Abstract
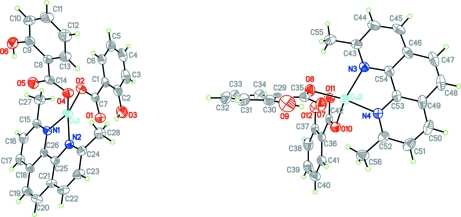
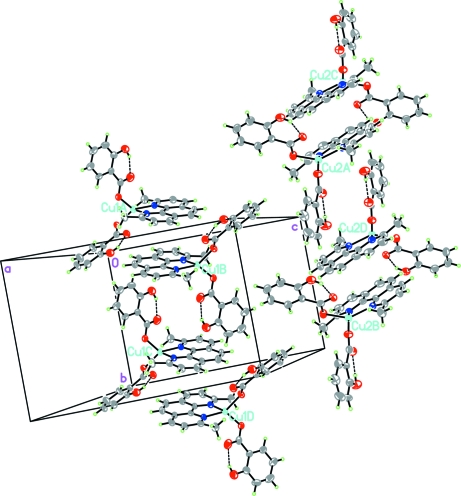
The CuII atoms in the two independent molecules of the title compound, [Cu(C7H5O3)2(C14H12N2)], are each coordinated by a bidentate 2,9-dimethyl-1,10-phenanthroline (dmphen) molecule and two monodentate 2-hydroxybenzoate anions in a distorted tetrahedral geometry. The crystal packing is stabilized by intramolecular hydrogen bonding and π–π interactions between the dmphen rings of neighboring molecules, with distances between their ring planes of 3.5670 (4) and 3.5181 (9) Å.
Related literature
For the features of metal–phenanthroline complexes, see: Naing et al. (1995 ▶); Wang et al. (1996 ▶); Wall et al. (1999 ▶). For related structures, see: Cheng et al. (2007 ▶); Xuan et al. (2007 ▶); Zhao et al. (2007 ▶).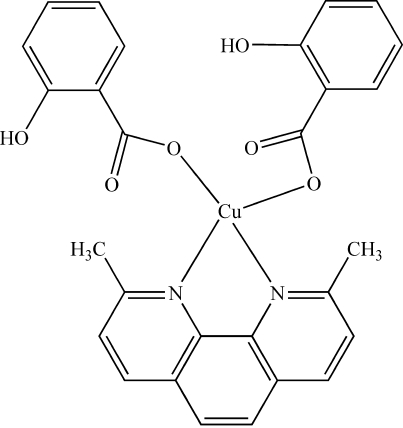
Experimental
Crystal data
[Cu(C7H5O3)2(C14H12N2)]
M r = 546.02
Monoclinic,
a = 23.819 (2) Å
b = 12.2576 (11) Å
c = 17.9084 (17) Å
β = 112.023 (1)°
V = 4847.0 (8) Å3
Z = 8
Mo Kα radiation
μ = 0.95 mm−1
T = 291 (2) K
0.30 × 0.21 × 0.19 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 2004 ▶) T min = 0.765, T max = 0.837
30637 measured reflections
8932 independent reflections
5274 reflections with I > 2σ(I)
R int = 0.060
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.121
S = 1.01
8932 reflections
675 parameters
H-atom parameters constrained
Δρmax = 0.38 e Å−3
Δρmin = −0.45 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: APEX2 and SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: publCIF (Westrip, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808036283/hg2438sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808036283/hg2438Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Selected geometric parameters (Å, °).
| Cu1—O2 | 1.931 (2) |
| Cu1—O4 | 1.946 (2) |
| Cu1—N2 | 1.994 (3) |
| Cu1—N1 | 2.022 (3) |
| Cu2—O10 | 1.945 (2) |
| Cu2—O8 | 1.956 (3) |
| Cu2—N4 | 1.992 (3) |
| Cu2—N3 | 2.044 (3) |
| O2—Cu1—O4 | 91.00 (11) |
| O2—Cu1—N2 | 152.67 (12) |
| O4—Cu1—N2 | 97.15 (11) |
| O2—Cu1—N1 | 104.90 (11) |
| O4—Cu1—N1 | 144.27 (11) |
| N2—Cu1—N1 | 83.30 (11) |
| O10—Cu2—O8 | 90.72 (11) |
| O10—Cu2—N4 | 94.59 (12) |
| O8—Cu2—N4 | 155.88 (12) |
| O10—Cu2—N3 | 143.61 (12) |
| O8—Cu2—N3 | 106.20 (12) |
| N4—Cu2—N3 | 82.98 (13) |
Table 2. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O12—H12⋯O11 | 0.82 | 1.82 | 2.549 (4) | 147 |
| O9—H9⋯O7 | 0.82 | 1.84 | 2.561 (4) | 146 |
| O6—H6⋯O5 | 0.82 | 1.85 | 2.572 (4) | 146 |
| O3—H3⋯O1 | 0.82 | 1.82 | 2.553 (5) | 148 |
Acknowledgments
Financial support from the Science Fund of Henan Province for Distinguished Young Scholars (No. 074100510005) is gratefully acknowledged.
supplementary crystallographic information
Comment
Metal-phenanthroline complexes have attracted much attention because of their peculiar features (Wang et al., 1996; Wall et al., 1999; Naing et al., 1995). Some Cu(II)-phenanthroline complexes have been synthesized and structures were determined (Cheng et al., 2007; Xuan et al., 2007; Zhao et al., 2007). Recently, we obtained the title compound copper(II) complex (I), by reaction of 2,9-dimethyl-1,10-phenanthroline, 2-hydroxybenzoic acid and Cu(NO3)2 in an ethanol/water mixture. The structure of the title compound, Cu(C14H12N2)(C6H4OHCOO)2,(I), is shown below.
There are two independent molecules in the asymmetric unit. Each CuII ion is four-coordinated by two N atoms from a 2,9-dimethyl-1,10-phenanthroline ligand, and two O atoms from two 2-hydroxybenzoic anions. The CuII ion locates in the center, and CuO2N2 unit forms a distorted tetrahedral geometry (Fig.1). The Cu—N and Cu—O bond lengths in two independent molecules different slightly (Table 1). The hydroxy directions of 2-hydroxybenzoic anions in the two independent molecules are also different.
An intramolecular hydrogen bond between the hydroxy group and uncoordinated carboxyl O atom stabilizes the conformation of the hydroxybenzoate ligands (Table 2). A partially overlapped arrangement of neighboring parallel Cu1A-dmphen [symmetry code: (Cu1A) x, y - 1, z] and Cu1B-dmphen rings [symmetry code: (Cu1B) -x + 1, -y + 1, -z + 1], Cu2A-dmphen [symmetry code: (Cu2A) -x + 2, y - 1/2, -z + 3/2] and Cu2C-dmphen rings [symmetry code: (Cu2C) x, -y - 1/2, z + 3/2] are observed in the structure of (I) (Fig.2). The shorter face-to-face separation of 3.5670 (4)Å and 3.5181 (9)Å indicates the existence of π—π stacking between the dmphen ligands.
Experimental
2-hydroxybenzoic acid (0.1389 g, 1 mmol) and NaOH (0.0370 g, 1 mmol) were dissolved in distilled water(10 ml) and Cu(NO3)2.3H2O (0.1222 g, 0.5 mmol) were added. This solution was added to a solution of 2,9-dimethyl-1,10-phenanthroline hemihydrate (C14H12N2.0.5H2O, 0.1090 g, 0.5 mmol) in ethanol (10 ml). The mixture was stirred at 323 K and then refluxed for 5 h, cooled to room temperature and filtered. Green single crystals of (I) were appeared over a period of eighteen days by slow evaporation at room temperature.
Refinement
Methyl H and hydroxy H atoms were placed in calculated positions,with C—H=0.96 and O—H=0.82 Å, and refined with free torsion angles to fit the electron density; Uiso(H) = 1.5Ueq(carrier). Other H atoms were placed in calculated positions, with C—H=0.93 Å, and refined in the riding-model approximation with Uiso(H) = 1.2Ueq(C).
Figures
Fig. 1.
The molecular structure of the title complex(I), with atom labels and 30% probability displacement ellipsoids.
Fig. 2.
π—π interactions of neighboring molecules and intramolecular hydrogen bonds in the crystal structure of (I).[symmetry code: (Cu1A) x, y - 1, z; (Cu1B) -x + 1, -y + 1, -z + 1; (Cu1D) -x + 1, -y + 2, -z + 1; (Cu2A) -x + 2, y - 1/2, -z + 3/2; (Cu2B) -x + 2, y + 1/2, -z + 3/2; (Cu2C) x, -y - 1/2, z + 3/2; (Cu2D) x, -y + 1/2, z + 3/2]
Crystal data
| [Cu(C7H5O3)2(C14H12N2)] | F000 = 2248 |
| Mr = 546.02 | Dx = 1.496 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 3270 reflections |
| a = 23.819 (2) Å | θ = 2.4–19.2º |
| b = 12.2576 (11) Å | µ = 0.95 mm−1 |
| c = 17.9084 (17) Å | T = 291 (2) K |
| β = 112.023 (1)º | Block, green |
| V = 4847.0 (8) Å3 | 0.30 × 0.21 × 0.19 mm |
| Z = 8 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 8932 independent reflections |
| Radiation source: fine-focus sealed tube | 5274 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.060 |
| T = 291(2) K | θmax = 25.5º |
| φ and ω scans | θmin = 2.4º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 2004) | h = −28→27 |
| Tmin = 0.765, Tmax = 0.837 | k = −14→14 |
| 30637 measured reflections | l = −21→21 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.045 | H-atom parameters constrained |
| wR(F2) = 0.122 | w = 1/[σ2(Fo2) + (0.051P)2 + 0.2481P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max = 0.001 |
| 8932 reflections | Δρmax = 0.38 e Å−3 |
| 675 parameters | Δρmin = −0.45 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes)are estimated using the full covariance matrix. The cell e.s.d.'s are takeninto account individually in the estimation of e.s.d.'s in distances, anglesand torsion angles; correlations between e.s.d.'s in cell parameters are onlyused when they are defined by crystal symmetry. An approximate (isotropic)treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR andgoodness of fit S are based on F2, conventional R-factors R are basedon F, with F set to zero for negative F2. The threshold expression ofF2 > σ(F2) is used only for calculating R-factors(gt) etc. and isnot relevant to the choice of reflections for refinement. R-factors basedon F2 are statistically about twice as large as those based on F, and R-factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Cu1 | 0.416752 (19) | 0.76816 (4) | 0.34347 (3) | 0.04372 (14) | |
| Cu2 | 0.91833 (2) | 0.24372 (4) | 0.06488 (3) | 0.04679 (15) | |
| O1 | 0.42604 (14) | 0.5478 (3) | 0.36158 (18) | 0.0828 (10) | |
| O2 | 0.37117 (12) | 0.6601 (2) | 0.26599 (15) | 0.0537 (7) | |
| O3 | 0.4003 (2) | 0.3447 (3) | 0.3513 (2) | 0.1039 (13) | |
| H3 | 0.4177 | 0.4014 | 0.3714 | 0.156* | |
| O4 | 0.42450 (12) | 0.8580 (2) | 0.25824 (15) | 0.0525 (7) | |
| O5 | 0.33691 (13) | 0.9202 (2) | 0.25654 (16) | 0.0636 (8) | |
| O6 | 0.26673 (13) | 1.0250 (3) | 0.13213 (18) | 0.0715 (8) | |
| H6 | 0.2774 | 0.9978 | 0.1772 | 0.107* | |
| O7 | 0.92602 (13) | 0.4499 (2) | 0.07908 (17) | 0.0690 (8) | |
| O8 | 0.85069 (12) | 0.3372 (2) | 0.06067 (17) | 0.0611 (7) | |
| O9 | 0.90651 (19) | 0.6548 (3) | 0.0833 (3) | 0.1015 (12) | |
| H9 | 0.9264 | 0.5995 | 0.0853 | 0.152* | |
| O10 | 0.92554 (11) | 0.1888 (2) | 0.16999 (15) | 0.0540 (7) | |
| O11 | 0.84406 (12) | 0.0976 (2) | 0.09313 (15) | 0.0568 (7) | |
| O12 | 0.79075 (13) | −0.0325 (2) | 0.15603 (18) | 0.0675 (8) | |
| H12 | 0.7973 | −0.0009 | 0.1198 | 0.101* | |
| N1 | 0.38290 (13) | 0.7629 (2) | 0.43145 (16) | 0.0390 (7) | |
| N2 | 0.49111 (13) | 0.8211 (2) | 0.43227 (17) | 0.0404 (7) | |
| N3 | 0.90189 (14) | 0.1985 (2) | −0.05127 (18) | 0.0461 (8) | |
| N4 | 1.00369 (13) | 0.2094 (2) | 0.08003 (19) | 0.0456 (8) | |
| C1 | 0.35133 (17) | 0.4709 (3) | 0.2453 (2) | 0.0479 (10) | |
| C2 | 0.3600 (2) | 0.3653 (4) | 0.2763 (3) | 0.0668 (12) | |
| C3 | 0.3263 (3) | 0.2798 (4) | 0.2294 (4) | 0.0814 (16) | |
| H3A | 0.3305 | 0.2098 | 0.2509 | 0.098* | |
| C4 | 0.2874 (2) | 0.2979 (4) | 0.1529 (4) | 0.0787 (15) | |
| H4 | 0.2660 | 0.2397 | 0.1220 | 0.094* | |
| C5 | 0.27904 (19) | 0.4010 (4) | 0.1202 (3) | 0.0681 (12) | |
| H5 | 0.2524 | 0.4125 | 0.0675 | 0.082* | |
| C6 | 0.31061 (17) | 0.4865 (3) | 0.1665 (2) | 0.0543 (10) | |
| H6A | 0.3047 | 0.5564 | 0.1448 | 0.065* | |
| C7 | 0.38553 (18) | 0.5648 (4) | 0.2941 (2) | 0.0521 (10) | |
| C8 | 0.36927 (17) | 0.9750 (3) | 0.1521 (2) | 0.0444 (9) | |
| C9 | 0.3138 (2) | 1.0240 (3) | 0.1071 (2) | 0.0552 (11) | |
| C10 | 0.3058 (2) | 1.0737 (4) | 0.0339 (3) | 0.0713 (13) | |
| H10 | 0.2688 | 1.1055 | 0.0037 | 0.086* | |
| C11 | 0.3519 (3) | 1.0761 (4) | 0.0061 (3) | 0.0842 (16) | |
| H11 | 0.3459 | 1.1088 | −0.0431 | 0.101* | |
| C12 | 0.4071 (2) | 1.0306 (4) | 0.0503 (3) | 0.0806 (14) | |
| H12A | 0.4386 | 1.0337 | 0.0317 | 0.097* | |
| C13 | 0.4154 (2) | 0.9797 (3) | 0.1232 (2) | 0.0606 (11) | |
| H13 | 0.4527 | 0.9483 | 0.1529 | 0.073* | |
| C14 | 0.37703 (19) | 0.9146 (3) | 0.2272 (2) | 0.0474 (10) | |
| C15 | 0.32818 (16) | 0.7343 (3) | 0.4286 (2) | 0.0445 (9) | |
| C16 | 0.31365 (18) | 0.7396 (3) | 0.4971 (2) | 0.0535 (10) | |
| H16 | 0.2753 | 0.7183 | 0.4938 | 0.064* | |
| C17 | 0.35443 (19) | 0.7751 (3) | 0.5680 (3) | 0.0545 (11) | |
| H17 | 0.3446 | 0.7770 | 0.6136 | 0.065* | |
| C18 | 0.41203 (17) | 0.8093 (3) | 0.5725 (2) | 0.0452 (9) | |
| C19 | 0.45829 (19) | 0.8514 (3) | 0.6437 (2) | 0.0568 (11) | |
| H19 | 0.4510 | 0.8555 | 0.6912 | 0.068* | |
| C20 | 0.5121 (2) | 0.8852 (3) | 0.6438 (2) | 0.0603 (11) | |
| H20 | 0.5408 | 0.9140 | 0.6905 | 0.072* | |
| C21 | 0.52535 (16) | 0.8772 (3) | 0.5721 (2) | 0.0467 (10) | |
| C22 | 0.58051 (18) | 0.9085 (3) | 0.5682 (2) | 0.0587 (11) | |
| H22 | 0.6104 | 0.9403 | 0.6125 | 0.070* | |
| C23 | 0.59019 (17) | 0.8921 (3) | 0.4987 (2) | 0.0579 (11) | |
| H23 | 0.6272 | 0.9118 | 0.4962 | 0.069* | |
| C24 | 0.54513 (16) | 0.8458 (3) | 0.4307 (2) | 0.0479 (10) | |
| C25 | 0.48185 (16) | 0.8339 (3) | 0.5027 (2) | 0.0389 (8) | |
| C26 | 0.42421 (16) | 0.8003 (3) | 0.5022 (2) | 0.0374 (8) | |
| C27 | 0.28159 (17) | 0.6985 (4) | 0.3492 (2) | 0.0632 (12) | |
| H27A | 0.2888 | 0.7348 | 0.3061 | 0.095* | |
| H27B | 0.2419 | 0.7169 | 0.3473 | 0.095* | |
| H27C | 0.2843 | 0.6210 | 0.3435 | 0.095* | |
| C28 | 0.55725 (18) | 0.8220 (4) | 0.3559 (2) | 0.0657 (12) | |
| H28A | 0.5362 | 0.7567 | 0.3311 | 0.099* | |
| H28B | 0.6000 | 0.8122 | 0.3698 | 0.099* | |
| H28C | 0.5433 | 0.8819 | 0.3190 | 0.099* | |
| C29 | 0.83703 (17) | 0.5201 (3) | 0.0916 (2) | 0.0480 (10) | |
| C30 | 0.8550 (2) | 0.6281 (4) | 0.0941 (2) | 0.0621 (12) | |
| C31 | 0.8204 (3) | 0.7101 (4) | 0.1074 (3) | 0.0879 (18) | |
| H31 | 0.8314 | 0.7825 | 0.1055 | 0.105* | |
| C32 | 0.7707 (3) | 0.6867 (5) | 0.1229 (3) | 0.097 (2) | |
| H32 | 0.7484 | 0.7430 | 0.1330 | 0.117* | |
| C33 | 0.7525 (2) | 0.5796 (5) | 0.1242 (3) | 0.0843 (16) | |
| H33 | 0.7186 | 0.5639 | 0.1364 | 0.101* | |
| C34 | 0.78519 (19) | 0.4953 (4) | 0.1070 (2) | 0.0644 (12) | |
| H34 | 0.7725 | 0.4233 | 0.1057 | 0.077* | |
| C35 | 0.87370 (19) | 0.4315 (3) | 0.0756 (2) | 0.0495 (10) | |
| C36 | 0.87985 (16) | 0.0701 (3) | 0.2347 (2) | 0.0441 (9) | |
| C37 | 0.83292 (18) | −0.0038 (3) | 0.2274 (3) | 0.0509 (10) | |
| C38 | 0.8293 (2) | −0.0506 (4) | 0.2951 (3) | 0.0703 (13) | |
| H38 | 0.7979 | −0.0984 | 0.2906 | 0.084* | |
| C39 | 0.8722 (2) | −0.0267 (4) | 0.3695 (3) | 0.0769 (14) | |
| H39 | 0.8692 | −0.0588 | 0.4150 | 0.092* | |
| C40 | 0.9200 (2) | 0.0443 (4) | 0.3788 (3) | 0.0710 (13) | |
| H40 | 0.9492 | 0.0587 | 0.4294 | 0.085* | |
| C41 | 0.92263 (18) | 0.0929 (3) | 0.3104 (2) | 0.0570 (11) | |
| H41 | 0.9537 | 0.1417 | 0.3153 | 0.068* | |
| C42 | 0.88268 (18) | 0.1215 (3) | 0.1610 (2) | 0.0460 (9) | |
| C43 | 0.84987 (19) | 0.1941 (3) | −0.1155 (2) | 0.0556 (11) | |
| C44 | 0.8485 (2) | 0.1465 (4) | −0.1871 (3) | 0.0698 (13) | |
| H44 | 0.8120 | 0.1430 | −0.2311 | 0.084* | |
| C45 | 0.8992 (2) | 0.1054 (4) | −0.1936 (3) | 0.0722 (13) | |
| H45 | 0.8973 | 0.0744 | −0.2419 | 0.087* | |
| C46 | 0.9546 (2) | 0.1095 (3) | −0.1280 (2) | 0.0569 (11) | |
| C47 | 1.0111 (3) | 0.0680 (3) | −0.1272 (3) | 0.0724 (14) | |
| H47 | 1.0125 | 0.0359 | −0.1735 | 0.087* | |
| C48 | 1.0621 (2) | 0.0744 (3) | −0.0611 (3) | 0.0724 (14) | |
| H48 | 1.0980 | 0.0468 | −0.0627 | 0.087* | |
| C49 | 1.0621 (2) | 0.1227 (3) | 0.0115 (3) | 0.0582 (11) | |
| C50 | 1.1135 (2) | 0.1331 (3) | 0.0828 (3) | 0.0708 (13) | |
| H50 | 1.1506 | 0.1060 | 0.0852 | 0.085* | |
| C51 | 1.1092 (2) | 0.1825 (4) | 0.1482 (3) | 0.0721 (13) | |
| H51 | 1.1435 | 0.1902 | 0.1950 | 0.087* | |
| C52 | 1.05307 (18) | 0.2222 (3) | 0.1457 (3) | 0.0544 (11) | |
| C53 | 1.00751 (17) | 0.1630 (3) | 0.0127 (2) | 0.0458 (9) | |
| C54 | 0.95339 (18) | 0.1572 (3) | −0.0572 (2) | 0.0472 (10) | |
| C55 | 0.7945 (2) | 0.2406 (4) | −0.1093 (3) | 0.0784 (14) | |
| H55A | 0.7885 | 0.2097 | −0.0636 | 0.118* | |
| H55B | 0.7602 | 0.2239 | −0.1574 | 0.118* | |
| H55C | 0.7987 | 0.3183 | −0.1030 | 0.118* | |
| C56 | 1.04876 (19) | 0.2811 (4) | 0.2161 (2) | 0.0688 (13) | |
| H56A | 1.0179 | 0.3361 | 0.1976 | 0.103* | |
| H56B | 1.0870 | 0.3148 | 0.2463 | 0.103* | |
| H56C | 1.0386 | 0.2303 | 0.2499 | 0.103* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Cu1 | 0.0443 (3) | 0.0543 (3) | 0.0349 (3) | −0.0088 (2) | 0.0174 (2) | −0.0012 (2) |
| Cu2 | 0.0473 (3) | 0.0509 (3) | 0.0462 (3) | 0.0020 (2) | 0.0221 (2) | −0.0010 (2) |
| O1 | 0.079 (2) | 0.090 (2) | 0.061 (2) | 0.0205 (18) | 0.0053 (18) | −0.0111 (17) |
| O2 | 0.0684 (18) | 0.0526 (17) | 0.0416 (15) | −0.0112 (14) | 0.0223 (14) | −0.0057 (13) |
| O3 | 0.164 (4) | 0.076 (2) | 0.073 (2) | 0.045 (3) | 0.046 (2) | 0.0203 (19) |
| O4 | 0.0520 (17) | 0.0604 (17) | 0.0450 (15) | −0.0038 (14) | 0.0180 (13) | 0.0105 (13) |
| O5 | 0.071 (2) | 0.0692 (19) | 0.0609 (18) | 0.0028 (15) | 0.0367 (16) | 0.0050 (15) |
| O6 | 0.071 (2) | 0.067 (2) | 0.074 (2) | 0.0095 (16) | 0.0252 (18) | 0.0059 (17) |
| O7 | 0.0567 (19) | 0.073 (2) | 0.085 (2) | −0.0001 (16) | 0.0352 (17) | −0.0041 (17) |
| O8 | 0.0611 (18) | 0.0504 (17) | 0.073 (2) | 0.0047 (14) | 0.0261 (15) | −0.0054 (15) |
| O9 | 0.119 (3) | 0.066 (2) | 0.121 (3) | −0.025 (2) | 0.048 (3) | −0.003 (2) |
| O10 | 0.0480 (16) | 0.0677 (18) | 0.0506 (17) | −0.0042 (14) | 0.0235 (13) | 0.0045 (14) |
| O11 | 0.0665 (18) | 0.0551 (17) | 0.0483 (17) | 0.0008 (14) | 0.0211 (15) | −0.0071 (13) |
| O12 | 0.066 (2) | 0.0548 (19) | 0.079 (2) | −0.0062 (15) | 0.0244 (18) | −0.0037 (16) |
| N1 | 0.0407 (17) | 0.0423 (17) | 0.0376 (17) | −0.0067 (14) | 0.0189 (14) | −0.0010 (14) |
| N2 | 0.0404 (18) | 0.0444 (18) | 0.0396 (18) | −0.0016 (14) | 0.0184 (14) | 0.0023 (14) |
| N3 | 0.055 (2) | 0.0425 (18) | 0.0437 (19) | −0.0013 (15) | 0.0212 (17) | 0.0019 (14) |
| N4 | 0.0475 (19) | 0.0410 (18) | 0.050 (2) | −0.0027 (14) | 0.0208 (17) | 0.0015 (15) |
| C1 | 0.053 (2) | 0.047 (2) | 0.055 (3) | −0.0010 (19) | 0.033 (2) | −0.006 (2) |
| C2 | 0.091 (4) | 0.058 (3) | 0.068 (3) | 0.009 (3) | 0.049 (3) | 0.003 (3) |
| C3 | 0.114 (5) | 0.047 (3) | 0.118 (5) | −0.001 (3) | 0.084 (4) | 0.001 (3) |
| C4 | 0.079 (4) | 0.067 (3) | 0.108 (4) | −0.017 (3) | 0.056 (3) | −0.032 (3) |
| C5 | 0.058 (3) | 0.066 (3) | 0.079 (3) | −0.005 (2) | 0.024 (2) | −0.020 (3) |
| C6 | 0.058 (3) | 0.048 (2) | 0.060 (3) | 0.000 (2) | 0.026 (2) | −0.008 (2) |
| C7 | 0.052 (3) | 0.068 (3) | 0.045 (3) | 0.008 (2) | 0.027 (2) | −0.004 (2) |
| C8 | 0.050 (2) | 0.043 (2) | 0.037 (2) | −0.0044 (18) | 0.0135 (19) | −0.0027 (17) |
| C9 | 0.070 (3) | 0.046 (2) | 0.049 (3) | −0.001 (2) | 0.021 (2) | −0.007 (2) |
| C10 | 0.082 (3) | 0.072 (3) | 0.047 (3) | 0.014 (3) | 0.009 (3) | 0.006 (2) |
| C11 | 0.121 (5) | 0.082 (4) | 0.048 (3) | 0.018 (3) | 0.032 (3) | 0.024 (3) |
| C12 | 0.100 (4) | 0.087 (4) | 0.067 (3) | 0.004 (3) | 0.046 (3) | 0.017 (3) |
| C13 | 0.069 (3) | 0.063 (3) | 0.051 (3) | 0.000 (2) | 0.024 (2) | 0.011 (2) |
| C14 | 0.056 (3) | 0.043 (2) | 0.041 (2) | −0.012 (2) | 0.016 (2) | −0.0079 (18) |
| C15 | 0.044 (2) | 0.045 (2) | 0.048 (2) | −0.0004 (18) | 0.0222 (19) | 0.0019 (18) |
| C16 | 0.048 (2) | 0.060 (3) | 0.063 (3) | −0.003 (2) | 0.033 (2) | 0.003 (2) |
| C17 | 0.067 (3) | 0.057 (3) | 0.056 (3) | 0.005 (2) | 0.042 (2) | 0.010 (2) |
| C18 | 0.056 (3) | 0.044 (2) | 0.043 (2) | −0.0002 (18) | 0.026 (2) | −0.0003 (18) |
| C19 | 0.073 (3) | 0.063 (3) | 0.039 (2) | −0.002 (2) | 0.026 (2) | −0.001 (2) |
| C20 | 0.068 (3) | 0.069 (3) | 0.040 (2) | −0.012 (2) | 0.015 (2) | −0.007 (2) |
| C21 | 0.044 (2) | 0.052 (2) | 0.039 (2) | −0.0060 (18) | 0.0098 (18) | 0.0031 (18) |
| C22 | 0.047 (3) | 0.068 (3) | 0.049 (3) | −0.010 (2) | 0.005 (2) | −0.001 (2) |
| C23 | 0.035 (2) | 0.079 (3) | 0.057 (3) | −0.009 (2) | 0.014 (2) | 0.011 (2) |
| C24 | 0.041 (2) | 0.058 (3) | 0.048 (2) | 0.0000 (19) | 0.020 (2) | 0.0086 (19) |
| C25 | 0.043 (2) | 0.040 (2) | 0.035 (2) | −0.0033 (16) | 0.0169 (18) | 0.0015 (16) |
| C26 | 0.044 (2) | 0.0333 (19) | 0.037 (2) | 0.0037 (16) | 0.0184 (18) | 0.0030 (16) |
| C27 | 0.042 (2) | 0.085 (3) | 0.062 (3) | −0.013 (2) | 0.020 (2) | −0.006 (2) |
| C28 | 0.050 (3) | 0.097 (3) | 0.060 (3) | −0.005 (2) | 0.032 (2) | 0.004 (2) |
| C29 | 0.052 (3) | 0.044 (2) | 0.038 (2) | 0.0078 (19) | 0.0053 (19) | −0.0018 (18) |
| C30 | 0.070 (3) | 0.054 (3) | 0.051 (3) | 0.003 (2) | 0.010 (2) | −0.004 (2) |
| C31 | 0.105 (4) | 0.053 (3) | 0.076 (4) | 0.026 (3) | 0.000 (3) | −0.013 (3) |
| C32 | 0.092 (4) | 0.092 (5) | 0.082 (4) | 0.051 (4) | 0.003 (3) | −0.019 (3) |
| C33 | 0.055 (3) | 0.117 (5) | 0.073 (3) | 0.030 (3) | 0.014 (3) | −0.005 (3) |
| C34 | 0.054 (3) | 0.076 (3) | 0.060 (3) | 0.013 (2) | 0.018 (2) | 0.000 (2) |
| C35 | 0.054 (3) | 0.053 (3) | 0.039 (2) | 0.006 (2) | 0.015 (2) | 0.0022 (19) |
| C36 | 0.046 (2) | 0.043 (2) | 0.049 (2) | 0.0101 (18) | 0.024 (2) | 0.0017 (18) |
| C37 | 0.055 (3) | 0.045 (2) | 0.063 (3) | 0.011 (2) | 0.034 (2) | 0.005 (2) |
| C38 | 0.079 (3) | 0.061 (3) | 0.088 (4) | 0.005 (2) | 0.052 (3) | 0.010 (3) |
| C39 | 0.107 (4) | 0.071 (3) | 0.078 (4) | 0.017 (3) | 0.064 (3) | 0.016 (3) |
| C40 | 0.086 (4) | 0.078 (3) | 0.051 (3) | 0.016 (3) | 0.027 (3) | 0.006 (2) |
| C41 | 0.064 (3) | 0.057 (3) | 0.054 (3) | 0.007 (2) | 0.027 (2) | 0.000 (2) |
| C42 | 0.049 (2) | 0.039 (2) | 0.057 (3) | 0.0128 (19) | 0.028 (2) | −0.0004 (19) |
| C43 | 0.063 (3) | 0.056 (3) | 0.043 (2) | 0.000 (2) | 0.015 (2) | 0.003 (2) |
| C44 | 0.087 (4) | 0.070 (3) | 0.044 (3) | 0.000 (3) | 0.016 (3) | −0.005 (2) |
| C45 | 0.115 (4) | 0.064 (3) | 0.042 (3) | 0.000 (3) | 0.033 (3) | −0.006 (2) |
| C46 | 0.090 (3) | 0.042 (2) | 0.053 (3) | −0.001 (2) | 0.043 (3) | −0.001 (2) |
| C47 | 0.109 (4) | 0.050 (3) | 0.088 (4) | 0.004 (3) | 0.071 (3) | −0.001 (3) |
| C48 | 0.080 (4) | 0.051 (3) | 0.116 (4) | 0.002 (2) | 0.071 (3) | −0.002 (3) |
| C49 | 0.067 (3) | 0.037 (2) | 0.087 (3) | 0.001 (2) | 0.049 (3) | 0.001 (2) |
| C50 | 0.051 (3) | 0.055 (3) | 0.114 (4) | 0.005 (2) | 0.040 (3) | 0.008 (3) |
| C51 | 0.053 (3) | 0.060 (3) | 0.092 (4) | −0.004 (2) | 0.014 (3) | 0.002 (3) |
| C52 | 0.047 (3) | 0.051 (2) | 0.063 (3) | −0.006 (2) | 0.017 (2) | 0.004 (2) |
| C53 | 0.056 (3) | 0.032 (2) | 0.060 (3) | −0.0012 (18) | 0.035 (2) | 0.0052 (18) |
| C54 | 0.062 (3) | 0.039 (2) | 0.049 (2) | −0.0037 (19) | 0.030 (2) | 0.0029 (18) |
| C55 | 0.056 (3) | 0.101 (4) | 0.064 (3) | 0.003 (3) | 0.006 (2) | −0.010 (3) |
| C56 | 0.060 (3) | 0.080 (3) | 0.060 (3) | −0.017 (2) | 0.015 (2) | −0.011 (2) |
Geometric parameters (Å, °)
| Cu1—O2 | 1.931 (2) | C20—C21 | 1.436 (5) |
| Cu1—O4 | 1.946 (2) | C20—H20 | 0.9300 |
| Cu1—N2 | 1.994 (3) | C21—C25 | 1.390 (5) |
| Cu1—N1 | 2.022 (3) | C21—C22 | 1.396 (5) |
| Cu2—O10 | 1.945 (2) | C22—C23 | 1.363 (5) |
| Cu2—O8 | 1.956 (3) | C22—H22 | 0.9300 |
| Cu2—N4 | 1.992 (3) | C23—C24 | 1.405 (5) |
| Cu2—N3 | 2.044 (3) | C23—H23 | 0.9300 |
| O1—C7 | 1.249 (4) | C24—C28 | 1.501 (5) |
| O2—C7 | 1.267 (4) | C25—C26 | 1.431 (5) |
| O3—C2 | 1.350 (5) | C27—H27A | 0.9600 |
| O3—H3 | 0.8200 | C27—H27B | 0.9600 |
| O4—C14 | 1.264 (4) | C27—H27C | 0.9600 |
| O5—C14 | 1.254 (4) | C28—H28A | 0.9600 |
| O6—C9 | 1.355 (5) | C28—H28B | 0.9600 |
| O6—H6 | 0.8200 | C28—H28C | 0.9600 |
| O7—C35 | 1.245 (4) | C29—C30 | 1.387 (6) |
| O8—C35 | 1.264 (4) | C29—C34 | 1.395 (5) |
| O9—C30 | 1.351 (5) | C29—C35 | 1.487 (5) |
| O9—H9 | 0.8200 | C30—C31 | 1.377 (6) |
| O10—C42 | 1.275 (4) | C31—C32 | 1.344 (7) |
| O11—C42 | 1.254 (4) | C31—H31 | 0.9300 |
| O12—C37 | 1.343 (4) | C32—C33 | 1.386 (8) |
| O12—H12 | 0.8200 | C32—H32 | 0.9300 |
| N1—C15 | 1.332 (4) | C33—C34 | 1.396 (6) |
| N1—C26 | 1.360 (4) | C33—H33 | 0.9300 |
| N2—C24 | 1.332 (4) | C34—H34 | 0.9300 |
| N2—C25 | 1.369 (4) | C36—C41 | 1.384 (5) |
| N3—C43 | 1.339 (5) | C36—C37 | 1.407 (5) |
| N3—C54 | 1.367 (4) | C36—C42 | 1.487 (5) |
| N4—C52 | 1.324 (5) | C37—C38 | 1.372 (5) |
| N4—C53 | 1.367 (4) | C38—C39 | 1.372 (6) |
| C1—C2 | 1.392 (5) | C38—H38 | 0.9300 |
| C1—C6 | 1.394 (5) | C39—C40 | 1.393 (6) |
| C1—C7 | 1.490 (5) | C39—H39 | 0.9300 |
| C2—C3 | 1.394 (6) | C40—C41 | 1.384 (5) |
| C3—C4 | 1.354 (6) | C40—H40 | 0.9300 |
| C3—H3A | 0.9300 | C41—H41 | 0.9300 |
| C4—C5 | 1.375 (6) | C43—C44 | 1.397 (5) |
| C4—H4 | 0.9300 | C43—C55 | 1.479 (6) |
| C5—C6 | 1.373 (5) | C44—C45 | 1.355 (6) |
| C5—H5 | 0.9300 | C44—H44 | 0.9300 |
| C6—H6A | 0.9300 | C45—C46 | 1.400 (6) |
| C8—C13 | 1.381 (5) | C45—H45 | 0.9300 |
| C8—C9 | 1.399 (5) | C46—C54 | 1.407 (5) |
| C8—C14 | 1.484 (5) | C46—C47 | 1.434 (6) |
| C9—C10 | 1.392 (6) | C47—C48 | 1.343 (6) |
| C10—C11 | 1.364 (6) | C47—H47 | 0.9300 |
| C10—H10 | 0.9300 | C48—C49 | 1.429 (6) |
| C11—C12 | 1.373 (6) | C48—H48 | 0.9300 |
| C11—H11 | 0.9300 | C49—C53 | 1.399 (5) |
| C12—C13 | 1.391 (5) | C49—C50 | 1.404 (6) |
| C12—H12A | 0.9300 | C50—C51 | 1.356 (6) |
| C13—H13 | 0.9300 | C50—H50 | 0.9300 |
| C15—C16 | 1.396 (5) | C51—C52 | 1.407 (6) |
| C15—C27 | 1.504 (5) | C51—H51 | 0.9300 |
| C16—C17 | 1.350 (5) | C52—C56 | 1.490 (6) |
| C16—H16 | 0.9300 | C53—C54 | 1.423 (5) |
| C17—C18 | 1.408 (5) | C55—H55A | 0.9600 |
| C17—H17 | 0.9300 | C55—H55B | 0.9600 |
| C18—C26 | 1.399 (5) | C55—H55C | 0.9600 |
| C18—C19 | 1.433 (5) | C56—H56A | 0.9600 |
| C19—C20 | 1.346 (5) | C56—H56B | 0.9600 |
| C19—H19 | 0.9300 | C56—H56C | 0.9600 |
| O2—Cu1—O4 | 91.00 (11) | C21—C25—C26 | 120.5 (3) |
| O2—Cu1—N2 | 152.67 (12) | N1—C26—C18 | 123.0 (3) |
| O4—Cu1—N2 | 97.15 (11) | N1—C26—C25 | 117.4 (3) |
| O2—Cu1—N1 | 104.90 (11) | C18—C26—C25 | 119.6 (3) |
| O4—Cu1—N1 | 144.27 (11) | C15—C27—H27A | 109.5 |
| N2—Cu1—N1 | 83.30 (11) | C15—C27—H27B | 109.5 |
| O10—Cu2—O8 | 90.72 (11) | H27A—C27—H27B | 109.5 |
| O10—Cu2—N4 | 94.59 (12) | C15—C27—H27C | 109.5 |
| O8—Cu2—N4 | 155.88 (12) | H27A—C27—H27C | 109.5 |
| O10—Cu2—N3 | 143.61 (12) | H27B—C27—H27C | 109.5 |
| O8—Cu2—N3 | 106.20 (12) | C24—C28—H28A | 109.5 |
| N4—Cu2—N3 | 82.98 (13) | C24—C28—H28B | 109.5 |
| C7—O2—Cu1 | 110.6 (2) | H28A—C28—H28B | 109.5 |
| C2—O3—H3 | 109.5 | C24—C28—H28C | 109.5 |
| C14—O4—Cu1 | 108.9 (2) | H28A—C28—H28C | 109.5 |
| C9—O6—H6 | 109.5 | H28B—C28—H28C | 109.5 |
| C35—O8—Cu2 | 104.0 (3) | C30—C29—C34 | 119.2 (4) |
| C30—O9—H9 | 109.5 | C30—C29—C35 | 120.4 (4) |
| C42—O10—Cu2 | 109.0 (2) | C34—C29—C35 | 120.4 (4) |
| C37—O12—H12 | 109.5 | O9—C30—C31 | 118.9 (5) |
| C15—N1—C26 | 118.7 (3) | O9—C30—C29 | 120.9 (4) |
| C15—N1—Cu1 | 130.4 (2) | C31—C30—C29 | 120.3 (5) |
| C26—N1—Cu1 | 110.8 (2) | C32—C31—C30 | 120.8 (5) |
| C24—N2—C25 | 118.9 (3) | C32—C31—H31 | 119.6 |
| C24—N2—Cu1 | 129.3 (3) | C30—C31—H31 | 119.6 |
| C25—N2—Cu1 | 111.8 (2) | C31—C32—C33 | 120.7 (5) |
| C43—N3—C54 | 119.1 (3) | C31—C32—H32 | 119.6 |
| C43—N3—Cu2 | 130.4 (3) | C33—C32—H32 | 119.6 |
| C54—N3—Cu2 | 110.1 (2) | C32—C33—C34 | 119.5 (5) |
| C52—N4—C53 | 120.1 (3) | C32—C33—H33 | 120.2 |
| C52—N4—Cu2 | 128.1 (3) | C34—C33—H33 | 120.2 |
| C53—N4—Cu2 | 111.7 (2) | C29—C34—C33 | 119.4 (5) |
| C2—C1—C6 | 118.3 (4) | C29—C34—H34 | 120.3 |
| C2—C1—C7 | 121.2 (4) | C33—C34—H34 | 120.3 |
| C6—C1—C7 | 120.5 (4) | O7—C35—O8 | 121.5 (4) |
| O3—C2—C1 | 121.0 (4) | O7—C35—C29 | 120.5 (4) |
| O3—C2—C3 | 119.5 (5) | O8—C35—C29 | 118.0 (4) |
| C1—C2—C3 | 119.5 (5) | C41—C36—C37 | 119.2 (4) |
| C4—C3—C2 | 120.5 (5) | C41—C36—C42 | 121.5 (4) |
| C4—C3—H3A | 119.8 | C37—C36—C42 | 119.3 (4) |
| C2—C3—H3A | 119.8 | O12—C37—C38 | 117.3 (4) |
| C3—C4—C5 | 121.1 (5) | O12—C37—C36 | 122.8 (4) |
| C3—C4—H4 | 119.5 | C38—C37—C36 | 119.9 (4) |
| C5—C4—H4 | 119.5 | C39—C38—C37 | 119.9 (5) |
| C6—C5—C4 | 119.1 (5) | C39—C38—H38 | 120.1 |
| C6—C5—H5 | 120.5 | C37—C38—H38 | 120.1 |
| C4—C5—H5 | 120.5 | C38—C39—C40 | 121.7 (4) |
| C5—C6—C1 | 121.5 (4) | C38—C39—H39 | 119.1 |
| C5—C6—H6A | 119.3 | C40—C39—H39 | 119.1 |
| C1—C6—H6A | 119.3 | C41—C40—C39 | 118.0 (4) |
| O1—C7—O2 | 122.3 (4) | C41—C40—H40 | 121.0 |
| O1—C7—C1 | 119.5 (4) | C39—C40—H40 | 121.0 |
| O2—C7—C1 | 118.2 (4) | C40—C41—C36 | 121.2 (4) |
| C13—C8—C9 | 118.6 (4) | C40—C41—H41 | 119.4 |
| C13—C8—C14 | 120.9 (4) | C36—C41—H41 | 119.4 |
| C9—C8—C14 | 120.4 (4) | O11—C42—O10 | 122.4 (4) |
| O6—C9—C10 | 118.0 (4) | O11—C42—C36 | 120.1 (4) |
| O6—C9—C8 | 122.2 (4) | O10—C42—C36 | 117.6 (4) |
| C10—C9—C8 | 119.8 (4) | N3—C43—C44 | 120.1 (4) |
| C11—C10—C9 | 120.5 (4) | N3—C43—C55 | 119.0 (4) |
| C11—C10—H10 | 119.7 | C44—C43—C55 | 120.9 (4) |
| C9—C10—H10 | 119.7 | C45—C44—C43 | 121.3 (4) |
| C10—C11—C12 | 120.5 (4) | C45—C44—H44 | 119.3 |
| C10—C11—H11 | 119.7 | C43—C44—H44 | 119.3 |
| C12—C11—H11 | 119.7 | C44—C45—C46 | 120.3 (4) |
| C11—C12—C13 | 119.6 (5) | C44—C45—H45 | 119.9 |
| C11—C12—H12A | 120.2 | C46—C45—H45 | 119.9 |
| C13—C12—H12A | 120.2 | C45—C46—C54 | 116.2 (4) |
| C8—C13—C12 | 121.0 (4) | C45—C46—C47 | 125.5 (4) |
| C8—C13—H13 | 119.5 | C54—C46—C47 | 118.3 (4) |
| C12—C13—H13 | 119.5 | C48—C47—C46 | 121.6 (4) |
| O5—C14—O4 | 122.8 (4) | C48—C47—H47 | 119.2 |
| O5—C14—C8 | 119.8 (4) | C46—C47—H47 | 119.2 |
| O4—C14—C8 | 117.4 (4) | C47—C48—C49 | 121.2 (4) |
| N1—C15—C16 | 121.0 (3) | C47—C48—H48 | 119.4 |
| N1—C15—C27 | 118.5 (3) | C49—C48—H48 | 119.4 |
| C16—C15—C27 | 120.4 (3) | C53—C49—C50 | 116.9 (4) |
| C17—C16—C15 | 121.0 (4) | C53—C49—C48 | 118.4 (4) |
| C17—C16—H16 | 119.5 | C50—C49—C48 | 124.7 (4) |
| C15—C16—H16 | 119.5 | C51—C50—C49 | 120.2 (4) |
| C16—C17—C18 | 119.5 (4) | C51—C50—H50 | 119.9 |
| C16—C17—H17 | 120.3 | C49—C50—H50 | 119.9 |
| C18—C17—H17 | 120.3 | C50—C51—C52 | 120.3 (4) |
| C26—C18—C17 | 116.9 (3) | C50—C51—H51 | 119.8 |
| C26—C18—C19 | 118.7 (4) | C52—C51—H51 | 119.8 |
| C17—C18—C19 | 124.5 (4) | N4—C52—C51 | 120.3 (4) |
| C20—C19—C18 | 121.7 (4) | N4—C52—C56 | 119.3 (4) |
| C20—C19—H19 | 119.2 | C51—C52—C56 | 120.4 (4) |
| C18—C19—H19 | 119.2 | N4—C53—C49 | 122.0 (4) |
| C19—C20—C21 | 120.5 (4) | N4—C53—C54 | 117.3 (3) |
| C19—C20—H20 | 119.8 | C49—C53—C54 | 120.7 (4) |
| C21—C20—H20 | 119.8 | N3—C54—C46 | 123.1 (4) |
| C25—C21—C22 | 117.4 (4) | N3—C54—C53 | 117.2 (3) |
| C25—C21—C20 | 119.0 (3) | C46—C54—C53 | 119.7 (4) |
| C22—C21—C20 | 123.6 (4) | C43—C55—H55A | 109.5 |
| C23—C22—C21 | 119.3 (4) | C43—C55—H55B | 109.5 |
| C23—C22—H22 | 120.3 | H55A—C55—H55B | 109.5 |
| C21—C22—H22 | 120.3 | C43—C55—H55C | 109.5 |
| C22—C23—C24 | 121.0 (4) | H55A—C55—H55C | 109.5 |
| C22—C23—H23 | 119.5 | H55B—C55—H55C | 109.5 |
| C24—C23—H23 | 119.5 | C52—C56—H56A | 109.5 |
| N2—C24—C23 | 120.2 (4) | C52—C56—H56B | 109.5 |
| N2—C24—C28 | 119.0 (3) | H56A—C56—H56B | 109.5 |
| C23—C24—C28 | 120.7 (3) | C52—C56—H56C | 109.5 |
| N2—C25—C21 | 123.0 (3) | H56A—C56—H56C | 109.5 |
| N2—C25—C26 | 116.6 (3) | H56B—C56—H56C | 109.5 |
| O4—Cu1—O2—C7 | 141.8 (3) | C24—N2—C25—C26 | −176.5 (3) |
| N2—Cu1—O2—C7 | 34.1 (4) | Cu1—N2—C25—C26 | 4.6 (4) |
| N1—Cu1—O2—C7 | −70.5 (3) | C22—C21—C25—N2 | 0.2 (5) |
| O2—Cu1—O4—C14 | 83.0 (2) | C20—C21—C25—N2 | −178.6 (3) |
| N2—Cu1—O4—C14 | −123.1 (2) | C22—C21—C25—C26 | −179.8 (3) |
| N1—Cu1—O4—C14 | −34.7 (3) | C20—C21—C25—C26 | 1.4 (5) |
| O10—Cu2—O8—C35 | 104.0 (2) | C15—N1—C26—C18 | 0.5 (5) |
| N4—Cu2—O8—C35 | 1.0 (4) | Cu1—N1—C26—C18 | 177.7 (3) |
| N3—Cu2—O8—C35 | −108.7 (2) | C15—N1—C26—C25 | −177.9 (3) |
| O8—Cu2—O10—C42 | 75.4 (2) | Cu1—N1—C26—C25 | −0.7 (4) |
| N4—Cu2—O10—C42 | −128.2 (2) | C17—C18—C26—N1 | 1.5 (5) |
| N3—Cu2—O10—C42 | −43.8 (3) | C19—C18—C26—N1 | −178.9 (3) |
| O2—Cu1—N1—C15 | −27.3 (3) | C17—C18—C26—C25 | 179.8 (3) |
| O4—Cu1—N1—C15 | 86.4 (3) | C19—C18—C26—C25 | −0.6 (5) |
| N2—Cu1—N1—C15 | 179.3 (3) | N2—C25—C26—N1 | −2.7 (5) |
| O2—Cu1—N1—C26 | 155.9 (2) | C21—C25—C26—N1 | 177.4 (3) |
| O4—Cu1—N1—C26 | −90.4 (3) | N2—C25—C26—C18 | 178.9 (3) |
| N2—Cu1—N1—C26 | 2.5 (2) | C21—C25—C26—C18 | −1.1 (5) |
| O2—Cu1—N2—C24 | 67.7 (4) | C34—C29—C30—O9 | 176.9 (4) |
| O4—Cu1—N2—C24 | −38.6 (3) | C35—C29—C30—O9 | −1.1 (6) |
| N1—Cu1—N2—C24 | 177.4 (3) | C34—C29—C30—C31 | −3.3 (6) |
| O2—Cu1—N2—C25 | −113.6 (3) | C35—C29—C30—C31 | 178.7 (4) |
| O4—Cu1—N2—C25 | 140.1 (2) | O9—C30—C31—C32 | −176.1 (5) |
| N1—Cu1—N2—C25 | −3.9 (2) | C29—C30—C31—C32 | 4.0 (7) |
| O10—Cu2—N3—C43 | 91.7 (4) | C30—C31—C32—C33 | −1.5 (8) |
| O8—Cu2—N3—C43 | −22.9 (4) | C31—C32—C33—C34 | −1.6 (8) |
| N4—Cu2—N3—C43 | 179.9 (3) | C30—C29—C34—C33 | 0.2 (6) |
| O10—Cu2—N3—C54 | −81.1 (3) | C35—C29—C34—C33 | 178.1 (4) |
| O8—Cu2—N3—C54 | 164.3 (2) | C32—C33—C34—C29 | 2.3 (7) |
| N4—Cu2—N3—C54 | 7.1 (2) | Cu2—O8—C35—O7 | 8.9 (4) |
| O10—Cu2—N4—C52 | −40.7 (3) | Cu2—O8—C35—C29 | −169.2 (3) |
| O8—Cu2—N4—C52 | 61.4 (5) | C30—C29—C35—O7 | 13.4 (6) |
| N3—Cu2—N4—C52 | 175.8 (3) | C34—C29—C35—O7 | −164.5 (4) |
| O10—Cu2—N4—C53 | 136.2 (2) | C30—C29—C35—O8 | −168.4 (4) |
| O8—Cu2—N4—C53 | −121.6 (3) | C34—C29—C35—O8 | 13.7 (5) |
| N3—Cu2—N4—C53 | −7.3 (2) | C41—C36—C37—O12 | −178.0 (3) |
| C6—C1—C2—O3 | 177.9 (4) | C42—C36—C37—O12 | 1.6 (5) |
| C7—C1—C2—O3 | −0.8 (6) | C41—C36—C37—C38 | 1.4 (5) |
| C6—C1—C2—C3 | −2.7 (6) | C42—C36—C37—C38 | −179.0 (3) |
| C7—C1—C2—C3 | 178.7 (4) | O12—C37—C38—C39 | 178.1 (4) |
| O3—C2—C3—C4 | −177.4 (5) | C36—C37—C38—C39 | −1.3 (6) |
| C1—C2—C3—C4 | 3.2 (7) | C37—C38—C39—C40 | −0.1 (7) |
| C2—C3—C4—C5 | −1.7 (7) | C38—C39—C40—C41 | 1.4 (7) |
| C3—C4—C5—C6 | −0.3 (7) | C39—C40—C41—C36 | −1.3 (6) |
| C4—C5—C6—C1 | 0.7 (6) | C37—C36—C41—C40 | −0.1 (6) |
| C2—C1—C6—C5 | 0.7 (6) | C42—C36—C41—C40 | −179.6 (4) |
| C7—C1—C6—C5 | 179.4 (4) | Cu2—O10—C42—O11 | 0.7 (4) |
| Cu1—O2—C7—O1 | −3.1 (5) | Cu2—O10—C42—C36 | −179.8 (2) |
| Cu1—O2—C7—C1 | 175.1 (3) | C41—C36—C42—O11 | 178.5 (3) |
| C2—C1—C7—O1 | 5.6 (6) | C37—C36—C42—O11 | −1.1 (5) |
| C6—C1—C7—O1 | −173.1 (4) | C41—C36—C42—O10 | −1.0 (5) |
| C2—C1—C7—O2 | −172.7 (4) | C37—C36—C42—O10 | 179.4 (3) |
| C6—C1—C7—O2 | 8.7 (5) | C54—N3—C43—C44 | 0.8 (6) |
| C13—C8—C9—O6 | −178.6 (4) | Cu2—N3—C43—C44 | −171.4 (3) |
| C14—C8—C9—O6 | 4.4 (5) | C54—N3—C43—C55 | −178.9 (4) |
| C13—C8—C9—C10 | 1.6 (6) | Cu2—N3—C43—C55 | 8.8 (6) |
| C14—C8—C9—C10 | −175.5 (3) | N3—C43—C44—C45 | −0.8 (7) |
| O6—C9—C10—C11 | 179.4 (4) | C55—C43—C44—C45 | 178.9 (4) |
| C8—C9—C10—C11 | −0.8 (6) | C43—C44—C45—C46 | 0.3 (7) |
| C9—C10—C11—C12 | −0.7 (8) | C44—C45—C46—C54 | 0.3 (6) |
| C10—C11—C12—C13 | 1.4 (8) | C44—C45—C46—C47 | 179.4 (4) |
| C9—C8—C13—C12 | −1.0 (6) | C45—C46—C47—C48 | −179.4 (4) |
| C14—C8—C13—C12 | 176.1 (4) | C54—C46—C47—C48 | −0.3 (6) |
| C11—C12—C13—C8 | −0.5 (7) | C46—C47—C48—C49 | 0.2 (7) |
| Cu1—O4—C14—O5 | 9.1 (4) | C47—C48—C49—C53 | 0.4 (6) |
| Cu1—O4—C14—C8 | −170.0 (2) | C47—C48—C49—C50 | −179.6 (4) |
| C13—C8—C14—O5 | 173.0 (4) | C53—C49—C50—C51 | −1.4 (6) |
| C9—C8—C14—O5 | −9.9 (5) | C48—C49—C50—C51 | 178.5 (4) |
| C13—C8—C14—O4 | −7.8 (5) | C49—C50—C51—C52 | 1.0 (7) |
| C9—C8—C14—O4 | 169.2 (3) | C53—N4—C52—C51 | −3.6 (6) |
| C26—N1—C15—C16 | −1.6 (5) | Cu2—N4—C52—C51 | 173.1 (3) |
| Cu1—N1—C15—C16 | −178.2 (3) | C53—N4—C52—C56 | 175.1 (3) |
| C26—N1—C15—C27 | 176.9 (3) | Cu2—N4—C52—C56 | −8.2 (5) |
| Cu1—N1—C15—C27 | 0.3 (5) | C50—C51—C52—N4 | 1.6 (6) |
| N1—C15—C16—C17 | 0.7 (6) | C50—C51—C52—C56 | −177.2 (4) |
| C27—C15—C16—C17 | −177.7 (4) | C52—N4—C53—C49 | 3.2 (5) |
| C15—C16—C17—C18 | 1.3 (6) | Cu2—N4—C53—C49 | −174.0 (3) |
| C16—C17—C18—C26 | −2.3 (5) | C52—N4—C53—C54 | −176.5 (3) |
| C16—C17—C18—C19 | 178.1 (4) | Cu2—N4—C53—C54 | 6.2 (4) |
| C26—C18—C19—C20 | 2.0 (6) | C50—C49—C53—N4 | −0.6 (5) |
| C17—C18—C19—C20 | −178.4 (4) | C48—C49—C53—N4 | 179.4 (3) |
| C18—C19—C20—C21 | −1.7 (6) | C50—C49—C53—C54 | 179.1 (3) |
| C19—C20—C21—C25 | 0.0 (6) | C48—C49—C53—C54 | −0.9 (5) |
| C19—C20—C21—C22 | −178.7 (4) | C43—N3—C54—C46 | −0.2 (5) |
| C25—C21—C22—C23 | −2.4 (6) | Cu2—N3—C54—C46 | 173.5 (3) |
| C20—C21—C22—C23 | 176.3 (4) | C43—N3—C54—C53 | −179.6 (3) |
| C21—C22—C23—C24 | 1.1 (6) | Cu2—N3—C54—C53 | −5.9 (4) |
| C25—N2—C24—C23 | −4.8 (5) | C45—C46—C54—N3 | −0.4 (5) |
| Cu1—N2—C24—C23 | 173.8 (3) | C47—C46—C54—N3 | −179.5 (3) |
| C25—N2—C24—C28 | 174.4 (3) | C45—C46—C54—C53 | 179.0 (4) |
| Cu1—N2—C24—C28 | −7.0 (5) | C47—C46—C54—C53 | −0.2 (5) |
| C22—C23—C24—N2 | 2.6 (6) | N4—C53—C54—N3 | −0.1 (5) |
| C22—C23—C24—C28 | −176.5 (4) | C49—C53—C54—N3 | −179.9 (3) |
| C24—N2—C25—C21 | 3.4 (5) | N4—C53—C54—C46 | −179.5 (3) |
| Cu1—N2—C25—C21 | −175.4 (3) | C49—C53—C54—C46 | 0.7 (5) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O12—H12···O11 | 0.82 | 1.82 | 2.549 (4) | 147 |
| O9—H9···O7 | 0.82 | 1.84 | 2.561 (4) | 146 |
| O6—H6···O5 | 0.82 | 1.85 | 2.572 (4) | 146 |
| O3—H3···O1 | 0.82 | 1.82 | 2.553 (5) | 148 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HG2438).
References
- Bruker (2004). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cheng, J.-K., Yin, P.-X., Li, Z.-J., Qin, Y.-Y. & Yao, Y.-G. (2007). Inorg. Chem. Commun.10, 808–810.
- Naing, K., Taniguchi, M., Takahashi, M. & Yamagishi, A. (1995). Inorg. Chem.34, 350–356.
- Sheldrick, G. M. (2004). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Wall, M., Linkletter, B., Williams, D., Lebuis, A.-M., Hynes, R. C. & Chin, J. (1999). J. Am. Chem. Soc.121, 4710–4711.
- Wang, J., Cai, X., Rivas, G., Shiraishi, H., Farias, P. A. M. & Dontha, N. (1996). Anal. Chem.68, 2629–2634. [DOI] [PubMed]
- Westrip, S. P. (2008). publCIF In preparation.
- Xuan, X.-P., Zhao, P.-Z. & Zhang, S.-X. (2007). Acta Cryst. E63, m1817.
- Zhao, P.-Z., Yan, F.-M., Xuan, X.-P. & Tang, Q.-H. (2007). Acta Cryst. E63, m2523.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808036283/hg2438sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808036283/hg2438Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report