Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breyer M. D., Jacobson H. R., Hebert R. L. Cellular mechanisms of prostaglandin E2 and vasopressin interactions in the collecting duct. Kidney Int. 1990 Oct;38(4):618–624. doi: 10.1038/ki.1990.251. [DOI] [PubMed] [Google Scholar]
- Brown D., Gluck S., Hartwig J. Structure of the novel membrane-coating material in proton-secreting epithelial cells and identification as an H+ATPase. J Cell Biol. 1987 Oct;105(4):1637–1648. doi: 10.1083/jcb.105.4.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Orci L. Vasopressin stimulates formation of coated pits in rat kidney collecting ducts. Nature. 1983 Mar 17;302(5905):253–255. doi: 10.1038/302253a0. [DOI] [PubMed] [Google Scholar]
- Coleman R. A., Harris H. W., Jr, Wade J. B. Visualization of endocytosed markers in freeze-fracture studies of toad urinary bladder. J Histochem Cytochem. 1987 Dec;35(12):1405–1414. doi: 10.1177/35.12.3119700. [DOI] [PubMed] [Google Scholar]
- Ding G., Franki N., Bourguet J., Hays R. M. Role of vesicular transport in ADH-stimulated aggregate delivery. Am J Physiol. 1988 Nov;255(5 Pt 1):C641–C652. doi: 10.1152/ajpcell.1988.255.5.C641. [DOI] [PubMed] [Google Scholar]
- Fettiplace R., Haydon D. A. Water permeability of lipid membranes. Physiol Rev. 1980 Apr;60(2):510–550. doi: 10.1152/physrev.1980.60.2.510. [DOI] [PubMed] [Google Scholar]
- Flamion B., Spring K. R. Water permeability of apical and basolateral cell membranes of rat inner medullary collecting duct. Am J Physiol. 1990 Dec;259(6 Pt 2):F986–F999. doi: 10.1152/ajprenal.1990.259.6.F986. [DOI] [PubMed] [Google Scholar]
- Gluck S., Al-Awqati Q. Vasopressin increases water permeability in inducing pores. Nature. 1980 Apr 17;284(5757):631–632. doi: 10.1038/284631a0. [DOI] [PubMed] [Google Scholar]
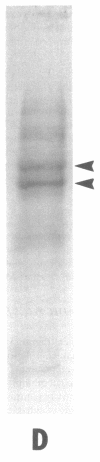
- Harris H. W., Jr Antidiuretic hormone modulates membrane phosphoproteins in toad urinary bladder and retrieved water channel containing apical membrane vesicles. J Am Soc Nephrol. 1991 Mar;1(9):1114–1122. doi: 10.1681/ASN.V191114. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Handler J. S., Blumenthal R. Apical membrane vesicles of ADH-stimulated toad bladder are highly water permeable. Am J Physiol. 1990 Feb;258(2 Pt 2):F237–F243. doi: 10.1152/ajprenal.1990.258.2.F237. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Handler J. S. The role of membrane turnover in the water permeability response to antidiuretic hormone. J Membr Biol. 1988 Aug;103(3):207–216. doi: 10.1007/BF01993980. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Kikeri D., Janoshazi A., Solomon A. K., Zeidel M. L. High proton flux through membranes containing antidiuretic hormone water channels. Am J Physiol. 1990 Aug;259(2 Pt 2):F366–F371. doi: 10.1152/ajprenal.1990.259.2.F366. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Murphy H. R., Willingham M. C., Handler J. S. Isolation and characterization of specialized regions of toad urinary bladder apical plasma membrane involved in the water permeability response to antidiuretic hormone. J Membr Biol. 1987;96(2):175–186. doi: 10.1007/BF01869243. [DOI] [PubMed] [Google Scholar]
- Harris H. W., Jr, Wade J. B., Handler J. S. Identification of specific apical membrane polypeptides associated with the antidiuretic hormone-elicited water permeability increase in the toad urinary bladder. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1942–1946. doi: 10.1073/pnas.85.6.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. W., Jr, Wade J. B., Handler J. S. Transepithelial water flow regulates apical membrane retrieval in antidiuretic hormone-stimulated toad urinary bladder. J Clin Invest. 1986 Sep;78(3):703–712. doi: 10.1172/JCI112630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert S. C., Andreoli T. E. Water movement across the mammalian cortical collecting duct. Kidney Int. 1982 Nov;22(5):526–535. doi: 10.1038/ki.1982.206. [DOI] [PubMed] [Google Scholar]
- Hoch B. S., Gorfien P. C., Linzer D., Fusco M. J., Levine S. D. Mercurial reagents inhibit flow through ADH-induced water channels in toad bladder. Am J Physiol. 1989 May;256(5 Pt 2):F948–F953. doi: 10.1152/ajprenal.1989.256.5.F948. [DOI] [PubMed] [Google Scholar]
- Humbert F., Montesano R., Grosso A., de Sousa R. C., Orci L. Particle aggregates in plasma and intracellular membranes of toad bladder (granular cell). Experientia. 1977 Oct 15;33(10):1364–1367. doi: 10.1007/BF01920184. [DOI] [PubMed] [Google Scholar]
- Kachadorian W. A., Muller J., Rudich S. W., DiScala V. A. Temperature dependence of ADH-induced water flow and intramembranous particle aggregates in toad bladder. Science. 1979 Aug 31;205(4409):910–913. doi: 10.1126/science.112678. [DOI] [PubMed] [Google Scholar]
- Kikeri D., Sun A., Zeidel M. L., Hebert S. C. Cell membranes impermeable to NH3. Nature. 1989 Jun 8;339(6224):478–480. doi: 10.1038/339478a0. [DOI] [PubMed] [Google Scholar]
- Lear J. D., Wasserman Z. R., DeGrado W. F. Synthetic amphiphilic peptide models for protein ion channels. Science. 1988 May 27;240(4856):1177–1181. doi: 10.1126/science.2453923. [DOI] [PubMed] [Google Scholar]
- Lencer W. I., Verkman A. S., Arnaout M. A., Ausiello D. A., Brown D. Endocytic vesicles from renal papilla which retrieve the vasopressin-sensitive water channel do not contain a functional H+ ATPase. J Cell Biol. 1990 Aug;111(2):379–389. doi: 10.1083/jcb.111.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S. D., Jacoby M., Finkelstein A. The water permeability of toad urinary bladder. II. The value of Pf/Pd(w) for the antidiuretic hormone-induced water permeation pathway. J Gen Physiol. 1984 Apr;83(4):543–561. doi: 10.1085/jgp.83.4.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey R. I. Transport of water and urea in red blood cells. Am J Physiol. 1984 Mar;246(3 Pt 1):C195–C203. doi: 10.1152/ajpcell.1984.246.3.C195. [DOI] [PubMed] [Google Scholar]
- Masur S. K., Cooper S., Rubin M. S. Effect of an osmotic gradient on antidiuretic hormone-induced endocytosis and hydroosmosis in the toad urinary bladder. Am J Physiol. 1984 Aug;247(2 Pt 2):F370–F379. doi: 10.1152/ajprenal.1984.247.2.F370. [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- Montal M. Molecular anatomy and molecular design of channel proteins. FASEB J. 1990 Jun;4(9):2623–2635. doi: 10.1096/fasebj.4.9.1693348. [DOI] [PubMed] [Google Scholar]
- Muller J., Kachadorian W. A. Aggregate-carrying membranes during ADH stimulation and washout in toad bladder. Am J Physiol. 1984 Jul;247(1 Pt 1):C90–C98. doi: 10.1152/ajpcell.1984.247.1.C90. [DOI] [PubMed] [Google Scholar]
- Palmer L. G., Lorenzen M. Antidiuretic hormone-dependent membrane capacitance and water permeability in the toad urinary bladder. Am J Physiol. 1983 Feb;244(2):F195–F204. doi: 10.1152/ajprenal.1983.244.2.F195. [DOI] [PubMed] [Google Scholar]
- Pearl M., Taylor A. Role of the cytoskeleton in the control of transcellular water flow by vasopressin in amphibian urinary bladder. Biol Cell. 1985;55(3):163–172. doi: 10.1111/j.1768-322x.1985.tb00421.x. [DOI] [PubMed] [Google Scholar]
- Reif M. C., Troutman S. L., Schafer J. A. Sustained response to vasopressin in isolated rat cortical collecting tubule. Kidney Int. 1984 Nov;26(5):725–732. doi: 10.1038/ki.1984.208. [DOI] [PubMed] [Google Scholar]
- Shi L. B., Brown D., Verkman A. S. Water, proton, and urea transport in toad bladder endosomes that contain the vasopressin-sensitive water channel. J Gen Physiol. 1990 May;95(5):941–960. doi: 10.1085/jgp.95.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L. B., Verkman A. S. Very high water permeability in vasopressin-induced endocytic vesicles from toad urinary bladder. J Gen Physiol. 1989 Dec;94(6):1101–1115. doi: 10.1085/jgp.94.6.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange K., Spring K. R. Cell membrane water permeability of rabbit cortical collecting duct. J Membr Biol. 1987;96(1):27–43. doi: 10.1007/BF01869332. [DOI] [PubMed] [Google Scholar]
- Strange K., Willingham M. C., Handler J. S., Harris H. W., Jr Apical membrane endocytosis via coated pits is stimulated by removal of antidiuretic hormone from isolated, perfused rabbit cortical collecting tubule. J Membr Biol. 1988 Jul;103(1):17–28. doi: 10.1007/BF01871929. [DOI] [PubMed] [Google Scholar]
- Vale R. D. Intracellular transport using microtubule-based motors. Annu Rev Cell Biol. 1987;3:347–378. doi: 10.1146/annurev.cb.03.110187.002023. [DOI] [PubMed] [Google Scholar]
- Valenti G., Guerra L., Casavola V., Svelto M. Fluorescence labeling of proteins related to ADH-induced change in frog bladder luminal membrane. Biol Cell. 1989;67(2):115–121. [PubMed] [Google Scholar]
- Verkman A. S., Lencer W. I., Brown D., Ausiello D. A. Endosomes from kidney collecting tubule cells contain the vasopressin-sensitive water channel. Nature. 1988 May 19;333(6170):268–269. doi: 10.1038/333268a0. [DOI] [PubMed] [Google Scholar]
- Verkman A. S. Mechanisms and regulation of water permeability in renal epithelia. Am J Physiol. 1989 Nov;257(5 Pt 1):C837–C850. doi: 10.1152/ajpcell.1989.257.5.C837. [DOI] [PubMed] [Google Scholar]
- Wade J. B., Stetson D. L., Lewis S. A. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]
- Zhang R. B., Verkman A. S. Water and urea permeability properties of Xenopus oocytes: expression of mRNA from toad urinary bladder. Am J Physiol. 1991 Jan;260(1 Pt 1):C26–C34. doi: 10.1152/ajpcell.1991.260.1.C26. [DOI] [PubMed] [Google Scholar]
- van Meer G. Lipid traffic in animal cells. Annu Rev Cell Biol. 1989;5:247–275. doi: 10.1146/annurev.cb.05.110189.001335. [DOI] [PubMed] [Google Scholar]