Abstract
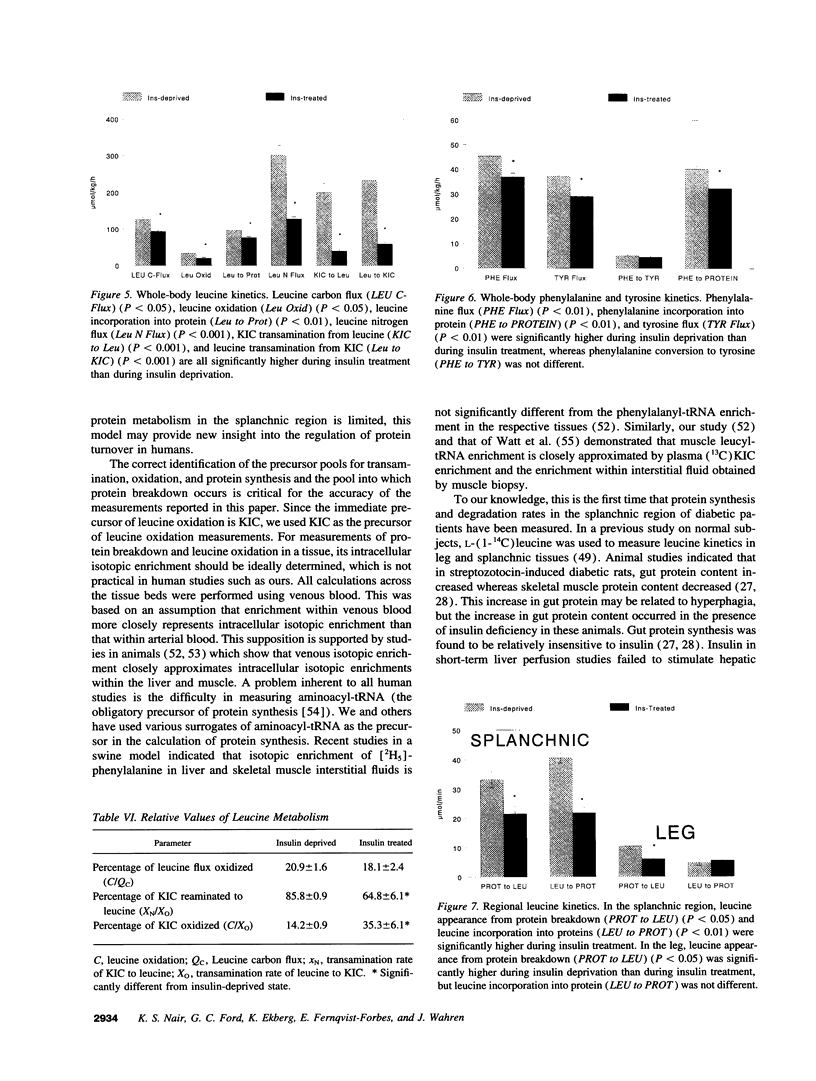
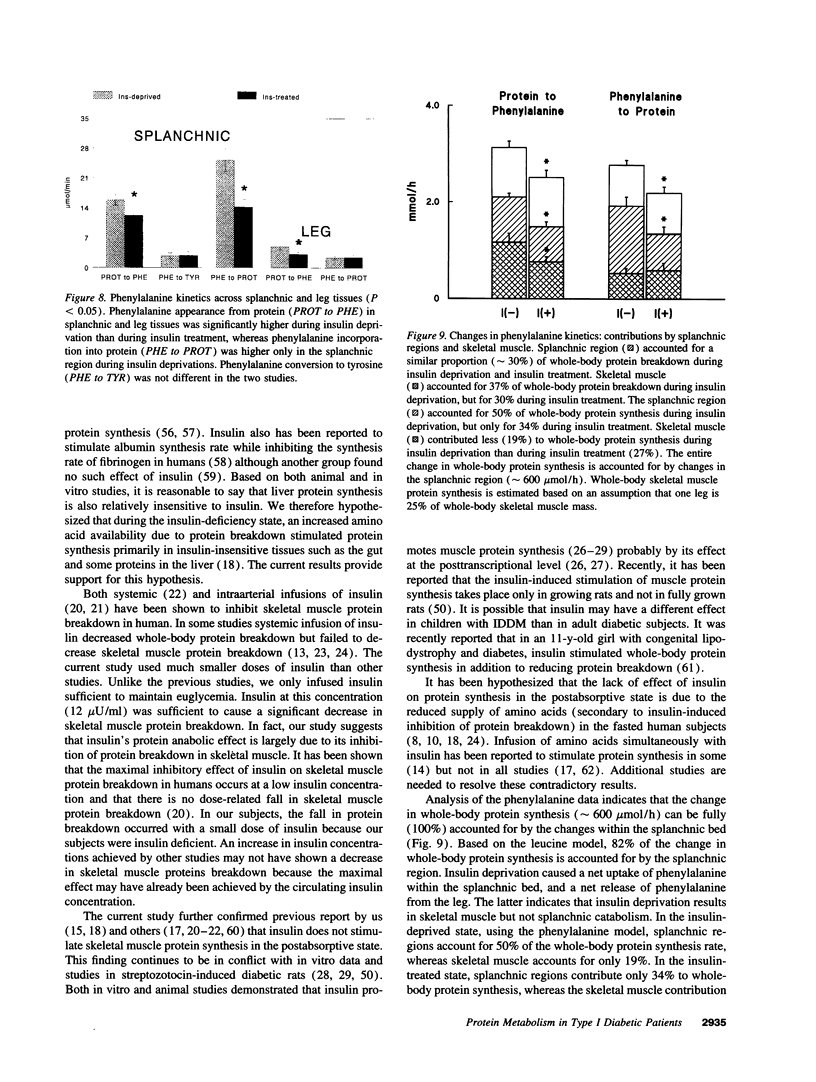
To elucidate the mechanism of insulin's anticatabolic effect in humans, protein dynamics were evaluated in the whole-body, splanchnic, and leg tissues in six C-peptide-negative type I diabetic male patients in the insulin-deprived and insulin-treated states using two separate amino acid models (leucine and phenylalanine). L-(1-13C,15N)leucine, L-(ring-2H5)phenylalanine, and L-(ring-2H2) tyrosine were infused intravenously, and isotopic enrichments of [1-13C,15N]-leucine, (13C)leucine, (13C)ketoisocaproate, (2H5)phenylalanine, [2H4]tyrosine, (2H2)tyrosine, and 13CO2 were measured in arterial, hepatic vein, and femoral vein samples. Whole-body leucine flux, phenylalanine flux, and tyrosine flux were decreased (< 0.01) by insulin treatment, indicating an inhibition of protein breakdown. Moreover, insulin decreased (< 0.05) the rates of leucine oxidation and leucine transamination (P < 0.01), but the percent rate of ketoisocaproate oxidation was increased by insulin (P < 0.01). Insulin also reduced (< 0.01) whole-body protein synthesis estimated from both the leucine model (nonoxidative leucine disposal) and the phenylalanine model (disposal of phenylalanine not accounted by its conversion to tyrosine). Regional studies demonstrated that changes in whole body protein breakdown are accounted for by changes in both splanchnic and leg tissues. The changes in whole-body protein synthesis were not associated with changes in skeletal muscle (leg) protein synthesis but could be accounted for by the splanchnic region. We conclude that though insulin decreases whole-body protein breakdown in patients with type I diabetes by inhibition of protein breakdown in splanchnic and leg tissues, it selectively decreases protein synthesis in splanchnic tissues, which accounted for the observed decrease in whole-body protein synthesis. Insulin also augmented anabolism by decreasing leucine transamination.
Full text
PDF

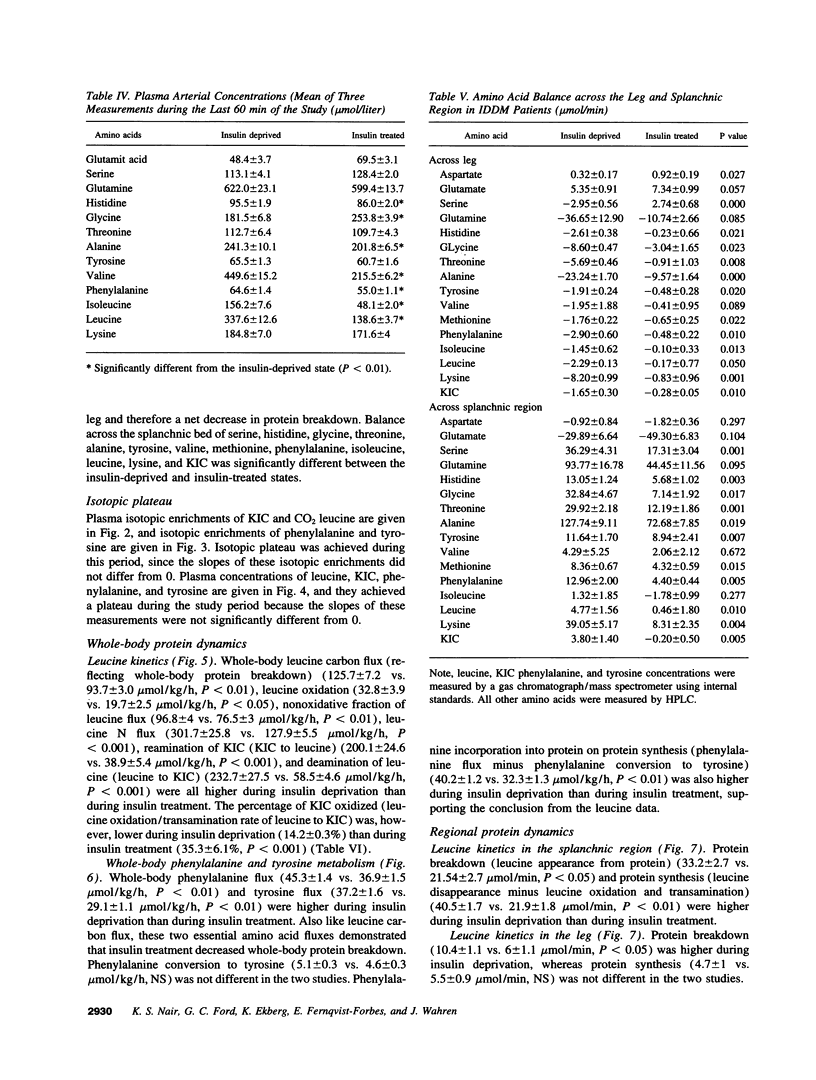
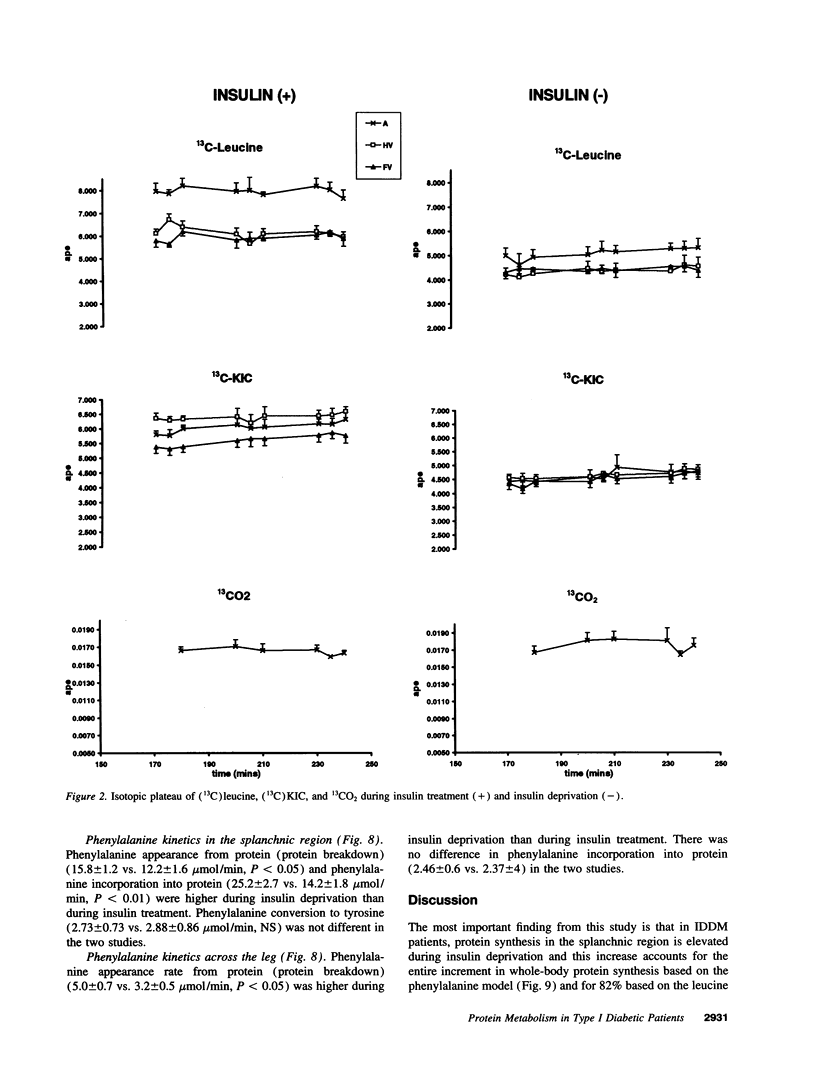
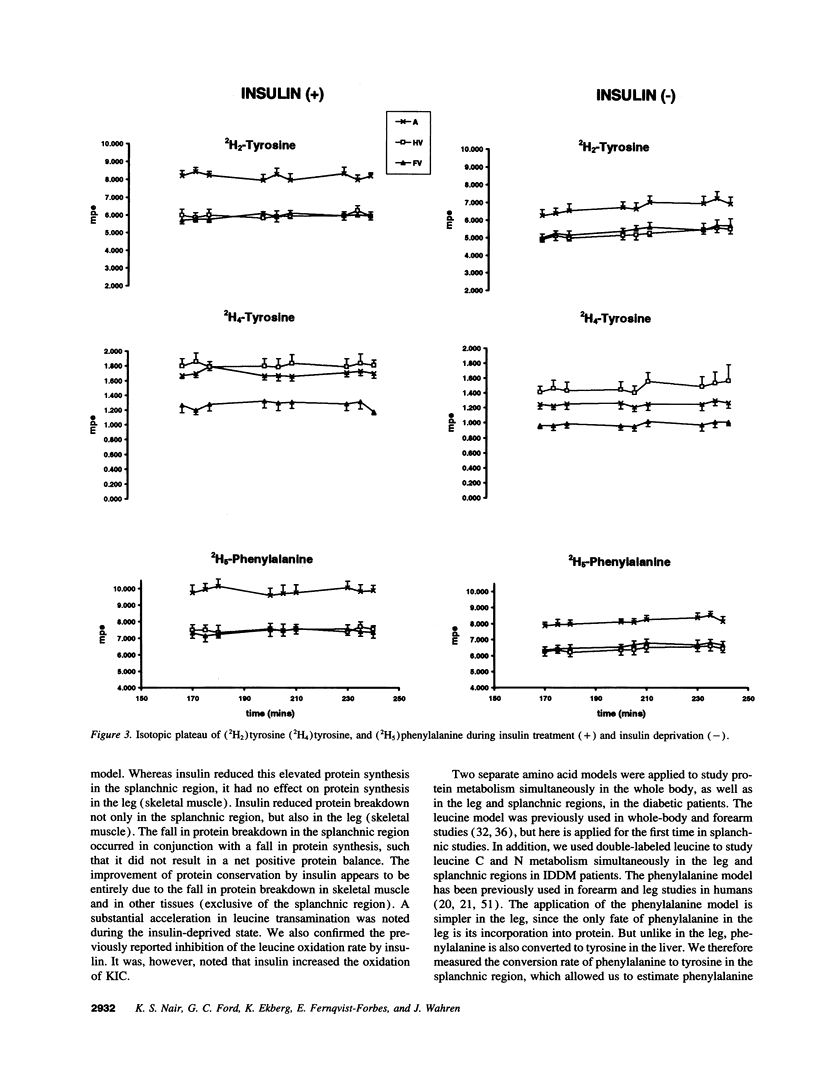
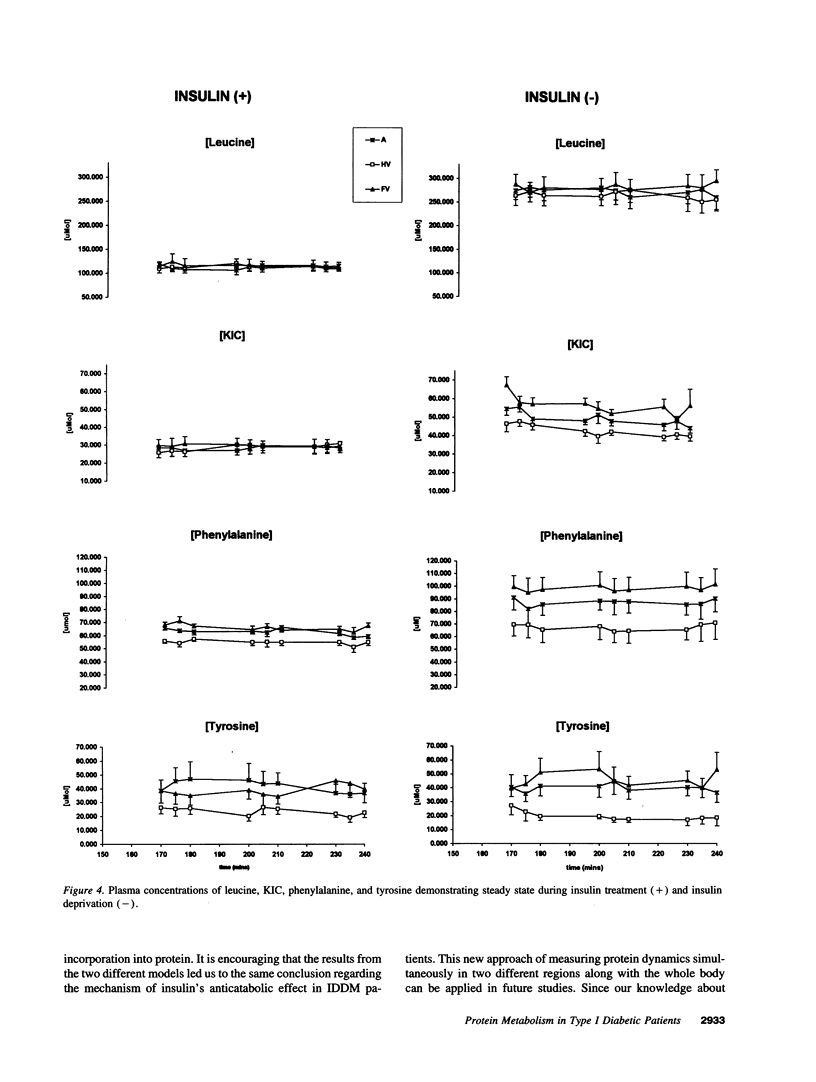


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arfvidsson B., Zachrisson H., Möller-Loswick A. C., Hyltander A., Sandström R., Lundholm K. Effect of systemic hyperinsulinemia on amino acid flux across human legs in postabsorptive state. Am J Physiol. 1991 Jan;260(1 Pt 1):E46–E52. doi: 10.1152/ajpendo.1991.260.1.E46. [DOI] [PubMed] [Google Scholar]
- Arnqvist H., Olsson P. O., von Schenck H. Free and total insulin as determined after precipitation with polyethylene glycol: analytical characteristics and effects of sample handling and storage. Clin Chem. 1987 Jan;33(1):93–96. [PubMed] [Google Scholar]
- Atchley D. W., Loeb R. F., Richards D. W., Benedict E. M., Driscoll M. E. ON DIABETIC ACIDOSIS: A Detailed Study of Electrolyte Balances Following the Withdrawal and Reestablishment of Insulin Therapy. J Clin Invest. 1933 Mar;12(2):297–326. doi: 10.1172/JCI100504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie A. G., Garlick P. J. Attenuated responses of muscle protein synthesis to fasting and insulin in adult female rats. Am J Physiol. 1992 Jan;262(1 Pt 1):E1–E5. doi: 10.1152/ajpendo.1992.262.1.E1. [DOI] [PubMed] [Google Scholar]
- Bang P., Eriksson U., Sara V., Wivall I. L., Hall K. Comparison of acid ethanol extraction and acid gel filtration prior to IGF-I and IGF-II radioimmunoassays: improvement of determinations in acid ethanol extracts by the use of truncated IGF-I as radioligand. Acta Endocrinol (Copenh) 1991 Jun;124(6):620–629. doi: 10.1530/acta.0.1240620. [DOI] [PubMed] [Google Scholar]
- Baumann G. Growth hormone binding proteins and various forms of growth hormone: implications for measurements. Acta Paediatr Scand Suppl. 1990;370:72–81. doi: 10.1111/j.1651-2227.1990.tb11677.x. [DOI] [PubMed] [Google Scholar]
- Baumann P. Q., Stirewalt W. S., O'Rourke B. D., Howard D., Nair K. S. Precursor pools of protein synthesis: a stable isotope study in a swine model. Am J Physiol. 1994 Aug;267(2 Pt 1):E203–E209. doi: 10.1152/ajpendo.1994.267.2.E203. [DOI] [PubMed] [Google Scholar]
- Block K. P., Heywood B. W., Buse M. G., Harper A. E. Activation of rat liver branched-chain 2-oxo acid dehydrogenase in vivo by glucagon and adrenaline. Biochem J. 1985 Dec 1;232(2):593–597. doi: 10.1042/bj2320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland K. C., Nair K. S., Kaplowitz P. B., Robbins D. C., Calles-Escandon J. Discordant metabolic actions of insulin in extreme lipodystrophy of childhood. J Clin Endocrinol Metab. 1993 Nov;77(5):1240–1245. doi: 10.1210/jcem.77.5.8077317. [DOI] [PubMed] [Google Scholar]
- De Feo P., Gaisano M. G., Haymond M. W. Differential effects of insulin deficiency on albumin and fibrinogen synthesis in humans. J Clin Invest. 1991 Sep;88(3):833–840. doi: 10.1172/JCI115384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denne S. C., Liechty E. A., Liu Y. M., Brechtel G., Baron A. D. Proteolysis in skeletal muscle and whole body in response to euglycemic hyperinsulinemia in normal adults. Am J Physiol. 1991 Dec;261(6 Pt 1):E809–E814. doi: 10.1152/ajpendo.1991.261.6.E809. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Sherwin R., Palaiologos G. Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med. 1977 Apr;137(4):507–513. [PubMed] [Google Scholar]
- Flakoll P. J., Kulaylat M., Frexes-Steed M., Hourani H., Brown L. L., Hill J. O., Abumrad N. N. Amino acids augment insulin's suppression of whole body proteolysis. Am J Physiol. 1989 Dec;257(6 Pt 1):E839–E847. doi: 10.1152/ajpendo.1989.257.6.E839. [DOI] [PubMed] [Google Scholar]
- Ford G. C., Cheng K. N., Halliday D. Analysis of (1-13C)leucine and (13C)KIC in plasma by capillary gas chromatography/mass spectrometry in protein turnover studies. Biomed Mass Spectrom. 1985 Aug;12(8):432–436. doi: 10.1002/bms.1200120814. [DOI] [PubMed] [Google Scholar]
- Gelfand R. A., Barrett E. J. Effect of physiologic hyperinsulinemia on skeletal muscle protein synthesis and breakdown in man. J Clin Invest. 1987 Jul;80(1):1–6. doi: 10.1172/JCI113033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand R. A., Glickman M. G., Castellino P., Louard R. J., DeFronzo R. A. Measurement of L-[1-14C]leucine kinetics in splanchnic and leg tissues in humans. Effect of amino acid infusion. Diabetes. 1988 Oct;37(10):1365–1372. doi: 10.2337/diab.37.10.1365. [DOI] [PubMed] [Google Scholar]
- Hagenfeldt L., Eriksson S., Wahren J. Influence of leucine on arterial concentrations and regional exchange of amino acids in healthy subjects. Clin Sci (Lond) 1980 Sep;59(3):173–181. doi: 10.1042/cs0590173. [DOI] [PubMed] [Google Scholar]
- Heslin M. J., Newman E., Wolf R. F., Pisters P. W., Brennan M. F. Effect of hyperinsulinemia on whole body and skeletal muscle leucine carbon kinetics in humans. Am J Physiol. 1992 Jun;262(6 Pt 1):E911–E918. doi: 10.1152/ajpendo.1992.262.6.E911. [DOI] [PubMed] [Google Scholar]
- Hjemdahl P., Daleskog M., Kahan T. Determination of plasma catecholamines by high performance liquid chromatography with electrochemical detection: comparison with a radioenzymatic method. Life Sci. 1979 Jul 9;25(2):131–138. doi: 10.1016/0024-3205(79)90384-9. [DOI] [PubMed] [Google Scholar]
- Jefferson L. S., Li J. B., Rannels S. R. Regulation by insulin of amino acid release and protein turnover in the perfused rat hemicorpus. J Biol Chem. 1977 Feb 25;252(4):1476–1483. [PubMed] [Google Scholar]
- Jefferson L. S. Lilly Lecture 1979: role of insulin in the regulation of protein synthesis. Diabetes. 1980 Jun;29(6):487–496. doi: 10.2337/diab.29.6.487. [DOI] [PubMed] [Google Scholar]
- Layman D. K., Wolfe R. R. Sample site selection for tracer studies applying a unidirectional circulatory approach. Am J Physiol. 1987 Aug;253(2 Pt 1):E173–E178. doi: 10.1152/ajpendo.1987.253.2.E173. [DOI] [PubMed] [Google Scholar]
- Louard R. J., Fryburg D. A., Gelfand R. A., Barrett E. J. Insulin sensitivity of protein and glucose metabolism in human forearm skeletal muscle. J Clin Invest. 1992 Dec;90(6):2348–2354. doi: 10.1172/JCI116124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzi L., Castellino P., Simonson D. C., Petrides A. S., DeFronzo R. A. Leucine metabolism in IDDM. Role of insulin and substrate availability. Diabetes. 1990 Jan;39(1):38–48. doi: 10.2337/diacare.39.1.38. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Bier D. M., Rennie M. J., Edwards R. H., Halliday D., Millward D. J., Clugston G. A. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981 Dec 4;214(4525):1129–1131. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Essén P., Thorell A., Calder A. G., Anderson S. E., Ljungqvist O., Sandgren A., Grant I., Tjäder I., Ballmer P. E. Response of protein synthesis in human skeletal muscle to insulin: an investigation with L-[2H5]phenylalanine. Am J Physiol. 1994 Jul;267(1 Pt 1):E102–E108. doi: 10.1152/ajpendo.1994.267.1.E102. [DOI] [PubMed] [Google Scholar]
- McNurlan M. A., Garlick P. J. Protein synthesis in liver and small intestine in protein deprivation and diabetes. Am J Physiol. 1981 Sep;241(3):E238–E245. doi: 10.1152/ajpendo.1981.241.3.E238. [DOI] [PubMed] [Google Scholar]
- Miles J., Glasscock R., Aikens J., Gerich J., Haymond M. A microfluorometric method for the determination of free fatty acids in plasma. J Lipid Res. 1983 Jan;24(1):96–99. [PubMed] [Google Scholar]
- Mortimore G. E., Mondon C. E. Inhibition by insulin of valine turnover in liver. Evidence for a general control of proteolysis. J Biol Chem. 1970 May 10;245(9):2375–2383. [PubMed] [Google Scholar]
- Nair K. S., Ford G. C., Halliday D. Effect of intravenous insulin treatment on in vivo whole body leucine kinetics and oxygen consumption in insulin-deprived type I diabetic patients. Metabolism. 1987 May;36(5):491–495. doi: 10.1016/0026-0495(87)90049-7. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Garrow J. S., Ford C., Mahler R. F., Halliday D. Effect of poor diabetic control and obesity on whole body protein metabolism in man. Diabetologia. 1983 Nov;25(5):400–403. doi: 10.1007/BF00282518. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Halliday D., Garrow J. S. Increased energy expenditure in poorly controlled Type 1 (insulin-dependent) diabetic patients. Diabetologia. 1984 Jul;27(1):13–16. doi: 10.1007/BF00253494. [DOI] [PubMed] [Google Scholar]
- Nair K. S., Schwartz R. G., Welle S. Leucine as a regulator of whole body and skeletal muscle protein metabolism in humans. Am J Physiol. 1992 Nov;263(5 Pt 1):E928–E934. doi: 10.1152/ajpendo.1992.263.5.E928. [DOI] [PubMed] [Google Scholar]
- Pacy P. J., Nair K. S., Ford C., Halliday D. Failure of insulin infusion to stimulate fractional muscle protein synthesis in type I diabetic patients. Anabolic effect of insulin and decreased proteolysis. Diabetes. 1989 May;38(5):618–624. doi: 10.2337/diab.38.5.618. [DOI] [PubMed] [Google Scholar]
- Pacy P. J., Read M., Halliday D. Influence of insulin on albumin and non-albumin protein fractional synthetic rates in post-absorptive type I diabetic patients. Eur J Clin Nutr. 1990 May;44(5):343–349. [PubMed] [Google Scholar]
- Pain V. M., Albertse E. C., Garlick P. J. Protein metabolism in skeletal muscle, diaphragm, and heart of diabetic rats. Am J Physiol. 1983 Dec;245(6):E604–E610. doi: 10.1152/ajpendo.1983.245.6.E604. [DOI] [PubMed] [Google Scholar]
- Pain V. M., Garlick P. J. Effect of streptozotocin diabetes and insulin treatment on the rate of protein synthesis in tissues of the rat in vivo. J Biol Chem. 1974 Jul 25;249(14):4510–4514. [PubMed] [Google Scholar]
- Proud C. G. Translation. Turned on by insulin. Nature. 1994 Oct 27;371(6500):747–748. doi: 10.1038/371747a0. [DOI] [PubMed] [Google Scholar]
- Póvoa G., Roovete A., Hall K. Cross-reaction of serum somatomedin-binding protein in a radioimmunoassay developed for somatomedin-binding protein isolated from human amniotic fluid. Acta Endocrinol (Copenh) 1984 Dec;107(4):563–570. doi: 10.1530/acta.0.1070563. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Beaufrere B., Koziet J., Desjeux J. F., Bier D. M., Young V. R., Lestradet H. Whole body de novo amino acid synthesis in type I (insulin-dependent) diabetes studied with stable isotope-labeled leucine, alanine, and glycine. Diabetes. 1985 Jan;34(1):67–73. doi: 10.2337/diab.34.1.67. [DOI] [PubMed] [Google Scholar]
- Schneible P. A., Airhart J., Low R. B. Differential compartmentation of leucine for oxidation and for protein synthesis in cultured skeletal muscle. J Biol Chem. 1981 May 25;256(10):4888–4894. [PubMed] [Google Scholar]
- Schwenk W. F., Berg P. J., Beaufrere B., Miles J. M., Haymond M. W. Use of t-butyldimethylsilylation in the gas chromatographic/mass spectrometric analysis of physiologic compounds found in plasma using electron-impact ionization. Anal Biochem. 1984 Aug 15;141(1):101–109. doi: 10.1016/0003-2697(84)90431-7. [DOI] [PubMed] [Google Scholar]
- Tessari P., Nosadini R., Trevisan R., De Kreutzenberg S. V., Inchiostro S., Duner E., Biolo G., Marescotti M. C., Tiengo A., Crepaldi G. Defective suppression by insulin of leucine-carbon appearance and oxidation in type 1, insulin-dependent diabetes mellitus. Evidence for insulin resistance involving glucose and amino acid metabolism. J Clin Invest. 1986 Jun;77(6):1797–1804. doi: 10.1172/JCI112504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G. N., Pacy P. J., Merritt H., Ford G. C., Read M. A., Cheng K. N., Halliday D. Rapid measurement of whole body and forearm protein turnover using a [2H5]phenylalanine model. Am J Physiol. 1989 May;256(5 Pt 1):E631–E639. doi: 10.1152/ajpendo.1989.256.5.E631. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C. H., Soler N. G., James H., Harvey T. C., Thomas B. J., Fremlin J. H., Fitzgerald M. G., Malins J. M. Studies in whole body potassium and whole body nitrogen in newly diagnosed diabetics. Q J Med. 1976 Apr;45(178):295–301. [PubMed] [Google Scholar]
- Watt P. W., Lindsay Y., Scrimgeour C. M., Chien P. A., Gibson J. N., Taylor D. J., Rennie M. J. Isolation of aminoacyl-tRNA and its labeling with stable-isotope tracers: Use in studies of human tissue protein synthesis. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5892–5896. doi: 10.1073/pnas.88.13.5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. R., Goodenough R. D., Wolfe M. H., Royle G. T., Nadel E. R. Isotopic analysis of leucine and urea metabolism in exercising humans. J Appl Physiol Respir Environ Exerc Physiol. 1982 Feb;52(2):458–466. doi: 10.1152/jappl.1982.52.2.458. [DOI] [PubMed] [Google Scholar]
- ZIERLER K. L., RABINOWITZ D. ROLES OF INSULIN AND GROWTH HORMONE, BASED ON STUDIES OF FOREARM METABOLISM IN MAN. Medicine (Baltimore) 1963 Nov;42:385–402. doi: 10.1097/00005792-196311000-00002. [DOI] [PubMed] [Google Scholar]