6—C5—H5 | 119.9 | C8—C9'—H9'1 | 111.5 |
| C4—C5—H5 | 119.9 | C10'—C9'—H9'2 | 111.5 |
| C5—C6—C1 | 121.7 (2) | C8—C9'—H9'2 | 111.5 |
| C5—C6—H6 | 119.2 | H9'1—C9'—H9'2 | 109.4 |
| C1—C6—H6 | 119.2 | C10'—C11—C7 | 103.0 (4) |
| N4—C7—C8 | 129.9 (2) | C7—C11—C10 | 103.5 (3) |
| N4—C7—C11 | 120.4 (2) | C10'—C11—H11A | 85.0 |
| C8—C7—C11 | 109.8 (2) | C7—C11—H11A | 111.1 |
| C7—C8—C9 | 104.8 (3) | C10—C11—H11A | 111.1 |
| C7—C8—C9' | 101.3 (4) | C10'—C11—H11B | 134.2 |
| C7—C8—H8A | 110.8 | C7—C11—H11B | 111.1 |
| C9—C8—H8A | 110.8 | C10—C11—H11B | 111.1 |
| C9'—C8—H8A | 91.1 | H11A—C11—H11B | 109.0 |
| C7—C8—H8B | 110.8 |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N3—H3···O2 | 0.86 | 1.99 | 2.605 (2) | 128 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FB2114).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
- Baughman, R. G., Martin, K. L., Singh, R. K. & Stoffer, J. O. (2004). Acta Cryst. C60, o103–o106. [DOI] [PubMed]
- Bruker (2003). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- El-Seify, F. A. & El-Dossoki, F. I. (2006). J. Korean Chem. Soc.50, 99–106.
- Kim, S. Y. & Yoon, N. M. (1998). Bull. Korean Chem. Soc.19, 891–893.
- Liang, Z.-P. (2007). Acta Cryst. E63, o2943.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Zare, H. R., Ardakani, M. M., Nasirizadah, N. & Safari, J. (2005). Bull. Korean Chem. Soc.26, 51–56.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808033345/fb2114sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808033345/fb2114Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report