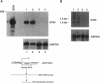
Abstract
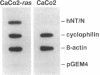
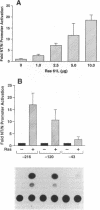
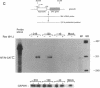
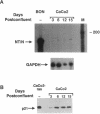
Ras regulates novel patterns of gene expression and the differentiation of various eukaryotic cell types. Stable transfection of Ha-ras into the human colon cancer line CaCo2 results in the morphologic differentiation to a small bowel phenotype. The purpose of our study was to determine whether the Ras regulatory pathway plays a role in the expression of the neurotensin gene (NT/N), a terminally differentiated endocrine product specifically localized in the gastrointestinal tract to the adult small bowel. We found that CaCo2-ras cells, but not parental CaCo2, express high levels of the human NT/N gene and, moreover, that this increase in gene expression is regulated at the level of transcription. Transfection experiments using NT/N-CAT mutation constructs identify the proximal 200 bp of NT/N flanking sequence as sufficient for maximal Ras-mediated NT/N reporter gene induction. Furthermore, a proximal AP-1/CRE motif is crucial for this Ras-mediated NT/N activation. Wild-type Ha-ras induces NT/N gene expression, albeit at lower levels than activated Ras; a dominant-negative Raf blocks this NT/N induction, suggesting that Raf lies down-stream of Ras in this pathway. In addition, postconfluent cultures of CaCo2 cells, which are differentiated to a small bowel phenotype, express the NT/N gene by 6 d after reaching confluency; this increase of NT/N expression is associated with concomitant increases of cellular p21ras protein. We conclude that Ras (both wild-type and activated) enhances expression of the NT/N gene in the gut-derived CaCo2 cell line, suggesting an important role for the Ras signaling pathway in NT/N gene transcription. Our results underscore the possibility that tissue-specific genes (such as NT/N) expressed in distinct subpopulations of the gut may be subject to Ras regulation. Finally, we speculate that the NT/N gene and the CaCo2 and CaCo2-ras cell systems will provide unique models to further define the cellular mechanisms leading to mammalian intestinal differentiation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Rosell S., Hjelmquist U., Chang D., Folkers K. Inhibition of gastric and intestinal motor activity in dogs by (Gln4) neurotensin. Acta Physiol Scand. 1977 Jun;100(2):231–235. doi: 10.1111/j.1748-1716.1977.tb05941.x. [DOI] [PubMed] [Google Scholar]
- Angel P., Hattori K., Smeal T., Karin M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP-1. Cell. 1988 Dec 2;55(5):875–885. doi: 10.1016/0092-8674(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Armstrong M. J., Parker M. C., Ferris C. F., Leeman S. E. Neurotensin stimulates [3H]oleic acid translocation across rat small intestine. Am J Physiol. 1986 Dec;251(6 Pt 1):G823–G829. doi: 10.1152/ajpgi.1986.251.6.G823. [DOI] [PubMed] [Google Scholar]
- Baca I., Feurle G. E., Schwab A., Mittmann U., Knauf W., Lehnert T. Effect of neurotensin on exocrine pancreatic secretion in dogs. Digestion. 1982;23(3):174–183. doi: 10.1159/000198725. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Bean A. J., Dagerlind A., Hökfelt T., Dobner P. R. Cloning of human neurotensin/neuromedin N genomic sequences and expression in the ventral mesencephalon of schizophrenics and age/sex matched controls. Neuroscience. 1992 Sep;50(2):259–268. doi: 10.1016/0306-4522(92)90421-w. [DOI] [PubMed] [Google Scholar]
- Bell L., Williams L. A scanning and transmission electron microscopical study of the morphogenesis of human colonic villi. Anat Embryol (Berl) 1982 Dec;165(3):437–455. doi: 10.1007/BF00305579. [DOI] [PubMed] [Google Scholar]
- Benito M., Porras A., Nebreda A. R., Santos E. Differentiation of 3T3-L1 fibroblasts to adipocytes induced by transfection of ras oncogenes. Science. 1991 Aug 2;253(5019):565–568. doi: 10.1126/science.1857988. [DOI] [PubMed] [Google Scholar]
- Binétruy B., Smeal T., Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991 May 9;351(6322):122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- Blenis J. Signal transduction via the MAP kinases: proceed at your own RSK. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):5889–5892. doi: 10.1073/pnas.90.13.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos J. L. The ras gene family and human carcinogenesis. Mutat Res. 1988 May;195(3):255–271. doi: 10.1016/0165-1110(88)90004-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruder J. T., Heidecker G., Rapp U. R. Serum-, TPA-, and Ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992 Apr;6(4):545–556. doi: 10.1101/gad.6.4.545. [DOI] [PubMed] [Google Scholar]
- Celano P., Berchtold C. M., Mabry M., Carroll M., Sidransky D., Casero R. A., Jr, Lupu R. Induction of markers of normal differentiation in human colon carcinoma cells by the v-rasH oncogene. Cell Growth Differ. 1993 Apr;4(4):341–347. [PubMed] [Google Scholar]
- Chantret I., Barbat A., Dussaulx E., Brattain M. G., Zweibaum A. Epithelial polarity, villin expression, and enterocytic differentiation of cultured human colon carcinoma cells: a survey of twenty cell lines. Cancer Res. 1988 Apr 1;48(7):1936–1942. [PubMed] [Google Scholar]
- Chung D. H., Evers B. M., Shimoda I., Townsend C. M., Jr, Rajaraman S., Thompson J. C. Effect of neurotensin on gut mucosal growth in rats with jejunal and ileal Thiry-Vella fistulas. Gastroenterology. 1992 Oct;103(4):1254–1259. doi: 10.1016/0016-5085(92)91512-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Conrad K. E., Oberwetter J. M., Vaillancourt R., Johnson G. L., Gutierrez-Hartmann A. Identification of the functional components of the Ras signaling pathway regulating pituitary cell-specific gene expression. Mol Cell Biol. 1994 Mar;14(3):1553–1565. doi: 10.1128/mcb.14.3.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Der C. J., Finkel T., Cooper G. M. Biological and biochemical properties of human rasH genes mutated at codon 61. Cell. 1986 Jan 17;44(1):167–176. doi: 10.1016/0092-8674(86)90495-2. [DOI] [PubMed] [Google Scholar]
- Der C. J., Pan B. T., Cooper G. M. rasH mutants deficient in GTP binding. Mol Cell Biol. 1986 Sep;6(9):3291–3294. doi: 10.1128/mcb.6.9.3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers B. M., Ehrenfried J. A., Wang X., Townsend C. M., Jr, Thompson J. C. Temporal-specific and spatial-specific patterns of neurotensin gene expression in the small bowel. Am J Physiol. 1994 Nov;267(5 Pt 1):G875–G882. doi: 10.1152/ajpgi.1994.267.5.G875. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Ishizuka J., Chung D. H., Townsend C. M., Jr, Thompson J. C. Neurotensin expression and release in human colon cancers. Ann Surg. 1992 Oct;216(4):423–431. doi: 10.1097/00000658-199210000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers B. M., Ishizuka J., Townsend C. M., Jr, Rajaraman S., Thompson J. C. Expression of neurotensin messenger RNA in a human carcinoid tumor. Ann Surg. 1991 Oct;214(4):448–455. doi: 10.1097/00000658-199110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers B. M., Izukura M., Chung D. H., Parekh D., Yoshinaga K., Greeley G. H., Jr, Uchida T., Townsend C. M., Jr, Thompson J. C. Neurotensin stimulates growth of colonic mucosa in young and aged rats. Gastroenterology. 1992 Jul;103(1):86–91. doi: 10.1016/0016-5085(92)91099-p. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Izukura M., Rajaraman S., Parekh D., Thakore K., Yoshinaga K., Uchida T., Townsend C. M., Jr, Thompson J. C. Effect of aging on neurotensin-stimulated growth of rat small intestine. Am J Physiol. 1994 Aug;267(2 Pt 1):G180–G186. doi: 10.1152/ajpgi.1994.267.2.G180. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Izukura M., Townsend C. M., Jr, Uchida T., Thompson J. C. Neurotensin prevents intestinal mucosal hypoplasia in rats fed an elemental diet. Dig Dis Sci. 1992 Mar;37(3):426–431. doi: 10.1007/BF01307738. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Rajaraman S., Chung D. H., Townsend C. M., Jr, Wang X., Graves K., Thompson J. C. Differential expression of the neurotensin gene in the developing rat and human gastrointestinal tract. Am J Physiol. 1993 Sep;265(3 Pt 1):G482–G490. doi: 10.1152/ajpgi.1993.265.3.G482. [DOI] [PubMed] [Google Scholar]
- Feurle G. E., Müller B., Rix E. Neurotensin induces hyperplasia of the pancreas and growth of the gastric antrum in rats. Gut. 1987;28 (Suppl):19–23. doi: 10.1136/gut.28.suppl.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furth M. E., Aldrich T. H., Cordon-Cardo C. Expression of ras proto-oncogene proteins in normal human tissues. Oncogene. 1987 Mar;1(1):47–58. [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Gutman A., Wasylyk B. Nuclear targets for transcription regulation by oncogenes. Trends Genet. 1991 Feb;7(2):49–54. doi: 10.1016/0168-9525(91)90231-E. [DOI] [PubMed] [Google Scholar]
- Hall A. A biochemical function for ras--at last. Science. 1994 Jun 3;264(5164):1413–1414. doi: 10.1126/science.8197454. [DOI] [PubMed] [Google Scholar]
- Hall C. V., Jacob P. E., Ringold G. M., Lee F. Expression and regulation of Escherichia coli lacZ gene fusions in mammalian cells. J Mol Appl Genet. 1983;2(1):101–109. [PubMed] [Google Scholar]
- Hauck W., Stanners C. P. Control of carcinoembryonic antigen gene family expression in a differentiating colon carcinoma cell line, Caco-2. Cancer Res. 1991 Jul 1;51(13):3526–3533. [PubMed] [Google Scholar]
- Karin M. Signal transduction from cell surface to nucleus in development and disease. FASEB J. 1992 May;6(8):2581–2590. doi: 10.1096/fasebj.6.8.1317309. [DOI] [PubMed] [Google Scholar]
- Kislauskis E., Dobner P. R. Mutually dependent response elements in the cis-regulatory region of the neurotensin/neuromedin N gene integrate environmental stimuli in PC12 cells. Neuron. 1990 May;4(5):783–795. doi: 10.1016/0896-6273(90)90205-t. [DOI] [PubMed] [Google Scholar]
- Koide H., Satoh T., Nakafuku M., Kaziro Y. GTP-dependent association of Raf-1 with Ha-Ras: identification of Raf as a target downstream of Ras in mammalian cells. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8683–8686. doi: 10.1073/pnas.90.18.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacroix B., Kedinger M., Simon-Assmann P., Rousset M., Zweibaum A., Haffen K. Developmental pattern of brush border enzymes in the human fetal colon. Correlation with some morphogenetic events. Early Hum Dev. 1984 Feb;9(2):95–103. doi: 10.1016/0378-3782(84)90089-6. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Willumsen B. M. Function and regulation of ras. Annu Rev Biochem. 1993;62:851–891. doi: 10.1146/annurev.bi.62.070193.004223. [DOI] [PubMed] [Google Scholar]
- Mabry M., Nakagawa T., Baylin S., Pettengill O., Sorenson G., Nelkin B. Insertion of the v-Ha-ras oncogene induces differentiation of calcitonin-producing human small cell lung cancer. J Clin Invest. 1989 Jul;84(1):194–199. doi: 10.1172/JCI114140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masquilier D., Sassone-Corsi P. Transcriptional cross-talk: nuclear factors CREM and CREB bind to AP-1 sites and inhibit activation by Jun. J Biol Chem. 1992 Nov 5;267(31):22460–22466. [PubMed] [Google Scholar]
- Muraki K., Mitra S. P., Dobner P. R., Carraway R. E. Enhanced expression of neurotensin/neuromedin N mRNA and products of NT/NMN precursor processing in neonatal rats. Peptides. 1993 Nov-Dec;14(6):1095–1102. doi: 10.1016/0196-9781(93)90161-9. [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Mabry M., de Bustros A., Ihle J. N., Nelkin B. D., Baylin S. B. Introduction of v-Ha-ras oncogene induces differentiation of cultured human medullary thyroid carcinoma cells. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5923–5927. doi: 10.1073/pnas.84.16.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida E., Gotoh Y. The MAP kinase cascade is essential for diverse signal transduction pathways. Trends Biochem Sci. 1993 Apr;18(4):128–131. doi: 10.1016/0968-0004(93)90019-j. [DOI] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Pelech S. L., Sanghera J. S. MAP kinases: charting the regulatory pathways. Science. 1992 Sep 4;257(5075):1355–1356. doi: 10.1126/science.1382311. [DOI] [PubMed] [Google Scholar]
- Reinecke M. Neurotensin. Immunohistochemical localization in central and peripheral nervous system and in endocrine cells and its functional role as neurotransmitter and endocrine hormone. Prog Histochem Cytochem. 1985;16(1):1–172. [PubMed] [Google Scholar]
- Rousset M., Laburthe M., Pinto M., Chevalier G., Rouyer-Fessard C., Dussaulx E., Trugnan G., Boige N., Brun J. L., Zweibaum A. Enterocytic differentiation and glucose utilization in the human colon tumor cell line Caco-2: modulation by forskolin. J Cell Physiol. 1985 Jun;123(3):377–385. doi: 10.1002/jcp.1041230313. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Der C. J., Verma I. M. ras-induced neuronal differentiation of PC12 cells: possible involvement of fos and jun. Mol Cell Biol. 1989 Aug;9(8):3174–3183. doi: 10.1128/mcb.9.8.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakafuku M., Kaziro Y. Function of Ras as a molecular switch in signal transduction. J Biol Chem. 1992 Dec 5;267(34):24149–24152. [PubMed] [Google Scholar]
- Schlessinger J. How receptor tyrosine kinases activate Ras. Trends Biochem Sci. 1993 Aug;18(8):273–275. doi: 10.1016/0968-0004(93)90031-h. [DOI] [PubMed] [Google Scholar]
- Schwab M., Alitalo K., Varmus H. E., Bishop J. M., George D. A cellular oncogene (c-Ki-ras) is amplified, overexpressed, and located within karyotypic abnormalities in mouse adrenocortical tumour cells. Nature. 1983 Jun 9;303(5917):497–501. doi: 10.1038/303497a0. [DOI] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D. A., Birrer M., Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991 Dec 12;354(6353):494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D., Grover-Bardwick A., Heidecker G., Rapp U. R., Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992 Aug;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szeberényi J., Cai H., Cooper G. M. Effect of a dominant inhibitory Ha-ras mutation on neuronal differentiation of PC12 cells. Mol Cell Biol. 1990 Oct;10(10):5324–5332. doi: 10.1128/mcb.10.10.5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thor K., Rosell S. Neurotensin increases colonic motility. Gastroenterology. 1986 Jan;90(1):27–31. doi: 10.1016/0016-5085(86)90070-3. [DOI] [PubMed] [Google Scholar]
- Thorburn A., Thorburn J., Chen S. Y., Powers S., Shubeita H. E., Feramisco J. R., Chien K. R. HRas-dependent pathways can activate morphological and genetic markers of cardiac muscle cell hypertrophy. J Biol Chem. 1993 Jan 25;268(3):2244–2249. [PubMed] [Google Scholar]
- Wood J. G., Hoang H. D., Bussjaeger L. J., Solomon T. E. Neurotensin stimulates growth of small intestine in rats. Am J Physiol. 1988 Dec;255(6 Pt 1):G813–G817. doi: 10.1152/ajpgi.1988.255.6.G813. [DOI] [PubMed] [Google Scholar]
- Xie W., Fletcher B. S., Andersen R. D., Herschman H. R. v-src induction of the TIS10/PGS2 prostaglandin synthase gene is mediated by an ATF/CRE transcription response element. Mol Cell Biol. 1994 Oct;14(10):6531–6539. doi: 10.1128/mcb.14.10.6531. [DOI] [PMC free article] [PubMed] [Google Scholar]