Abstract
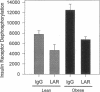
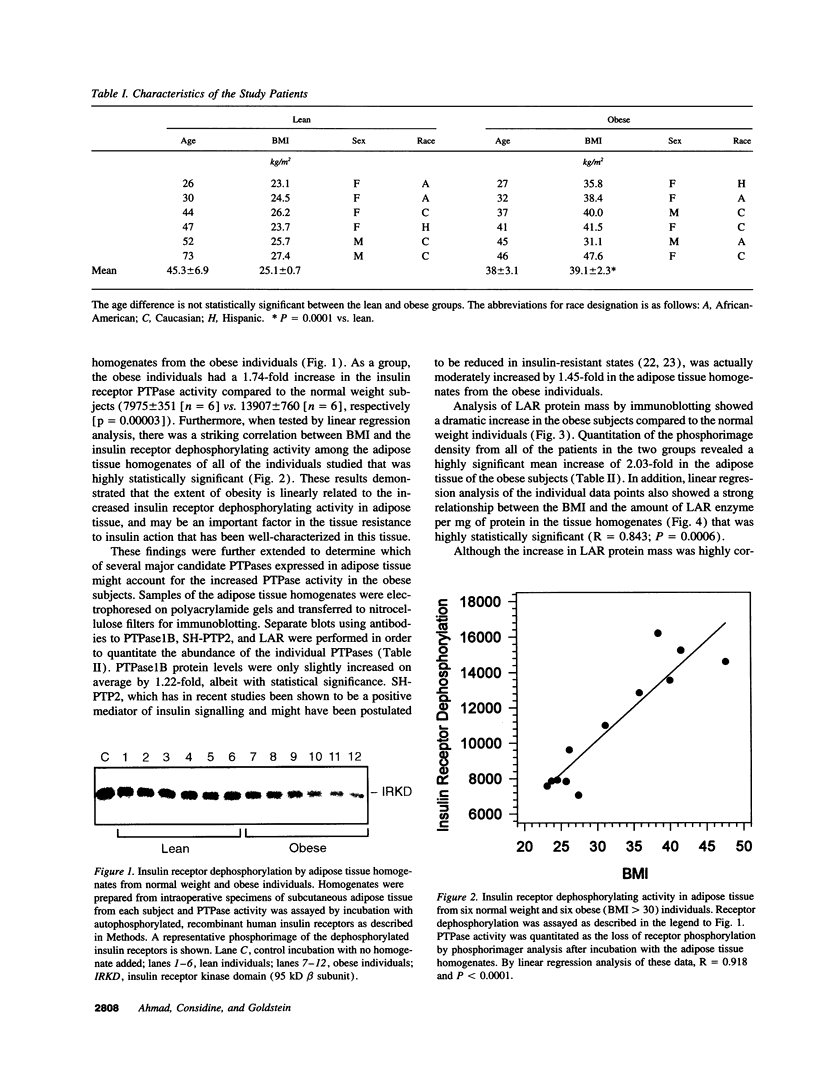
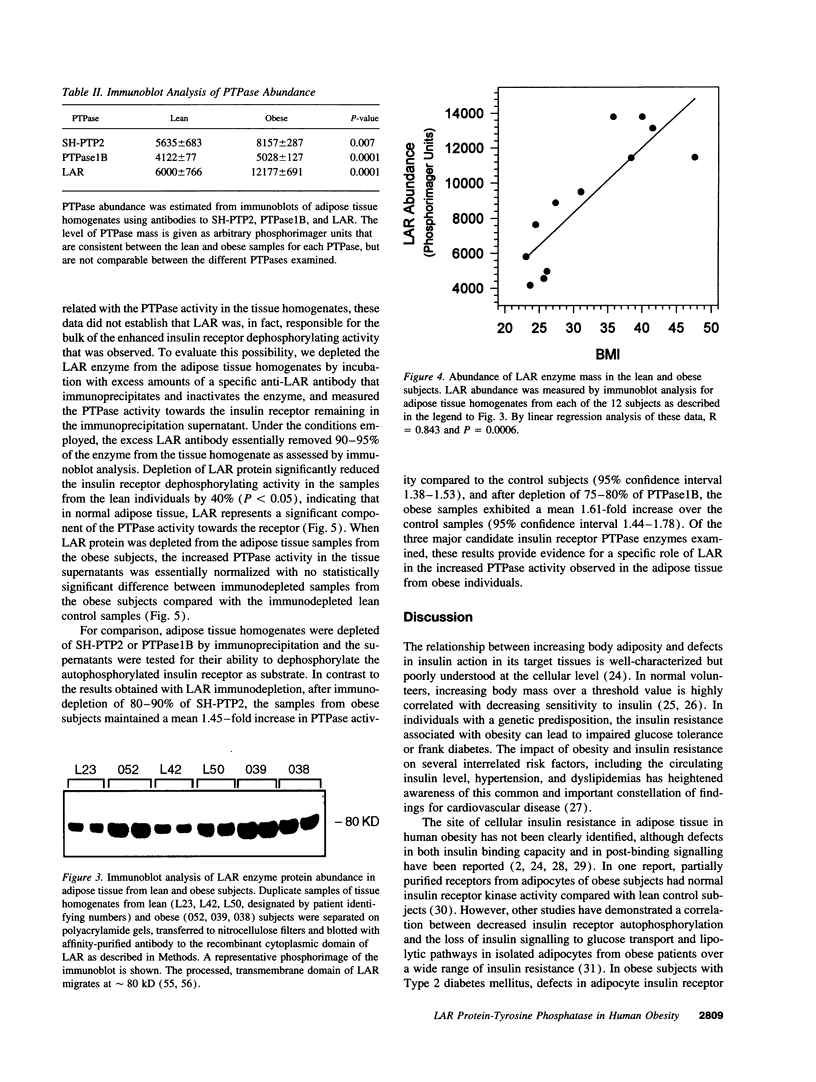
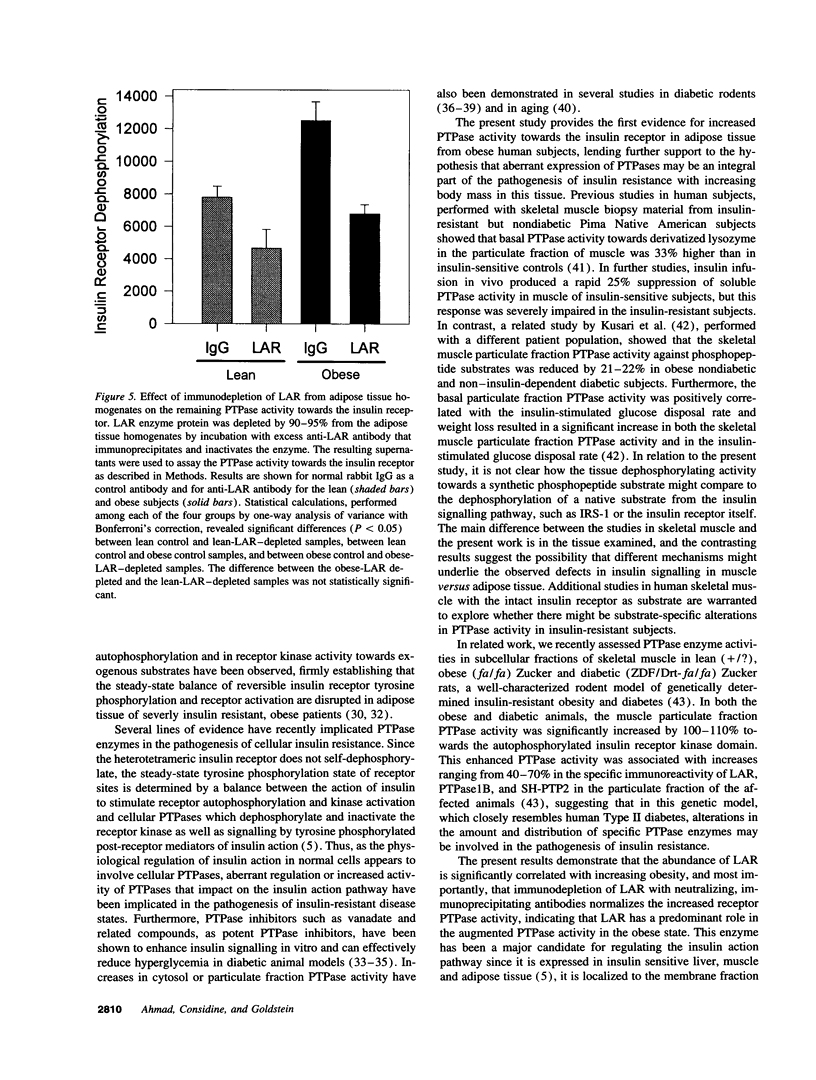
Protein-tyrosine phosphatases (PTPases) have an essential role in the regulation of the steady-state phosphorylation of the insulin receptor and other proteins in the insulin signalling pathway. To examine whether increased PTPase activity is associated with adipose tissue insulin resistance in human obesity we measured PTPase enzyme activity towards the insulin receptor in homogenates of subcutaneous adipose tissue from a series of six lean and six nondiabetic, obese (body mass index > 30) subjects. The obese subjects had a mean 1.74-fold increase in PTPase activity (P < 0.0001) with a striking positive correlation by linear regression analysis between PTPase activity and body mass index among all of the samples (R = 0.918; P < 0.0001). The abundance of three candidate insulin receptor PTPases in adipose tissue was also estimated by immunoblot analysis. The most prominent increase was a 2.03-fold rise in the transmembrane PTPase LAR (P < 0.001). Of the three PTPase examined, only immunodepletion of LAR protein from the homogenates with neutralizing antibodies resulted in normalization of the PTPase activity towards the insulin receptor, demonstrating that the increase in LAR was responsible for the enhanced PTPase activity in the adipose tissue from obese subjects. These studies suggest that increased PTPase activity towards the insulin receptor is a pathogenetic factor in the insulin resistance of adipose tissue in human obesity and provide evidence for a potential role of the LAR PTPase in the regulation of insulin signalling in disease states.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad S., Banville D., Zhao Z., Fischer E. H., Shen S. H. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum N., Sussman K. E., Draznin B. Differential effects of diabetes on adipocyte and liver phosphotyrosine and phosphoserine phosphatase activities. Diabetes. 1991 Dec;40(12):1620–1629. doi: 10.2337/diab.40.12.1620. [DOI] [PubMed] [Google Scholar]
- Bernier M., Liotta A. S., Kole H. K., Shock D. D., Roth J. Dynamic regulation of intact and C-terminal truncated insulin receptor phosphorylation in permeabilized cells. Biochemistry. 1994 Apr 12;33(14):4343–4351. doi: 10.1021/bi00180a031. [DOI] [PubMed] [Google Scholar]
- Boden G., Chen X., DeSantis R. A., Kendrick Z. Effects of age and body fat on insulin resistance in healthy men. Diabetes Care. 1993 May;16(5):728–733. doi: 10.2337/diacare.16.5.728. [DOI] [PubMed] [Google Scholar]
- Boylan J. M., Brautigan D. L., Madden J., Raven T., Ellis L., Gruppuso P. A. Differential regulation of multiple hepatic protein tyrosine phosphatases in alloxan diabetic rats. J Clin Invest. 1992 Jul;90(1):174–179. doi: 10.1172/JCI115833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Campbell P. J., Gerich J. E. Impact of obesity on insulin action in volunteers with normal glucose tolerance: demonstration of a threshold for the adverse effect of obesity. J Clin Endocrinol Metab. 1990 Apr;70(4):1114–1118. doi: 10.1210/jcem-70-4-1114. [DOI] [PubMed] [Google Scholar]
- Caro J. F. Clinical review 26: Insulin resistance in obese and nonobese man. J Clin Endocrinol Metab. 1991 Oct;73(4):691–695. doi: 10.1210/jcem-73-4-691. [DOI] [PubMed] [Google Scholar]
- Caro J. F., Dohm L. G., Pories W. J., Sinha M. K. Cellular alterations in liver, skeletal muscle, and adipose tissue responsible for insulin resistance in obesity and type II diabetes. Diabetes Metab Rev. 1989 Dec;5(8):665–689. doi: 10.1002/dmr.5610050804. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Tonks N. K., Kumar S., Diltz C. D., Harrylock M., Cool D. E., Krebs E. G., Fischer E. H., Walsh K. A. Human placenta protein-tyrosine-phosphatase: amino acid sequence and relationship to a family of receptor-like proteins. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5252–5256. doi: 10.1073/pnas.86.14.5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernoff J., Schievella A. R., Jost C. A., Erikson R. L., Neel B. G. Cloning of a cDNA for a major human protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2735–2739. doi: 10.1073/pnas.87.7.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaraldi T. P., Molina J. M., Olefsky J. M. Insulin action kinetics in adipocytes from obese and noninsulin-dependent diabetes mellitus subjects: identification of multiple cellular defects in glucose transport. J Clin Endocrinol Metab. 1991 Apr;72(4):876–882. doi: 10.1210/jcem-72-4-876. [DOI] [PubMed] [Google Scholar]
- Ding W., Zhang W. R., Sullivan K., Hashimoto N., Goldstein B. J. Identification of protein-tyrosine phosphatases prevalent in adipocytes by molecular cloning. Biochem Biophys Res Commun. 1994 Jul 29;202(2):902–907. doi: 10.1006/bbrc.1994.2015. [DOI] [PubMed] [Google Scholar]
- Fischer E. H., Charbonneau H., Tonks N. K. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991 Jul 26;253(5018):401–406. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- Freeman R. M., Jr, Plutzky J., Neel B. G. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freidenberg G. R., Henry R. R., Klein H. H., Reichart D. R., Olefsky J. M. Decreased kinase activity of insulin receptors from adipocytes of non-insulin-dependent diabetic subjects. J Clin Invest. 1987 Jan;79(1):240–250. doi: 10.1172/JCI112789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B. J. Regulation of insulin receptor signaling by protein-tyrosine dephosphorylation. Receptor. 1993 Spring;3(1):1–15. [PubMed] [Google Scholar]
- Goren H. J., Boland D. The 180000 molecular weight plasma membrane insulin receptor substrate is a protein tyrosine phosphatase and is elevated in diabetic plasma membranes. Biochem Biophys Res Commun. 1991 Oct 31;180(2):463–469. doi: 10.1016/s0006-291x(05)81087-3. [DOI] [PubMed] [Google Scholar]
- Guan K. L., Haun R. S., Watson S. J., Geahlen R. L., Dixon J. E. Cloning and expression of a protein-tyrosine-phosphatase. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1501–1505. doi: 10.1073/pnas.87.4.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto N., Feener E. P., Zhang W. R., Goldstein B. J. Insulin receptor protein-tyrosine phosphatases. Leukocyte common antigen-related phosphatase rapidly deactivates the insulin receptor kinase by preferential dephosphorylation of the receptor regulatory domain. J Biol Chem. 1992 Jul 15;267(20):13811–13814. [PubMed] [Google Scholar]
- Hashimoto N., Zhang W. R., Goldstein B. J. Insulin receptor and epidermal growth factor receptor dephosphorylation by three major rat liver protein-tyrosine phosphatases expressed in a recombinant bacterial system. Biochem J. 1992 Jun 1;284(Pt 2):569–576. doi: 10.1042/bj2840569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga M., White M. F., Kahn C. R. Phosphorylation of the insulin receptor in cultured hepatoma cells and a solubilized system. Methods Enzymol. 1985;109:609–621. doi: 10.1016/0076-6879(85)09118-2. [DOI] [PubMed] [Google Scholar]
- Kolterman O. G., Insel J., Saekow M., Olefsky J. M. Mechanisms of insulin resistance in human obesity: evidence for receptor and postreceptor defects. J Clin Invest. 1980 Jun;65(6):1272–1284. doi: 10.1172/JCI109790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhné M. R., Pawson T., Lienhard G. E., Feng G. S. The insulin receptor substrate 1 associates with the SH2-containing phosphotyrosine phosphatase Syp. J Biol Chem. 1993 Jun 5;268(16):11479–11481. [PubMed] [Google Scholar]
- Kuhné M. R., Zhao Z., Rowles J., Lavan B. E., Shen S. H., Fischer E. H., Lienhard G. E. Dephosphorylation of insulin receptor substrate 1 by the tyrosine phosphatase PTP2C. J Biol Chem. 1994 Jun 3;269(22):15833–15837. [PubMed] [Google Scholar]
- Kulas D. T., Zhang W. R., Goldstein B. J., Furlanetto R. W., Mooney R. A. Insulin receptor signaling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J Biol Chem. 1995 Feb 10;270(6):2435–2438. doi: 10.1074/jbc.270.6.2435. [DOI] [PubMed] [Google Scholar]
- Kusari J., Kenner K. A., Suh K. I., Hill D. E., Henry R. R. Skeletal muscle protein tyrosine phosphatase activity and tyrosine phosphatase 1B protein content are associated with insulin action and resistance. J Clin Invest. 1994 Mar;93(3):1156–1162. doi: 10.1172/JCI117068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lammers R., Bossenmaier B., Cool D. E., Tonks N. K., Schlessinger J., Fischer E. H., Ullrich A. Differential activities of protein tyrosine phosphatases in intact cells. J Biol Chem. 1993 Oct 25;268(30):22456–22462. [PubMed] [Google Scholar]
- Maegawa H., Ugi S., Adachi M., Hinoda Y., Kikkawa R., Yachi A., Shigeta Y., Kashiwagi A. Insulin receptor kinase phosphorylates protein tyrosine phosphatase containing Src homology 2 regions and modulates its PTPase activity in vitro. Biochem Biophys Res Commun. 1994 Mar 15;199(2):780–785. doi: 10.1006/bbrc.1994.1297. [DOI] [PubMed] [Google Scholar]
- Maegawa H., Ugi S., Ishibashi O., Tachikawa-Ide R., Takahara N., Tanaka Y., Takagi Y., Kikkawa R., Shigeta Y., Kashiwagi A. Src homology 2 domains of protein tyrosine phosphatase are phosphorylated by insulin receptor kinase and bind to the COOH-terminus of insulin receptors in vitro. Biochem Biophys Res Commun. 1993 Jul 15;194(1):208–214. doi: 10.1006/bbrc.1993.1805. [DOI] [PubMed] [Google Scholar]
- McGuire M. C., Fields R. M., Nyomba B. L., Raz I., Bogardus C., Tonks N. K., Sommercorn J. Abnormal regulation of protein tyrosine phosphatase activities in skeletal muscle of insulin-resistant humans. Diabetes. 1991 Jul;40(7):939–942. doi: 10.2337/diab.40.7.939. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Backer J. M., Kahn C. R. Hepatic phosphotyrosine phosphatase activity and its alterations in diabetic rats. J Clin Invest. 1989 Sep;84(3):976–983. doi: 10.1172/JCI114261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerovitch J., Rothenberg P., Shechter Y., Bonner-Weir S., Kahn C. R. Vanadate normalizes hyperglycemia in two mouse models of non-insulin-dependent diabetes mellitus. J Clin Invest. 1991 Apr;87(4):1286–1294. doi: 10.1172/JCI115131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milarski K. L., Saltiel A. R. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994 Aug 19;269(33):21239–21243. [PubMed] [Google Scholar]
- Mooney R. A., Anderson D. L. Phosphorylation of the insulin receptor in permeabilized adipocytes is coupled to a rapid dephosphorylation reaction. J Biol Chem. 1989 Apr 25;264(12):6850–6857. [PubMed] [Google Scholar]
- Nadiv O., Shinitzky M., Manu H., Hecht D., Roberts C. T., Jr, LeRoith D., Zick Y. Elevated protein tyrosine phosphatase activity and increased membrane viscosity are associated with impaired activation of the insulin receptor kinase in old rats. Biochem J. 1994 Mar 1;298(Pt 2):443–450. doi: 10.1042/bj2980443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky J. M. Decreased insulin binding to adipocytes and circulating monocytes from obese subjects. J Clin Invest. 1976 May;57(5):1165–1172. doi: 10.1172/JCI108384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner B. I., Faure R., Burgess J. W., Bevan A. P., Lachance D., Zhang-Sun G., Fantus I. G., Ng J. B., Hall D. A., Lum B. S. Peroxovanadium compounds. A new class of potent phosphotyrosine phosphatase inhibitors which are insulin mimetics. J Biol Chem. 1994 Feb 11;269(6):4596–4604. [PubMed] [Google Scholar]
- Rabinowitz D. Some endocrine and metabolic aspects of obesity. Annu Rev Med. 1970;21:241–258. doi: 10.1146/annurev.me.21.020170.001325. [DOI] [PubMed] [Google Scholar]
- Reaven G. M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes. 1988 Dec;37(12):1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- Shisheva A., Ikonomov O., Shechter Y. The protein tyrosine phosphatase inhibitor, pervanadate, is a powerful antidiabetic agent in streptozotocin-treated diabetic rats. Endocrinology. 1994 Jan;134(1):507–510. doi: 10.1210/endo.134.1.8275968. [DOI] [PubMed] [Google Scholar]
- Sinha M. K., Pories W. J., Flickinger E. G., Meelheim D., Caro J. F. Insulin-receptor kinase activity of adipose tissue from morbidly obese humans with and without NIDDM. Diabetes. 1987 May;36(5):620–625. doi: 10.2337/diab.36.5.620. [DOI] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Ariniello P. D., Tang M., Munro J. M., Blattler W. A., Adler D. A., Disteche C. M., Saito H. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992 Mar;11(3):897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F., Saito H. A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J Exp Med. 1988 Nov 1;168(5):1523–1530. doi: 10.1084/jem.168.5.1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto S., Wandless T. J., Shoelson S. E., Neel B. G., Walsh C. T. Activation of the SH2-containing protein tyrosine phosphatase, SH-PTP2, by phosphotyrosine-containing peptides derived from insulin receptor substrate-1. J Biol Chem. 1994 May 6;269(18):13614–13622. [PubMed] [Google Scholar]
- Sun X. J., Crimmins D. L., Myers M. G., Jr, Miralpeix M., White M. F. Pleiotropic insulin signals are engaged by multisite phosphorylation of IRS-1. Mol Cell Biol. 1993 Dec;13(12):7418–7428. doi: 10.1128/mcb.13.12.7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S., Kahn C. R., Kubo K., Foley J. E. Alterations in insulin receptor autophosphorylation in insulin resistance: correlation with altered sensitivity to glucose transport and antilipolysis to insulin. J Clin Endocrinol Metab. 1988 May;66(5):992–999. doi: 10.1210/jcem-66-5-992. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugi S., Maegawa H., Olefsky J. M., Shigeta Y., Kashiwagi A. Src homology 2 domains of protein tyrosine phosphatase are associated in vitro with both the insulin receptor and insulin receptor substrate-1 via different phosphotyrosine motifs. FEBS Lett. 1994 Mar 7;340(3):216–220. doi: 10.1016/0014-5793(94)80141-x. [DOI] [PubMed] [Google Scholar]
- Vogel W., Lammers R., Huang J., Ullrich A. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993 Mar 12;259(5101):1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- White M. F., Kahn C. R. The insulin signaling system. J Biol Chem. 1994 Jan 7;269(1):1–4. [PubMed] [Google Scholar]
- Xiao S., Rose D. W., Sasaoka T., Maegawa H., Burke T. R., Jr, Roller P. P., Shoelson S. E., Olefsky J. M. Syp (SH-PTP2) is a positive mediator of growth factor-stimulated mitogenic signal transduction. J Biol Chem. 1994 Aug 19;269(33):21244–21248. [PubMed] [Google Scholar]
- Yu Q., Lenardo T., Weinberg R. A. The N-terminal and C-terminal domains of a receptor tyrosine phosphatase are associated by non-covalent linkage. Oncogene. 1992 Jun;7(6):1051–1057. [PubMed] [Google Scholar]