Abstract
In the title compound, C13H14N2·C14H10O5, a 1:1 cocrystal of 1,3-di-4-pyridylpropane (bpp) and 4,4′-oxydibenzoic acid (H2oba), the dihedral angle between the two benzene rings of the flexible H2oba molecule is 57.07 (1)°; the two pyridine rings of bpp make a dihedral angle of 27.52 (1)°. Strong intermolecular O—H⋯N hydrogen bonds link the molecules into chains, which are then linked into a three-dimensional network through intermolecular C—H⋯O and π–π stacking interactions [centroid–centroid distance = 3.7838 (3) Å].
Related literature
For the use of co-crystallization reactions in developing new methodologies in supramolecular synthesis, see: Desiraju (2003 ▶); Shan et al. (2002 ▶). For hydrogen bonding and π–π stacking in molecular synthesis, see: Shattock et al. (2005 ▶). For a related structure, see: Ma et al. (2006 ▶). An independent determination of this structure is reported in the preceeding paper (Li et al., 2008 ▶).
Experimental
Crystal data
C13H14N2·C14H10O5
M r = 456.48
Triclinic,
a = 6.8927 (12) Å
b = 11.5788 (19) Å
c = 14.974 (3) Å
α = 86.638 (3)°
β = 81.205 (3)°
γ = 73.963 (3)°
V = 1134.9 (3) Å3
Z = 2
Mo Kα radiation
μ = 0.09 mm−1
T = 293 (2) K
0.38 × 0.20 × 0.16 mm
Data collection
Bruker SMART CCD diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.966, T max = 0.985
5767 measured reflections
3965 independent reflections
1530 reflections with I > 2σ(I)
R int = 0.034
Refinement
R[F 2 > 2σ(F 2)] = 0.073
wR(F 2) = 0.232
S = 1.01
3965 reflections
309 parameters
H-atom parameters constrained
Δρmax = 0.64 e Å−3
Δρmin = −0.22 e Å−3
Data collection: SMART (Bruker, 1997 ▶); cell refinement: SAINT (Bruker, 1997 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808034971/fl2224sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808034971/fl2224Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯N2i | 0.82 | 1.86 | 2.679 (5) | 174 |
| O5—H5⋯N1ii | 0.82 | 1.75 | 2.566 (5) | 175 |
| C4—H4⋯O3iii | 0.93 | 2.55 | 3.418 (6) | 155 |
| C5—H5A⋯O3iv | 0.93 | 2.48 | 3.160 (6) | 130 |
| C12—H12⋯O4v | 0.93 | 2.45 | 3.174 (7) | 135 |
Symmetry codes: (i) 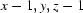 ; (ii)
; (ii) 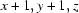 ; (iii)
; (iii) 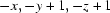 ; (iv)
; (iv) 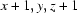 ; (v)
; (v) 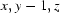 .
.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (No. 20773104), the Program for New Century Excellent Talents in Universities (NCET-06–0891), the Key Project of the Chinese Ministry of Education (208143) and the Important Project of Hubei Provincial Education Office (09HB81).
supplementary crystallographic information
Comment
Co-crystallization reactions provide helpful means for probing the importance and balance between different intermolecular interactions, and thus offer practical guidelines for developing new methodologies in supramolecular synthesis (Desiraju,2003; Shan et al., 2002). The role of hydrogen bonding and π–π stacking for these purposes is well established (Shattock et al.,2005). We attempted to synthesize a CdII complex with the mixed ligand using hydrothermal synthesis conditions. However, we were not successful and a new co-crystal, (bpp)(H2oba)(I), was isolated instead and its structure is reported here. A similar structure has been reported (Ma et al., 2006) and an independent determination of the structure of (I) is reported in the preceeding paper (Li et al., 2008).
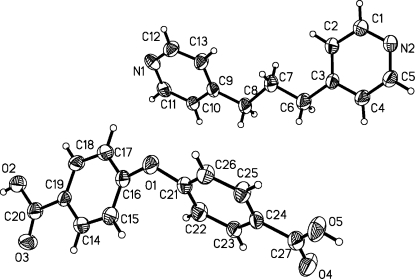
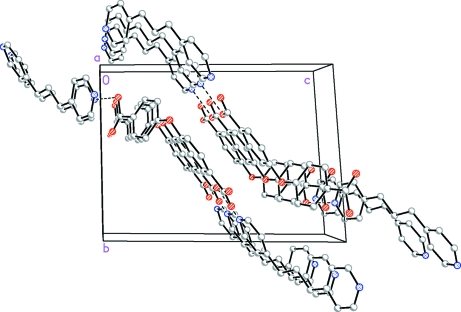
The asymmetric unit consists of one bpp and one H2oba as shown in Fig 1. The dihedral angle between the two phenyl rings of the flexible H2oba molecule is 57.07°, while it is 27.52° for the two phenyl rings of the bpp. The COOH group(O4—C27—O5) is co-planar with the phenyl ring and the other COOH group(O2—C20—O3) is slightly twisted with a the twist angle is 10.507 (8)°. In (I), the protonated carboxylate O2 of the flexible H2oba molecule forms two kinds of strong intermolecular hydrogen bonds with atoms N1 and N2 of the bpp molecule (Table 1), linking the molecules into one-dimensional chains. C—H···O hydrogen bonds involving the bpp carbon atoms (C4,C5 and C12) and uncoordinated carboxy oxygen atoms (O3 and O4) provide additional attractive forces between adjacent chains. Furthermore, there are π-π aromatic stacking interactions involving bpp ligands of adjacent units [centroid-centroid distance = 3.7838 (3) Å] that taken together with the C-H···O interactions form a three-dimensional supramolecular motif (Fig. 2).
Experimental
All chemicals were of reagent grade quality obtained from commercial sources and used without further purification. H2oba (0.5 mmol, 0.129 g), Cd(CH3COO)2.2H2O(1.5 mmol, 0.400 g), bpp(0.5 mmol, 0.099 g) and water (15 ml) were placed in a 25 ml Teflon-lined stainless steel reactor and heated at 453 K for five days, and then cooled slowly to 298 K at which time colourless crystals were obtained.The crystal used for data collection was obtained directly from the reaction mixture on cooling without further re-crystallization.
Refinement
All H atoms were positioned geometrically (C—H = 0.93 ?and O—H = 0.82 ?) and allowed to ride on their parent atoms, with Uiso(H)values equal to 1.2Ueq(C) or 1.5Ueq(O).
Figures
Fig. 1.
The structure of (I), with the atom-numbering scheme for the asymmetric unit, showing displacement ellipsoids at the 30% probability level.
Fig. 2.
Supramolecular network formed by hydrogen-bonding and π–π stacking interactions.
Crystal data
| C27H24N2O5 | Z = 2 |
| Mr = 456.48 | F000 = 480 |
| Triclinic, P1 | Dx = 1.336 Mg m−3 |
| a = 6.8927 (12) Å | Mo Kα radiation λ = 0.71073 Å |
| b = 11.5788 (19) Å | θ = 1.8–25.1º |
| c = 14.974 (3) Å | µ = 0.09 mm−1 |
| α = 86.638 (3)º | T = 293 (2) K |
| β = 81.205 (3)º | Prism, colorless |
| γ = 73.963 (3)º | 0.38 × 0.20 × 0.16 mm |
| V = 1134.9 (3) Å3 |
Data collection
| Bruker SMART CCD diffractometer | 3965 independent reflections |
| Radiation source: fine-focus sealed tube | 1530 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.034 |
| T = 293(2) K | θmax = 25.1º |
| φ and ω scans | θmin = 1.8º |
| Absorption correction: Multi-scan(SADABS; Sheldrick, 1996) | h = −4→8 |
| Tmin = 0.966, Tmax = 0.985 | k = −11→13 |
| 5767 measured reflections | l = −17→17 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.074 | H-atom parameters constrained |
| wR(F2) = 0.232 | w = 1/[σ2(Fo2) + (0.09P)2 + 0.05P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.01 | (Δ/σ)max < 0.001 |
| 3965 reflections | Δρmax = 0.64 e Å−3 |
| 309 parameters | Δρmin = −0.22 e Å−3 |
| Primary atom site location: structure-invariant direct methods | Extinction correction: none |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | −0.1091 (7) | −0.1160 (4) | 0.5739 (3) | 0.0694 (12) | |
| N2 | 0.6798 (6) | 0.1948 (4) | 0.9734 (2) | 0.0664 (12) | |
| O1 | 0.6076 (5) | 0.3326 (3) | 0.2752 (2) | 0.0738 (10) | |
| O2 | −0.0318 (6) | 0.1895 (3) | 0.0761 (2) | 0.0776 (11) | |
| H2 | −0.1262 | 0.1911 | 0.0483 | 0.116* | |
| O3 | −0.1240 (6) | 0.3872 (3) | 0.0493 (2) | 0.0835 (12) | |
| O4 | 0.5299 (6) | 0.7923 (3) | 0.5091 (3) | 0.0970 (13) | |
| O5 | 0.8627 (5) | 0.7071 (3) | 0.4860 (3) | 0.0918 (12) | |
| H5 | 0.8652 | 0.7642 | 0.5152 | 0.138* | |
| C1 | 0.6402 (8) | 0.1023 (4) | 0.9399 (3) | 0.0731 (15) | |
| H1 | 0.7252 | 0.0259 | 0.9485 | 0.088* | |
| C2 | 0.4779 (8) | 0.1132 (4) | 0.8923 (3) | 0.0720 (15) | |
| H2A | 0.4552 | 0.0449 | 0.8711 | 0.086* | |
| C3 | 0.3505 (7) | 0.2248 (4) | 0.8766 (3) | 0.0563 (12) | |
| C4 | 0.3945 (7) | 0.3207 (4) | 0.9109 (3) | 0.0665 (14) | |
| H4 | 0.3132 | 0.3982 | 0.9026 | 0.080* | |
| C5 | 0.5569 (8) | 0.3026 (4) | 0.9570 (3) | 0.0717 (15) | |
| H5A | 0.5836 | 0.3696 | 0.9783 | 0.086* | |
| C6 | 0.1679 (7) | 0.2448 (4) | 0.8272 (3) | 0.0726 (15) | |
| H6A | 0.0457 | 0.2712 | 0.8707 | 0.087* | |
| H6B | 0.1705 | 0.3100 | 0.7837 | 0.087* | |
| C7 | 0.1503 (7) | 0.1392 (4) | 0.7780 (3) | 0.0723 (15) | |
| H7A | 0.1395 | 0.0749 | 0.8213 | 0.087* | |
| H7B | 0.2735 | 0.1103 | 0.7355 | 0.087* | |
| C8 | −0.0324 (7) | 0.1698 (4) | 0.7273 (3) | 0.0658 (14) | |
| H8A | −0.1547 | 0.1987 | 0.7703 | 0.079* | |
| H8B | −0.0214 | 0.2351 | 0.6849 | 0.079* | |
| C9 | −0.0580 (7) | 0.0684 (4) | 0.6761 (3) | 0.0579 (13) | |
| C10 | −0.2435 (7) | 0.0733 (4) | 0.6501 (3) | 0.0617 (13) | |
| H10 | −0.3557 | 0.1381 | 0.6664 | 0.074* | |
| C11 | −0.2627 (8) | −0.0177 (5) | 0.6000 (3) | 0.0684 (14) | |
| H11 | −0.3895 | −0.0112 | 0.5829 | 0.082* | |
| C12 | 0.0675 (8) | −0.1175 (4) | 0.5988 (3) | 0.0741 (15) | |
| H12 | 0.1782 | −0.1829 | 0.5818 | 0.089* | |
| C13 | 0.1000 (8) | −0.0295 (4) | 0.6480 (3) | 0.0710 (15) | |
| H13 | 0.2295 | −0.0365 | 0.6622 | 0.085* | |
| C14 | 0.1947 (7) | 0.4155 (4) | 0.1356 (3) | 0.0669 (14) | |
| H14 | 0.1244 | 0.4808 | 0.1035 | 0.080* | |
| C15 | 0.3456 (8) | 0.4285 (4) | 0.1817 (3) | 0.0679 (14) | |
| H15 | 0.3811 | 0.5007 | 0.1787 | 0.081* | |
| C16 | 0.4428 (7) | 0.3329 (4) | 0.2322 (3) | 0.0600 (13) | |
| C17 | 0.3950 (7) | 0.2251 (4) | 0.2337 (3) | 0.0686 (15) | |
| H17 | 0.4633 | 0.1602 | 0.2668 | 0.082* | |
| C18 | 0.2452 (7) | 0.2132 (4) | 0.1858 (3) | 0.0642 (14) | |
| H18 | 0.2123 | 0.1403 | 0.1876 | 0.077* | |
| C19 | 0.1451 (7) | 0.3072 (4) | 0.1360 (3) | 0.0520 (12) | |
| C20 | −0.0181 (8) | 0.3011 (5) | 0.0835 (3) | 0.0645 (14) | |
| C21 | 0.6111 (8) | 0.4317 (4) | 0.3224 (3) | 0.0572 (12) | |
| C22 | 0.4418 (8) | 0.5167 (4) | 0.3598 (3) | 0.0655 (14) | |
| H22 | 0.3126 | 0.5129 | 0.3518 | 0.079* | |
| C23 | 0.4631 (7) | 0.6079 (4) | 0.4093 (3) | 0.0618 (13) | |
| H23 | 0.3483 | 0.6667 | 0.4336 | 0.074* | |
| C24 | 0.6554 (7) | 0.6123 (4) | 0.4232 (3) | 0.0533 (12) | |
| C25 | 0.8227 (7) | 0.5255 (4) | 0.3868 (3) | 0.0659 (14) | |
| H25 | 0.9519 | 0.5276 | 0.3962 | 0.079* | |
| C26 | 0.8021 (7) | 0.4350 (4) | 0.3364 (3) | 0.0656 (13) | |
| H26 | 0.9168 | 0.3763 | 0.3120 | 0.079* | |
| C27 | 0.6727 (9) | 0.7134 (5) | 0.4775 (3) | 0.0678 (14) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.055 (3) | 0.088 (3) | 0.072 (3) | −0.025 (3) | −0.024 (2) | 0.011 (2) |
| N2 | 0.069 (3) | 0.065 (3) | 0.075 (3) | −0.024 (2) | −0.024 (2) | −0.006 (2) |
| O1 | 0.070 (2) | 0.069 (2) | 0.090 (3) | −0.0147 (18) | −0.036 (2) | −0.0197 (18) |
| O2 | 0.090 (3) | 0.060 (2) | 0.098 (3) | −0.0266 (19) | −0.045 (2) | −0.0052 (17) |
| O3 | 0.088 (3) | 0.066 (2) | 0.105 (3) | −0.015 (2) | −0.055 (2) | 0.007 (2) |
| O4 | 0.073 (3) | 0.086 (3) | 0.132 (3) | −0.001 (2) | −0.034 (3) | −0.050 (2) |
| O5 | 0.071 (3) | 0.096 (3) | 0.119 (3) | −0.026 (2) | −0.027 (2) | −0.041 (2) |
| C1 | 0.084 (4) | 0.060 (3) | 0.086 (4) | −0.025 (3) | −0.032 (3) | −0.002 (3) |
| C2 | 0.086 (4) | 0.061 (3) | 0.082 (4) | −0.027 (3) | −0.035 (3) | −0.005 (3) |
| C3 | 0.058 (3) | 0.056 (3) | 0.058 (3) | −0.019 (3) | −0.011 (3) | −0.011 (2) |
| C4 | 0.058 (3) | 0.057 (3) | 0.087 (4) | −0.013 (3) | −0.019 (3) | −0.013 (3) |
| C5 | 0.073 (4) | 0.059 (3) | 0.089 (4) | −0.019 (3) | −0.022 (3) | −0.015 (3) |
| C6 | 0.068 (4) | 0.072 (3) | 0.083 (4) | −0.021 (3) | −0.020 (3) | −0.014 (3) |
| C7 | 0.074 (4) | 0.074 (3) | 0.080 (4) | −0.027 (3) | −0.026 (3) | −0.013 (3) |
| C8 | 0.062 (3) | 0.072 (3) | 0.069 (3) | −0.018 (3) | −0.025 (3) | −0.012 (3) |
| C9 | 0.058 (4) | 0.067 (3) | 0.053 (3) | −0.018 (3) | −0.020 (3) | −0.004 (2) |
| C10 | 0.059 (4) | 0.059 (3) | 0.071 (3) | −0.012 (3) | −0.026 (3) | −0.008 (2) |
| C11 | 0.054 (4) | 0.080 (3) | 0.079 (4) | −0.019 (3) | −0.032 (3) | −0.001 (3) |
| C12 | 0.062 (4) | 0.071 (3) | 0.084 (4) | −0.007 (3) | −0.008 (3) | −0.019 (3) |
| C13 | 0.055 (4) | 0.075 (3) | 0.085 (4) | −0.010 (3) | −0.021 (3) | −0.026 (3) |
| C14 | 0.078 (4) | 0.059 (3) | 0.070 (3) | −0.019 (3) | −0.030 (3) | −0.001 (2) |
| C15 | 0.079 (4) | 0.063 (3) | 0.077 (4) | −0.033 (3) | −0.029 (3) | −0.003 (3) |
| C16 | 0.066 (4) | 0.059 (3) | 0.062 (3) | −0.018 (3) | −0.024 (3) | −0.009 (2) |
| C17 | 0.075 (4) | 0.056 (3) | 0.080 (4) | −0.012 (3) | −0.034 (3) | −0.005 (3) |
| C18 | 0.074 (4) | 0.047 (3) | 0.080 (4) | −0.021 (3) | −0.027 (3) | −0.003 (2) |
| C19 | 0.055 (3) | 0.050 (3) | 0.055 (3) | −0.016 (2) | −0.014 (2) | −0.010 (2) |
| C20 | 0.066 (4) | 0.063 (3) | 0.071 (4) | −0.022 (3) | −0.019 (3) | −0.007 (3) |
| C21 | 0.062 (4) | 0.058 (3) | 0.059 (3) | −0.021 (3) | −0.021 (3) | −0.002 (2) |
| C22 | 0.049 (3) | 0.079 (3) | 0.075 (4) | −0.021 (3) | −0.019 (3) | −0.009 (3) |
| C23 | 0.056 (3) | 0.060 (3) | 0.068 (3) | −0.002 (3) | −0.026 (3) | −0.011 (2) |
| C24 | 0.057 (3) | 0.060 (3) | 0.050 (3) | −0.020 (3) | −0.017 (3) | −0.005 (2) |
| C25 | 0.050 (3) | 0.078 (3) | 0.075 (4) | −0.024 (3) | −0.008 (3) | −0.020 (3) |
| C26 | 0.047 (3) | 0.072 (3) | 0.076 (4) | −0.009 (3) | −0.010 (3) | −0.018 (3) |
| C27 | 0.065 (4) | 0.070 (4) | 0.075 (4) | −0.019 (3) | −0.025 (3) | −0.007 (3) |
Geometric parameters (Å, °)
| N1—C12 | 1.321 (6) | C9—C13 | 1.372 (6) |
| N1—C11 | 1.352 (6) | C9—C10 | 1.379 (5) |
| N2—C1 | 1.320 (5) | C10—C11 | 1.375 (6) |
| N2—C5 | 1.333 (5) | C10—H10 | 0.9300 |
| O1—C16 | 1.387 (5) | C11—H11 | 0.9300 |
| O1—C21 | 1.390 (5) | C12—C13 | 1.376 (6) |
| O2—C20 | 1.333 (5) | C12—H12 | 0.9300 |
| O2—H2 | 0.8200 | C13—H13 | 0.9300 |
| O3—C20 | 1.201 (5) | C14—C15 | 1.378 (6) |
| O4—C27 | 1.200 (5) | C14—C19 | 1.387 (5) |
| O5—C27 | 1.317 (6) | C14—H14 | 0.9300 |
| O5—H5 | 0.8200 | C15—C16 | 1.376 (6) |
| C1—C2 | 1.388 (6) | C15—H15 | 0.9300 |
| C1—H1 | 0.9300 | C16—C17 | 1.374 (6) |
| C2—C3 | 1.377 (6) | C17—C18 | 1.384 (6) |
| C2—H2A | 0.9300 | C17—H17 | 0.9300 |
| C3—C4 | 1.374 (5) | C18—C19 | 1.365 (6) |
| C3—C6 | 1.513 (6) | C18—H18 | 0.9300 |
| C4—C5 | 1.365 (6) | C19—C20 | 1.487 (6) |
| C4—H4 | 0.9300 | C21—C22 | 1.369 (6) |
| C5—H5A | 0.9300 | C21—C26 | 1.375 (6) |
| C6—C7 | 1.505 (5) | C22—C23 | 1.378 (5) |
| C6—H6A | 0.9700 | C22—H22 | 0.9300 |
| C6—H6B | 0.9700 | C23—C24 | 1.387 (6) |
| C7—C8 | 1.518 (6) | C23—H23 | 0.9300 |
| C7—H7A | 0.9700 | C24—C25 | 1.367 (6) |
| C7—H7B | 0.9700 | C24—C27 | 1.505 (6) |
| C8—C9 | 1.502 (5) | C25—C26 | 1.377 (6) |
| C8—H8A | 0.9700 | C25—H25 | 0.9300 |
| C8—H8B | 0.9700 | C26—H26 | 0.9300 |
| C12—N1—C11 | 114.4 (4) | N1—C12—H12 | 117.5 |
| C1—N2—C5 | 116.0 (4) | C13—C12—H12 | 117.5 |
| C16—O1—C21 | 121.5 (4) | C9—C13—C12 | 120.3 (5) |
| C20—O2—H2 | 109.5 | C9—C13—H13 | 119.9 |
| C27—O5—H5 | 109.5 | C12—C13—H13 | 119.9 |
| N2—C1—C2 | 123.2 (5) | C15—C14—C19 | 121.6 (4) |
| N2—C1—H1 | 118.4 | C15—C14—H14 | 119.2 |
| C2—C1—H1 | 118.4 | C19—C14—H14 | 119.2 |
| C3—C2—C1 | 120.3 (4) | C16—C15—C14 | 118.8 (4) |
| C3—C2—H2A | 119.9 | C16—C15—H15 | 120.6 |
| C1—C2—H2A | 119.9 | C14—C15—H15 | 120.6 |
| C4—C3—C2 | 116.0 (4) | C17—C16—C15 | 120.3 (4) |
| C4—C3—C6 | 120.2 (4) | C17—C16—O1 | 115.6 (4) |
| C2—C3—C6 | 123.8 (4) | C15—C16—O1 | 123.7 (4) |
| C5—C4—C3 | 120.2 (4) | C16—C17—C18 | 120.0 (4) |
| C5—C4—H4 | 119.9 | C16—C17—H17 | 120.0 |
| C3—C4—H4 | 119.9 | C18—C17—H17 | 120.0 |
| N2—C5—C4 | 124.2 (4) | C19—C18—C17 | 120.7 (4) |
| N2—C5—H5A | 117.9 | C19—C18—H18 | 119.6 |
| C4—C5—H5A | 117.9 | C17—C18—H18 | 119.6 |
| C7—C6—C3 | 116.9 (4) | C18—C19—C14 | 118.5 (4) |
| C7—C6—H6A | 108.1 | C18—C19—C20 | 123.6 (4) |
| C3—C6—H6A | 108.1 | C14—C19—C20 | 117.9 (4) |
| C7—C6—H6B | 108.1 | O3—C20—O2 | 123.2 (4) |
| C3—C6—H6B | 108.1 | O3—C20—C19 | 123.7 (5) |
| H6A—C6—H6B | 107.3 | O2—C20—C19 | 113.1 (4) |
| C6—C7—C8 | 112.9 (4) | C22—C21—C26 | 120.2 (4) |
| C6—C7—H7A | 109.0 | C22—C21—O1 | 124.9 (4) |
| C8—C7—H7A | 109.0 | C26—C21—O1 | 114.7 (4) |
| C6—C7—H7B | 109.0 | C21—C22—C23 | 119.9 (4) |
| C8—C7—H7B | 109.0 | C21—C22—H22 | 120.0 |
| H7A—C7—H7B | 107.8 | C23—C22—H22 | 120.0 |
| C9—C8—C7 | 115.6 (4) | C22—C23—C24 | 120.2 (4) |
| C9—C8—H8A | 108.4 | C22—C23—H23 | 119.9 |
| C7—C8—H8A | 108.4 | C24—C23—H23 | 119.9 |
| C9—C8—H8B | 108.4 | C25—C24—C23 | 119.2 (4) |
| C7—C8—H8B | 108.4 | C25—C24—C27 | 122.1 (4) |
| H8A—C8—H8B | 107.4 | C23—C24—C27 | 118.7 (5) |
| C13—C9—C10 | 116.1 (4) | C24—C25—C26 | 120.8 (4) |
| C13—C9—C8 | 123.2 (4) | C24—C25—H25 | 119.6 |
| C10—C9—C8 | 120.6 (4) | C26—C25—H25 | 119.6 |
| C11—C10—C9 | 120.1 (4) | C21—C26—C25 | 119.7 (5) |
| C11—C10—H10 | 120.0 | C21—C26—H26 | 120.1 |
| C9—C10—H10 | 120.0 | C25—C26—H26 | 120.1 |
| N1—C11—C10 | 124.2 (4) | O4—C27—O5 | 123.2 (5) |
| N1—C11—H11 | 117.9 | O4—C27—C24 | 124.1 (5) |
| C10—C11—H11 | 117.9 | O5—C27—C24 | 112.7 (5) |
| N1—C12—C13 | 125.0 (5) | ||
| C5—N2—C1—C2 | −1.8 (7) | C15—C16—C17—C18 | −1.5 (8) |
| N2—C1—C2—C3 | 1.2 (8) | O1—C16—C17—C18 | −174.2 (4) |
| C1—C2—C3—C4 | −0.4 (7) | C16—C17—C18—C19 | 0.8 (8) |
| C1—C2—C3—C6 | −178.7 (5) | C17—C18—C19—C14 | −1.0 (7) |
| C2—C3—C4—C5 | 0.4 (7) | C17—C18—C19—C20 | −179.6 (4) |
| C6—C3—C4—C5 | 178.8 (4) | C15—C14—C19—C18 | 2.1 (7) |
| C1—N2—C5—C4 | 1.8 (7) | C15—C14—C19—C20 | −179.3 (4) |
| C3—C4—C5—N2 | −1.1 (8) | C18—C19—C20—O3 | 170.0 (5) |
| C4—C3—C6—C7 | 169.8 (4) | C14—C19—C20—O3 | −8.5 (7) |
| C2—C3—C6—C7 | −11.9 (7) | C18—C19—C20—O2 | −11.6 (7) |
| C3—C6—C7—C8 | −177.5 (4) | C14—C19—C20—O2 | 169.8 (4) |
| C6—C7—C8—C9 | 179.6 (4) | C16—O1—C21—C22 | 24.9 (7) |
| C7—C8—C9—C13 | −23.0 (7) | C16—O1—C21—C26 | −160.3 (4) |
| C7—C8—C9—C10 | 160.9 (4) | C26—C21—C22—C23 | 2.0 (7) |
| C13—C9—C10—C11 | 0.9 (7) | O1—C21—C22—C23 | 176.6 (4) |
| C8—C9—C10—C11 | 177.2 (4) | C21—C22—C23—C24 | −1.5 (7) |
| C12—N1—C11—C10 | −1.6 (7) | C22—C23—C24—C25 | 0.2 (7) |
| C9—C10—C11—N1 | 0.8 (7) | C22—C23—C24—C27 | 179.9 (4) |
| C11—N1—C12—C13 | 0.8 (7) | C23—C24—C25—C26 | 0.5 (7) |
| C10—C9—C13—C12 | −1.7 (7) | C27—C24—C25—C26 | −179.2 (4) |
| C8—C9—C13—C12 | −177.9 (4) | C22—C21—C26—C25 | −1.3 (7) |
| N1—C12—C13—C9 | 0.9 (8) | O1—C21—C26—C25 | −176.4 (4) |
| C19—C14—C15—C16 | −2.8 (8) | C24—C25—C26—C21 | 0.0 (7) |
| C14—C15—C16—C17 | 2.4 (8) | C25—C24—C27—O4 | 178.6 (5) |
| C14—C15—C16—O1 | 174.5 (4) | C23—C24—C27—O4 | −1.1 (8) |
| C21—O1—C16—C17 | −144.3 (4) | C25—C24—C27—O5 | −0.5 (7) |
| C21—O1—C16—C15 | 43.3 (7) | C23—C24—C27—O5 | 179.8 (4) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···N2i | 0.82 | 1.86 | 2.679 (5) | 174 |
| O5—H5···N1ii | 0.82 | 1.75 | 2.566 (5) | 175 |
| C4—H4···O3iii | 0.93 | 2.55 | 3.418 (6) | 155 |
| C5—H5A···O3iv | 0.93 | 2.48 | 3.160 (6) | 130 |
| C12—H12···O4v | 0.93 | 2.45 | 3.174 (7) | 135 |
Symmetry codes: (i) x−1, y, z−1; (ii) x+1, y+1, z; (iii) −x, −y+1, −z+1; (iv) x+1, y, z+1; (v) x, y−1, z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FL2224).
References
- Bruker (1997). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Desiraju, G. R. (2003). J. Mol. Struct.656, 5–15.
- Li, G., Salim, C. & Hinode, H. (2008). Acta Cryst. E64, o2251. [DOI] [PMC free article] [PubMed]
- Ma, Z.-C., Ma, A.-Q. & Wang, G.-P. (2006). Acta Cryst. E62, o1165–o1166.
- Shan, N., Bond, A. D. & Jones, W. (2002). Tetrahedron Lett 43, 3101–3104.
- Shattock, T. R., Vishweshwar, P., Wang, Z. & Zaworotko, M. J. (2005). Cryst. Growth Des.5, 2046–2049.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I, global. DOI: 10.1107/S1600536808034971/fl2224sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808034971/fl2224Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report