Abstract
The title compound, C29H35N3, is the product of the condensation reaction between 2,6-diacetylpyridine and 2,6-diethylaniline. In the molecule, the pyridyl ring is coplanar with the imino functional groups [torsion angles in the range 177.1 (2)–179.9 (2)°. The two 2,6-diethyl-substituted benzene rings are approximately perpendicular to the ethylidenepyridine central core, the dihedral angles being 88.7 (1) and 88.4 (1)°, respectively.
Related literature
For applications of pyridine derivatives, see: Tang & VanSlyke (1987 ▶); Wang (2001 ▶). For the synthesis of the title molecule, see: Fan et al. (2004 ▶). For structures of other imino derivatives, see: Mentes et al. (2001 ▶); Huang et al. (2006 ▶).
Experimental
Crystal data
C29H35N3
M r = 425.60
Monoclinic,

a = 7.9390 (8) Å
b = 12.3208 (13) Å
c = 25.998 (3) Å
β = 96.234 (2)°
V = 2528.0 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.07 mm−1
T = 193 (2) K
0.26 × 0.24 × 0.20 mm
Data collection
Bruker SMART APEX CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 1998 ▶) T min = 0.983, T max = 0.987
13906 measured reflections
4938 independent reflections
2362 reflections with I > 2σ(I)
R int = 0.077
Refinement
R[F 2 > 2σ(F 2)] = 0.057
wR(F 2) = 0.101
S = 0.95
4938 reflections
289 parameters
H-atom parameters constrained
Δρmax = 0.30 e Å−3
Δρmin = −0.19 e Å−3
Data collection: SMART (Bruker, 1998 ▶); cell refinement: SAINT (Bruker, 1998 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808036842/bh2205sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808036842/bh2205Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected bond lengths (Å).
| N1—C1 | 1.272 (3) |
| N1—C10 | 1.431 (3) |
| N2—C2 | 1.333 (2) |
| N2—C6 | 1.348 (2) |
| N3—C7 | 1.275 (2) |
| N3—C20 | 1.424 (3) |
Acknowledgments
This work was supported by the National Natural Science Foundation of China (20671025 and 20771030), the Young Foundation of Heilongjiang Province in China (QC06C029), Heilongjiang Natural Science Foundation (B200603) and the Science Innovation Special Foundation of Harbin City, China (2006RFQXG037).
supplementary crystallographic information
Comment
Luminescent coordination compounds based on pyridine-type ligands have attracted intensive attention due to their potential application in areas of sensor technologies and electro-luminescent devices (Tang & VanSlyke, 1987; Wang, 2001). In order to explore potential luminescent complexes of this type, we prepared a series of bis(iminoalkyl)pyridine ligands by the condensation of 2,6-diacetylpyridine with the corresponding aniline in methanol (Fan et al., 2004). We report here the crystal structure of one of them, (I).
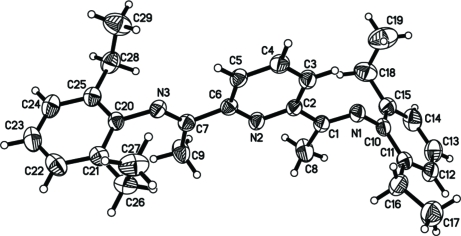
The molecular structure of (I) is shown in Fig. 1 and selected bond distances are given in Table 1. The pyridyl ring is coplanar with the two imino functional groups. The two imino C═N bonds have typical double-bond characteristics, with bond lengths of 1.272 (3) and 1.275 (2) Å, which are similar to that in BIP1, 1.266 (4) (Mentes et al., 2001) and in 2,6-bis[1-(2,6-dimethylphenylimino)ethyl]pyridine, 1.265 (2) and 1.271 (2) Å (Huang et al., 2006). Compound (I) possesses a structure which approximates Cs symmetry about a plane bisecting the central pyridyl ring. The two 2,6-diethyl-substituted phenyl rings are approximately perpendicular to the ethylidenepyridine ring, with the dihedral angles being 88.7° and 88.4°.
Experimental
The title compound was synthesized according to the literature method of Fan et al. (2004). To a solution of 2,6-diethylpyridine (1.5 g, 9.2 mmol) in absolute methanol (40 ml) was added 2,6-diethylaniline (4.6 ml, 27.7 mmol). After the addition of several drops of formic acid, the reaction mixture was refluxed for 24 h and then allowed to cool down to room temperature. The crude product precipitated as a yellow powder. Pure (I) was obtained as yellow block crystals in 84% yield (3.3 g) upon recrystallization from methanol, giving single crystals suitable for X-ray diffraction.
Refinement
The C-bound H atoms were positioned geometrically with C—H = 0.93–0.97 Å, and allowed to ride on their parent atoms with Uiso(H) = 1.5Ueq(carrier C) for methyl groups and Uiso(H) = 1.2Ueq(carrier C) otherwise.
Figures
Fig. 1.
View of the molecule of (I) showing the atom-labelling scheme. Displacement ellipsoids are drawn at the 50% probability level.
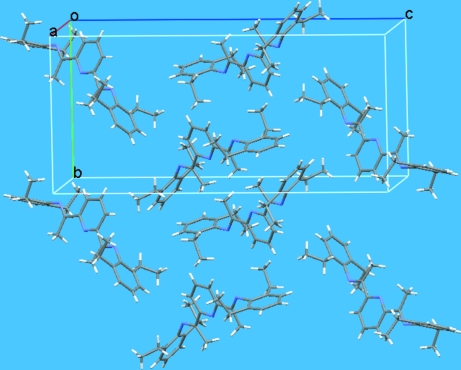
Fig. 2.
Packing of (I) along a cell axis direction.
Crystal data
| C29H35N3 | F000 = 920 |
| Mr = 425.60 | Dx = 1.118 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 13906 reflections |
| a = 7.9390 (8) Å | θ = 1.6–26.0º |
| b = 12.3208 (13) Å | µ = 0.07 mm−1 |
| c = 25.998 (3) Å | T = 193 (2) K |
| β = 96.234 (2)º | Block, yellow |
| V = 2528.0 (5) Å3 | 0.26 × 0.24 × 0.20 mm |
| Z = 4 |
Data collection
| Bruker SMART APEX CCD area-detector diffractometer | 4938 independent reflections |
| Radiation source: fine-focus sealed tube | 2362 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.077 |
| T = 193(2) K | θmax = 26.0º |
| φ and ω scans | θmin = 1.6º |
| Absorption correction: multi-scan(SADABS; Bruker, 1998) | h = −9→9 |
| Tmin = 0.983, Tmax = 0.987 | k = −15→12 |
| 13906 measured reflections | l = −31→32 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.057 | H-atom parameters constrained |
| wR(F2) = 0.101 | w = 1/[σ2(Fo2) + (0.02P)2] where P = (Fo2 + 2Fc2)/3 |
| S = 0.95 | (Δ/σ)max < 0.001 |
| 4938 reflections | Δρmax = 0.30 e Å−3 |
| 289 parameters | Δρmin = −0.19 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | −0.5544 (2) | 0.61937 (15) | −0.15400 (7) | 0.0377 (5) | |
| N2 | −0.2647 (2) | 0.69242 (15) | −0.04651 (7) | 0.0342 (5) | |
| N3 | 0.0206 (2) | 0.83861 (14) | 0.04282 (7) | 0.0328 (5) | |
| C1 | −0.4636 (3) | 0.60967 (19) | −0.11083 (9) | 0.0353 (6) | |
| C2 | −0.3645 (3) | 0.70650 (19) | −0.09065 (9) | 0.0315 (6) | |
| C3 | −0.3771 (3) | 0.80441 (18) | −0.11695 (9) | 0.0379 (7) | |
| H3B | −0.4480 | 0.8113 | −0.1477 | 0.045* | |
| C4 | −0.2830 (3) | 0.89137 (19) | −0.09692 (9) | 0.0401 (7) | |
| H4A | −0.2893 | 0.9580 | −0.1139 | 0.048* | |
| C5 | −0.1790 (3) | 0.87807 (18) | −0.05110 (9) | 0.0346 (6) | |
| H5A | −0.1140 | 0.9355 | −0.0367 | 0.041* | |
| C6 | −0.1734 (3) | 0.77816 (18) | −0.02709 (9) | 0.0321 (6) | |
| C7 | −0.0646 (3) | 0.75836 (19) | 0.02294 (9) | 0.0328 (6) | |
| C8 | −0.4473 (3) | 0.50849 (18) | −0.07891 (9) | 0.0551 (8) | |
| H8A | −0.5166 | 0.4524 | −0.0959 | 0.083* | |
| H8B | −0.4837 | 0.5227 | −0.0455 | 0.083* | |
| H8C | −0.3311 | 0.4854 | −0.0748 | 0.083* | |
| C9 | −0.0684 (3) | 0.64705 (18) | 0.04624 (9) | 0.0516 (8) | |
| H9A | 0.0057 | 0.6450 | 0.0780 | 0.077* | |
| H9B | −0.0314 | 0.5947 | 0.0225 | 0.077* | |
| H9C | −0.1818 | 0.6302 | 0.0531 | 0.077* | |
| C10 | −0.6522 (3) | 0.52973 (18) | −0.17576 (9) | 0.0350 (6) | |
| C11 | −0.5797 (3) | 0.46024 (19) | −0.20928 (9) | 0.0354 (6) | |
| C12 | −0.6793 (3) | 0.3774 (2) | −0.23284 (9) | 0.0460 (7) | |
| H12A | −0.6339 | 0.3312 | −0.2560 | 0.055* | |
| C13 | −0.8439 (4) | 0.3630 (2) | −0.22238 (10) | 0.0529 (8) | |
| H13A | −0.9089 | 0.3071 | −0.2382 | 0.063* | |
| C14 | −0.9118 (3) | 0.4313 (2) | −0.18850 (10) | 0.0531 (8) | |
| H14A | −1.0227 | 0.4202 | −0.1813 | 0.064* | |
| C15 | −0.8197 (3) | 0.5162 (2) | −0.16479 (10) | 0.0442 (7) | |
| C16 | −0.3966 (3) | 0.4759 (2) | −0.21860 (9) | 0.0542 (8) | |
| H16A | −0.3738 | 0.5533 | −0.2186 | 0.065* | |
| H16B | −0.3261 | 0.4447 | −0.1895 | 0.065* | |
| C17 | −0.3415 (3) | 0.4290 (2) | −0.26723 (10) | 0.0677 (9) | |
| H17A | −0.2233 | 0.4437 | −0.2685 | 0.102* | |
| H17B | −0.4061 | 0.4613 | −0.2967 | 0.102* | |
| H17C | −0.3599 | 0.3520 | −0.2677 | 0.102* | |
| C18 | −0.8989 (3) | 0.5920 (2) | −0.12862 (10) | 0.0595 (8) | |
| H18A | −0.9946 | 0.5565 | −0.1155 | 0.071* | |
| H18B | −0.8167 | 0.6094 | −0.0994 | 0.071* | |
| C19 | −0.9567 (4) | 0.6936 (2) | −0.15592 (11) | 0.0843 (11) | |
| H19A | −1.0065 | 0.7408 | −0.1324 | 0.126* | |
| H19B | −1.0392 | 0.6764 | −0.1845 | 0.126* | |
| H19C | −0.8616 | 0.7292 | −0.1685 | 0.126* | |
| C20 | 0.1215 (3) | 0.82874 (17) | 0.09135 (9) | 0.0314 (6) | |
| C21 | 0.2917 (3) | 0.79922 (18) | 0.09253 (10) | 0.0366 (6) | |
| C22 | 0.3912 (3) | 0.80093 (19) | 0.14006 (11) | 0.0481 (7) | |
| H22A | 0.5045 | 0.7806 | 0.1417 | 0.058* | |
| C23 | 0.3256 (4) | 0.8321 (2) | 0.18473 (11) | 0.0512 (8) | |
| H23A | 0.3947 | 0.8347 | 0.2160 | 0.061* | |
| C24 | 0.1569 (4) | 0.85952 (19) | 0.18268 (10) | 0.0466 (7) | |
| H24A | 0.1129 | 0.8799 | 0.2130 | 0.056* | |
| C25 | 0.0504 (3) | 0.85759 (18) | 0.13639 (9) | 0.0367 (6) | |
| C26 | 0.3694 (3) | 0.76989 (19) | 0.04370 (9) | 0.0458 (7) | |
| H26A | 0.2924 | 0.7225 | 0.0227 | 0.055* | |
| H26B | 0.4738 | 0.7302 | 0.0530 | 0.055* | |
| C27 | 0.4070 (3) | 0.86848 (19) | 0.01206 (10) | 0.0595 (8) | |
| H27A | 0.4547 | 0.8456 | −0.0185 | 0.089* | |
| H27B | 0.4862 | 0.9146 | 0.0323 | 0.089* | |
| H27C | 0.3040 | 0.9077 | 0.0024 | 0.089* | |
| C28 | −0.1336 (3) | 0.8865 (2) | 0.13489 (9) | 0.0482 (7) | |
| H28A | −0.1670 | 0.8812 | 0.1696 | 0.058* | |
| H28B | −0.1999 | 0.8342 | 0.1134 | 0.058* | |
| C29 | −0.1747 (4) | 0.9995 (2) | 0.11419 (10) | 0.0685 (9) | |
| H29A | −0.2938 | 1.0131 | 0.1142 | 0.103* | |
| H29B | −0.1447 | 1.0051 | 0.0795 | 0.103* | |
| H29C | −0.1117 | 1.0520 | 0.1357 | 0.103* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0406 (14) | 0.0347 (13) | 0.0358 (13) | −0.0017 (10) | −0.0047 (11) | −0.0046 (10) |
| N2 | 0.0406 (14) | 0.0297 (12) | 0.0312 (12) | −0.0026 (10) | −0.0010 (10) | −0.0022 (10) |
| N3 | 0.0371 (13) | 0.0301 (12) | 0.0309 (12) | −0.0024 (10) | 0.0030 (10) | −0.0034 (10) |
| C1 | 0.0411 (17) | 0.0318 (15) | 0.0320 (15) | 0.0018 (12) | −0.0001 (13) | −0.0012 (12) |
| C2 | 0.0369 (16) | 0.0278 (15) | 0.0293 (15) | 0.0002 (12) | 0.0020 (12) | −0.0022 (12) |
| C3 | 0.0455 (18) | 0.0329 (15) | 0.0340 (15) | −0.0013 (13) | −0.0009 (13) | −0.0007 (13) |
| C4 | 0.0536 (18) | 0.0265 (15) | 0.0396 (16) | −0.0012 (13) | 0.0026 (14) | 0.0026 (12) |
| C5 | 0.0408 (16) | 0.0291 (15) | 0.0337 (15) | −0.0042 (12) | 0.0029 (13) | −0.0028 (12) |
| C6 | 0.0357 (16) | 0.0269 (14) | 0.0337 (15) | −0.0014 (12) | 0.0044 (12) | −0.0011 (12) |
| C7 | 0.0369 (16) | 0.0295 (15) | 0.0318 (15) | −0.0001 (12) | 0.0039 (13) | 0.0007 (12) |
| C8 | 0.072 (2) | 0.0370 (16) | 0.0509 (18) | −0.0122 (15) | −0.0163 (16) | 0.0078 (14) |
| C9 | 0.064 (2) | 0.0381 (16) | 0.0479 (17) | −0.0114 (14) | −0.0170 (15) | 0.0096 (14) |
| C10 | 0.0386 (17) | 0.0332 (15) | 0.0311 (15) | −0.0026 (13) | −0.0057 (13) | 0.0008 (12) |
| C11 | 0.0316 (16) | 0.0389 (16) | 0.0344 (15) | −0.0021 (13) | −0.0021 (13) | −0.0005 (13) |
| C12 | 0.053 (2) | 0.0466 (17) | 0.0372 (16) | −0.0019 (15) | −0.0017 (14) | −0.0079 (13) |
| C13 | 0.052 (2) | 0.055 (2) | 0.0492 (18) | −0.0161 (16) | −0.0035 (16) | −0.0064 (15) |
| C14 | 0.0335 (18) | 0.071 (2) | 0.0537 (19) | −0.0119 (16) | 0.0004 (15) | −0.0030 (17) |
| C15 | 0.0375 (18) | 0.0504 (18) | 0.0439 (17) | 0.0016 (14) | 0.0000 (14) | −0.0038 (14) |
| C16 | 0.0444 (19) | 0.071 (2) | 0.0471 (18) | 0.0011 (15) | 0.0047 (14) | −0.0214 (15) |
| C17 | 0.063 (2) | 0.087 (2) | 0.054 (2) | 0.0008 (18) | 0.0091 (16) | −0.0043 (17) |
| C18 | 0.049 (2) | 0.061 (2) | 0.070 (2) | 0.0079 (16) | 0.0126 (16) | 0.0072 (17) |
| C19 | 0.110 (3) | 0.054 (2) | 0.096 (3) | 0.007 (2) | 0.042 (2) | 0.006 (2) |
| C20 | 0.0345 (16) | 0.0256 (14) | 0.0327 (15) | −0.0062 (12) | −0.0028 (13) | 0.0009 (11) |
| C21 | 0.0391 (17) | 0.0291 (15) | 0.0410 (16) | −0.0041 (12) | 0.0016 (14) | 0.0062 (12) |
| C22 | 0.0389 (18) | 0.0431 (17) | 0.060 (2) | −0.0043 (13) | −0.0057 (16) | 0.0111 (15) |
| C23 | 0.056 (2) | 0.0497 (18) | 0.0442 (19) | −0.0110 (16) | −0.0113 (16) | 0.0052 (15) |
| C24 | 0.062 (2) | 0.0439 (17) | 0.0340 (16) | −0.0109 (15) | 0.0035 (15) | −0.0012 (13) |
| C25 | 0.0407 (17) | 0.0341 (15) | 0.0351 (16) | −0.0057 (13) | 0.0038 (14) | 0.0008 (12) |
| C26 | 0.0416 (18) | 0.0397 (16) | 0.0567 (18) | 0.0045 (13) | 0.0072 (14) | 0.0047 (14) |
| C27 | 0.070 (2) | 0.0473 (18) | 0.066 (2) | 0.0092 (15) | 0.0282 (17) | 0.0120 (15) |
| C28 | 0.051 (2) | 0.0554 (19) | 0.0396 (16) | −0.0051 (15) | 0.0118 (14) | −0.0067 (14) |
| C29 | 0.064 (2) | 0.076 (2) | 0.068 (2) | 0.0192 (17) | 0.0232 (17) | 0.0168 (18) |
Geometric parameters (Å, °)
| N1—C1 | 1.272 (3) | C16—H16A | 0.9700 |
| N1—C10 | 1.431 (3) | C16—H16B | 0.9700 |
| N2—C2 | 1.333 (2) | C17—H17A | 0.9600 |
| N2—C6 | 1.348 (2) | C17—H17B | 0.9600 |
| N3—C7 | 1.275 (2) | C17—H17C | 0.9600 |
| N3—C20 | 1.424 (3) | C18—C19 | 1.486 (3) |
| C1—C2 | 1.493 (3) | C18—H18A | 0.9700 |
| C1—C8 | 1.495 (3) | C18—H18B | 0.9700 |
| C2—C3 | 1.385 (3) | C19—H19A | 0.9600 |
| C3—C4 | 1.376 (3) | C19—H19B | 0.9600 |
| C3—H3B | 0.9300 | C19—H19C | 0.9600 |
| C4—C5 | 1.383 (3) | C20—C21 | 1.397 (3) |
| C4—H4A | 0.9300 | C20—C25 | 1.400 (3) |
| C5—C6 | 1.379 (3) | C21—C22 | 1.393 (3) |
| C5—H5A | 0.9300 | C21—C26 | 1.514 (3) |
| C6—C7 | 1.501 (3) | C22—C23 | 1.378 (3) |
| C7—C9 | 1.501 (3) | C22—H22A | 0.9300 |
| C8—H8A | 0.9600 | C23—C24 | 1.376 (3) |
| C8—H8B | 0.9600 | C23—H23A | 0.9300 |
| C8—H8C | 0.9600 | C24—C25 | 1.394 (3) |
| C9—H9A | 0.9600 | C24—H24A | 0.9300 |
| C9—H9B | 0.9600 | C25—C28 | 1.500 (3) |
| C9—H9C | 0.9600 | C26—C27 | 1.515 (3) |
| C10—C11 | 1.390 (3) | C26—H26A | 0.9700 |
| C10—C15 | 1.399 (3) | C26—H26B | 0.9700 |
| C11—C12 | 1.391 (3) | C27—H27A | 0.9600 |
| C11—C16 | 1.511 (3) | C27—H27B | 0.9600 |
| C12—C13 | 1.375 (3) | C27—H27C | 0.9600 |
| C12—H12A | 0.9300 | C28—C29 | 1.515 (3) |
| C13—C14 | 1.370 (3) | C28—H28A | 0.9700 |
| C13—H13A | 0.9300 | C28—H28B | 0.9700 |
| C14—C15 | 1.383 (3) | C29—H29A | 0.9600 |
| C14—H14A | 0.9300 | C29—H29B | 0.9600 |
| C15—C18 | 1.511 (3) | C29—H29C | 0.9600 |
| C16—C17 | 1.498 (3) | ||
| C1—N1—C10 | 120.5 (2) | C16—C17—H17A | 109.5 |
| C2—N2—C6 | 117.7 (2) | C16—C17—H17B | 109.5 |
| C7—N3—C20 | 121.0 (2) | H17A—C17—H17B | 109.5 |
| N1—C1—C2 | 117.5 (2) | C16—C17—H17C | 109.5 |
| N1—C1—C8 | 125.1 (2) | H17A—C17—H17C | 109.5 |
| C2—C1—C8 | 117.4 (2) | H17B—C17—H17C | 109.5 |
| N2—C2—C3 | 122.9 (2) | C19—C18—C15 | 110.6 (2) |
| N2—C2—C1 | 116.1 (2) | C19—C18—H18A | 109.5 |
| C3—C2—C1 | 121.0 (2) | C15—C18—H18A | 109.5 |
| C4—C3—C2 | 119.0 (2) | C19—C18—H18B | 109.5 |
| C4—C3—H3B | 120.5 | C15—C18—H18B | 109.5 |
| C2—C3—H3B | 120.5 | H18A—C18—H18B | 108.1 |
| C3—C4—C5 | 118.8 (2) | C18—C19—H19A | 109.5 |
| C3—C4—H4A | 120.6 | C18—C19—H19B | 109.5 |
| C5—C4—H4A | 120.6 | H19A—C19—H19B | 109.5 |
| C6—C5—C4 | 118.9 (2) | C18—C19—H19C | 109.5 |
| C6—C5—H5A | 120.6 | H19A—C19—H19C | 109.5 |
| C4—C5—H5A | 120.6 | H19B—C19—H19C | 109.5 |
| N2—C6—C5 | 122.7 (2) | C21—C20—C25 | 121.7 (2) |
| N2—C6—C7 | 115.6 (2) | C21—C20—N3 | 119.4 (2) |
| C5—C6—C7 | 121.7 (2) | C25—C20—N3 | 118.7 (2) |
| N3—C7—C6 | 117.1 (2) | C22—C21—C20 | 118.0 (2) |
| N3—C7—C9 | 125.3 (2) | C22—C21—C26 | 120.3 (2) |
| C6—C7—C9 | 117.6 (2) | C20—C21—C26 | 121.7 (2) |
| C1—C8—H8A | 109.5 | C23—C22—C21 | 121.5 (3) |
| C1—C8—H8B | 109.5 | C23—C22—H22A | 119.3 |
| H8A—C8—H8B | 109.5 | C21—C22—H22A | 119.3 |
| C1—C8—H8C | 109.5 | C24—C23—C22 | 119.4 (3) |
| H8A—C8—H8C | 109.5 | C24—C23—H23A | 120.3 |
| H8B—C8—H8C | 109.5 | C22—C23—H23A | 120.3 |
| C7—C9—H9A | 109.5 | C23—C24—C25 | 121.7 (3) |
| C7—C9—H9B | 109.5 | C23—C24—H24A | 119.1 |
| H9A—C9—H9B | 109.5 | C25—C24—H24A | 119.1 |
| C7—C9—H9C | 109.5 | C24—C25—C20 | 117.7 (2) |
| H9A—C9—H9C | 109.5 | C24—C25—C28 | 121.1 (2) |
| H9B—C9—H9C | 109.5 | C20—C25—C28 | 121.2 (2) |
| C11—C10—C15 | 121.4 (2) | C21—C26—C27 | 112.7 (2) |
| C11—C10—N1 | 118.6 (2) | C21—C26—H26A | 109.0 |
| C15—C10—N1 | 119.9 (2) | C27—C26—H26A | 109.0 |
| C10—C11—C12 | 118.2 (2) | C21—C26—H26B | 109.0 |
| C10—C11—C16 | 119.5 (2) | C27—C26—H26B | 109.0 |
| C12—C11—C16 | 122.2 (2) | H26A—C26—H26B | 107.8 |
| C13—C12—C11 | 121.0 (3) | C26—C27—H27A | 109.5 |
| C13—C12—H12A | 119.5 | C26—C27—H27B | 109.5 |
| C11—C12—H12A | 119.5 | H27A—C27—H27B | 109.5 |
| C14—C13—C12 | 119.8 (3) | C26—C27—H27C | 109.5 |
| C14—C13—H13A | 120.1 | H27A—C27—H27C | 109.5 |
| C12—C13—H13A | 120.1 | H27B—C27—H27C | 109.5 |
| C13—C14—C15 | 121.7 (3) | C25—C28—C29 | 113.5 (2) |
| C13—C14—H14A | 119.2 | C25—C28—H28A | 108.9 |
| C15—C14—H14A | 119.2 | C29—C28—H28A | 108.9 |
| C14—C15—C10 | 117.9 (2) | C25—C28—H28B | 108.9 |
| C14—C15—C18 | 120.7 (3) | C29—C28—H28B | 108.9 |
| C10—C15—C18 | 121.4 (2) | H28A—C28—H28B | 107.7 |
| C17—C16—C11 | 117.4 (2) | C28—C29—H29A | 109.5 |
| C17—C16—H16A | 108.0 | C28—C29—H29B | 109.5 |
| C11—C16—H16A | 108.0 | H29A—C29—H29B | 109.5 |
| C17—C16—H16B | 108.0 | C28—C29—H29C | 109.5 |
| C11—C16—H16B | 108.0 | H29A—C29—H29C | 109.5 |
| H16A—C16—H16B | 107.2 | H29B—C29—H29C | 109.5 |
| C10—N1—C1—C2 | 179.8 (2) | C12—C13—C14—C15 | 1.0 (4) |
| C10—N1—C1—C8 | 0.3 (4) | C13—C14—C15—C10 | −1.0 (4) |
| C6—N2—C2—C3 | 0.2 (3) | C13—C14—C15—C18 | 178.4 (2) |
| C6—N2—C2—C1 | −179.9 (2) | C11—C10—C15—C14 | −0.2 (4) |
| N1—C1—C2—N2 | −177.1 (2) | N1—C10—C15—C14 | 177.2 (2) |
| C8—C1—C2—N2 | 2.4 (3) | C11—C10—C15—C18 | −179.6 (2) |
| N1—C1—C2—C3 | 2.8 (3) | N1—C10—C15—C18 | −2.3 (4) |
| C8—C1—C2—C3 | −177.7 (2) | C10—C11—C16—C17 | −158.5 (2) |
| N2—C2—C3—C4 | 0.0 (4) | C12—C11—C16—C17 | 22.1 (4) |
| C1—C2—C3—C4 | −179.9 (2) | C14—C15—C18—C19 | −98.9 (3) |
| C2—C3—C4—C5 | 0.0 (4) | C10—C15—C18—C19 | 80.6 (3) |
| C3—C4—C5—C6 | −0.1 (3) | C7—N3—C20—C21 | 91.8 (3) |
| C2—N2—C6—C5 | −0.3 (3) | C7—N3—C20—C25 | −93.3 (3) |
| C2—N2—C6—C7 | 179.4 (2) | C25—C20—C21—C22 | −1.1 (3) |
| C4—C5—C6—N2 | 0.3 (3) | N3—C20—C21—C22 | 173.6 (2) |
| C4—C5—C6—C7 | −179.4 (2) | C25—C20—C21—C26 | −178.9 (2) |
| C20—N3—C7—C6 | 177.2 (2) | N3—C20—C21—C26 | −4.2 (3) |
| C20—N3—C7—C9 | −1.0 (4) | C20—C21—C22—C23 | −0.9 (4) |
| N2—C6—C7—N3 | −178.6 (2) | C26—C21—C22—C23 | 177.0 (2) |
| C5—C6—C7—N3 | 1.1 (3) | C21—C22—C23—C24 | 1.7 (4) |
| N2—C6—C7—C9 | −0.2 (3) | C22—C23—C24—C25 | −0.6 (4) |
| C5—C6—C7—C9 | 179.5 (2) | C23—C24—C25—C20 | −1.3 (4) |
| C1—N1—C10—C11 | −91.4 (3) | C23—C24—C25—C28 | 179.4 (2) |
| C1—N1—C10—C15 | 91.2 (3) | C21—C20—C25—C24 | 2.1 (3) |
| C15—C10—C11—C12 | 1.4 (3) | N3—C20—C25—C24 | −172.6 (2) |
| N1—C10—C11—C12 | −175.95 (19) | C21—C20—C25—C28 | −178.5 (2) |
| C15—C10—C11—C16 | −178.0 (2) | N3—C20—C25—C28 | 6.8 (3) |
| N1—C10—C11—C16 | 4.6 (3) | C22—C21—C26—C27 | −100.8 (3) |
| C10—C11—C12—C13 | −1.5 (4) | C20—C21—C26—C27 | 77.0 (3) |
| C16—C11—C12—C13 | 177.9 (2) | C24—C25—C28—C29 | 102.6 (3) |
| C11—C12—C13—C14 | 0.3 (4) | C20—C25—C28—C29 | −76.7 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: BH2205).
References
- Bruker (1998). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Fan, R.-Q., Zhu, D.-S., Mu, Y., Li, G.-H., Yang, Y.-L., Su, Q. & Feng, S.-H. (2004). Eur. J. Inorg. Chem. pp. 4891–4897.
- Huang, Y.-B., Ma, X.-L., Zheng, S.-N., Chen, J.-X. & Wei, C.-X. (2006). Acta Cryst. E62, o3044–o3045.
- Mentes, A., Fawcett, J. & Kemmitt, R. D. W. (2001). Acta Cryst. E57, o424–o425.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tang, C. W. & VanSlyke, S. A. (1987). Appl. Phys. Lett.51, 913–915.
- Wang, S. (2001). Coord. Chem. Rev.215, 79–98.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808036842/bh2205sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808036842/bh2205Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report