Abstract
There are two molecules in the asymmetric unit of the title compound, C19H21BrClNO, with dihedral angles between the aromatic rings of 70.0 (2) and 81.9 (3)°. The crystal structure is stabilized by intermolecular C—H⋯π and C—Br⋯π interactions. In additional, the stacked molecules exhibit intramolecular O—H⋯N hydrogen bonds.
Related literature
For the synthesis, see: Chang et al. (1998 ▶). For Schiff base compounds in coordination chemistry, see: Pu (2008 ▶). For Schiff base compounds containing salicylidene, see: Figuet et al. (2001 ▶); Kennedy & Reglinski (2001 ▶); Thamotharan et al. (2003 ▶). For related structures, see: Lin et al. (2005 ▶); Chen & Ye (2008 ▶).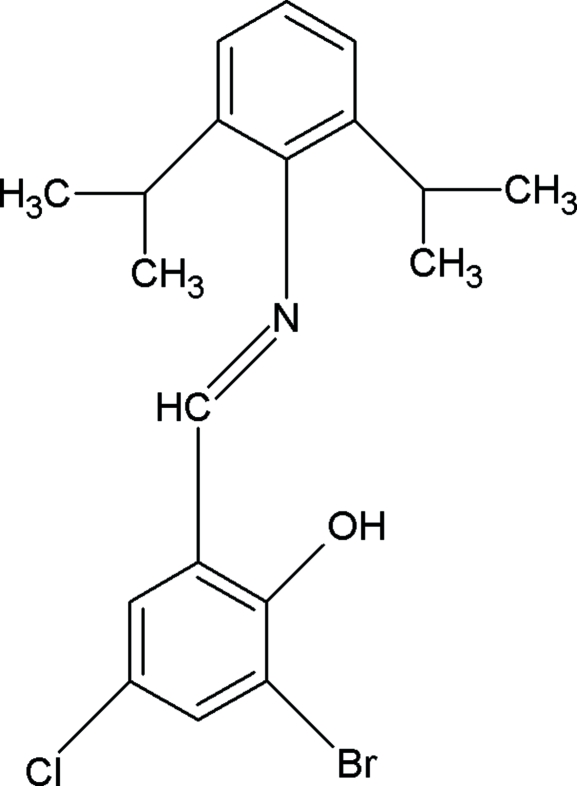
Experimental
Crystal data
C19H21BrClNO
M r = 394.73
Monoclinic,
a = 11.356 (2) Å
b = 15.045 (3) Å
c = 22.660 (5) Å
β = 91.36 (3)°
V = 3870.4 (13) Å3
Z = 8
Mo Kα radiation
μ = 2.27 mm−1
T = 293 (2) K
0.26 × 0.15 × 0.15 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.672, T max = 0.712
36408 measured reflections
6820 independent reflections
4111 reflections with I > 2σ(I)
R int = 0.045
Refinement
R[F 2 > 2σ(F 2)] = 0.045
wR(F 2) = 0.146
S = 1.04
6820 reflections
424 parameters
H-atom parameters constrained
Δρmax = 0.45 e Å−3
Δρmin = −0.60 e Å−3
Data collection: APEX2 (Bruker, 2004 ▶); cell refinement: APEX2 and SAINT (Bruker, 2004 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, (1997 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2003 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808035071/lx2070sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808035071/lx2070Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1A⋯N1 | 0.82 | 1.87 | 2.598 (4) | 147 |
| O2—H2A⋯N2 | 0.82 | 1.88 | 2.610 (4) | 147 |
| C28—H28A⋯Cg1i | 0.96 | 2.96 | 3.773 (6) | 144 |
| C16—Br1⋯Cg4i | 1.88 | 3.53 | 4.75 (2) | 120 |
Symmetry code: (i) 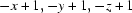 . Cg1 is the centroid of the C14–C19 benzene ring and Cg4 is the centroid of the C33–C38 benzene ring..
. Cg1 is the centroid of the C14–C19 benzene ring and Cg4 is the centroid of the C33–C38 benzene ring..
Acknowledgments
The authors are grateful to Dr. J. Jothi Kumar, Principle of Presidency College (Autonomous), Chennai, for providing the computer and internet facilities. Dr Babu Varghese, SAIF, IIT, Madras, India, is thanked for collecting the X-ray intensity data.
supplementary crystallographic information
Comment
Schiff base compounds have been of great interest for many years. These compounds play an important role in the development of coordination chemistry related to catalysis and enzymatic reactions, magnetism and molecular architectures (Pu, 2008). As a part of our ongoing investigation in this field we have determined the crystal structure of the title compound, (I).
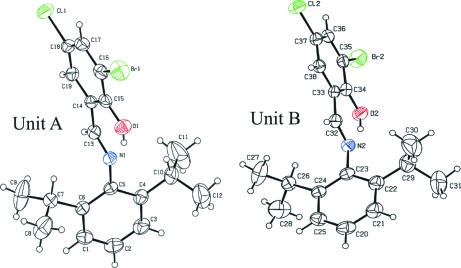
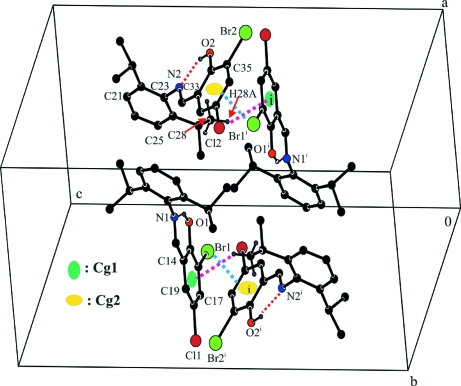
Fig.1 shows the asymmetric unit consisting of two molecules of (I) viz. unit A and unit B. The two crystallographically independent molecules have the same geometrical parameters within the precision of the experiments. The bond lengths and angles in (I) are comparable to the corresponding values in the related structure, 2-Bezyliminomethyl-6-bromo-4-chloro-phenol (Pu, 2008). Like other Schiff base compounds containing salicylidene (Figuet et al., 2001; Kennedy & Reglinski, 2001; Thamotharan et al., 2003) the hydroxyl groups form intramolecular hydrogen bonds with the N atoms, thereby completing six-membered rings (Fig. 2). The molecular packing is stabilized by intermolecular C—H···π and C—Br···π interactions, with a C28—H28A···Cg1i separation of 2.96 Å and a C16—Br1···Cg2i separation of 3.532 (5) Å (Fig. 2 and Table 1; Cg1 and Cg2 are the centroids of the C14-C19 and C33-C38 benzene rings, respectively, symmetry code as in Fig. 2). In addition, the molecular packing is further stabilized by two intramolecular O—H···N hydrogen bonds (Table 1).
Experimental
The title compound was synthesized by refluxing an ethanol solution (20 ml) of 5-bromo-3-chloro-2-hydroxybenzaldehyde (1.72 g, 10 mmol) and 2,6-diisopropylaniline (1.72 g, 10 mmol), at 80°C for 2 h. Upon cooling to 0°C, a yellow solid crystalline product was obtained. The precipitate was filtered off and washed with cold ethanol. Single crystal of good diffraction quality was obtained by the recrystallization of compound with ethanol solution by slow evaporation method.
Refinement
All H atoms were fixed geometrically and allowed to ride on their parent C atoms, with C—H distances fixed in the range (0.82–0.97)Å with Uiso(H)= 1.5Ueq(methyl H) and 1.2Ueq(for other H atoms).
Figures
Fig. 1.
The molecular structure of title compound showing 30% probability displacement ellipsoids.
Fig. 2.
C—H···π, C—Br···π and O—H···N interactions (dotted lines) in the title compound. Cg denotes the ring centroid. [Symmetry code: (i) -x+1, -y+1, -z+1.]
Crystal data
| C19H21BrClNO | F000 = 1616 |
| Mr = 394.73 | Dx = 1.355 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 9432 reflections |
| a = 11.356 (2) Å | θ = 1.6–28.1º |
| b = 15.045 (3) Å | µ = 2.27 mm−1 |
| c = 22.660 (5) Å | T = 293 (2) K |
| β = 91.36 (3)º | Block, colourless |
| V = 3870.4 (13) Å3 | 0.26 × 0.15 × 0.15 mm |
| Z = 8 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 6820 independent reflections |
| Radiation source: fine-focus sealed tube | 4111 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.045 |
| Detector resolution: 10.0 pixels mm-1 | θmax = 25.0º |
| T = 293(2) K | θmin = 1.6º |
| ω and φ scans | h = −13→13 |
| Absorption correction: Multi-scan(SADABS; Sheldrick, 1996) | k = −17→17 |
| Tmin = 0.672, Tmax = 0.712 | l = −26→26 |
| 36408 measured reflections |
Refinement
| Refinement on F2 | Hydrogen site location: difference Fourier map |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.045 | w = 1/[σ2(Fo2) + (0.0678P)2 + 2.2687P] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.146 | (Δ/σ)max < 0.001 |
| S = 1.04 | Δρmax = 0.45 e Å−3 |
| 6820 reflections | Δρmin = −0.60 e Å−3 |
| 424 parameters | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.0011 (2) |
| Secondary atom site location: difference Fourier map |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.40344 (6) | 0.77524 (4) | 0.52632 (2) | 0.0958 (2) | |
| Br2 | 0.84887 (5) | −0.02832 (4) | 0.51695 (3) | 0.0954 (2) | |
| Cl1 | −0.06647 (12) | 0.75082 (9) | 0.58127 (6) | 0.0945 (5) | |
| Cl2 | 0.39884 (10) | 0.00891 (9) | 0.60014 (6) | 0.0805 (4) | |
| O1 | 0.4129 (2) | 0.60711 (19) | 0.59597 (13) | 0.0682 (8) | |
| H1A | 0.4147 | 0.5609 | 0.6152 | 0.102* | |
| O2 | 0.8822 (2) | 0.14048 (19) | 0.58425 (13) | 0.0672 (8) | |
| H2A | 0.8905 | 0.1875 | 0.6022 | 0.101* | |
| N1 | 0.3289 (3) | 0.4748 (2) | 0.65542 (13) | 0.0535 (8) | |
| N2 | 0.8189 (3) | 0.2768 (2) | 0.64732 (14) | 0.0564 (8) | |
| C1 | 0.3129 (4) | 0.2336 (3) | 0.68334 (19) | 0.0645 (11) | |
| H1 | 0.2833 | 0.1820 | 0.6660 | 0.077* | |
| C2 | 0.3718 (4) | 0.2290 (3) | 0.7371 (2) | 0.0744 (13) | |
| H2 | 0.3799 | 0.1745 | 0.7561 | 0.089* | |
| C3 | 0.4184 (4) | 0.3039 (3) | 0.76285 (19) | 0.0748 (13) | |
| H3 | 0.4600 | 0.2991 | 0.7986 | 0.090* | |
| C4 | 0.4050 (4) | 0.3865 (3) | 0.73692 (17) | 0.0624 (11) | |
| C5 | 0.3417 (3) | 0.3900 (3) | 0.68311 (16) | 0.0499 (9) | |
| C6 | 0.2973 (3) | 0.3146 (3) | 0.65476 (16) | 0.0512 (9) | |
| C7 | 0.2381 (4) | 0.3171 (3) | 0.59384 (19) | 0.0658 (11) | |
| H7 | 0.2328 | 0.3797 | 0.5821 | 0.079* | |
| C8 | 0.3125 (5) | 0.2709 (5) | 0.5489 (2) | 0.118 (2) | |
| H8A | 0.3185 | 0.2089 | 0.5585 | 0.178* | |
| H8B | 0.3897 | 0.2969 | 0.5493 | 0.178* | |
| H8C | 0.2766 | 0.2776 | 0.5104 | 0.178* | |
| C9 | 0.1148 (5) | 0.2808 (6) | 0.5929 (3) | 0.145 (3) | |
| H9A | 0.1171 | 0.2179 | 0.5997 | 0.218* | |
| H9B | 0.0779 | 0.2925 | 0.5551 | 0.218* | |
| H9C | 0.0705 | 0.3090 | 0.6232 | 0.218* | |
| C10 | 0.4545 (5) | 0.4695 (3) | 0.7655 (2) | 0.0890 (16) | |
| H10 | 0.4494 | 0.5173 | 0.7363 | 0.107* | |
| C11 | 0.3828 (7) | 0.4963 (6) | 0.8163 (4) | 0.205 (5) | |
| H11A | 0.3029 | 0.5062 | 0.8032 | 0.307* | |
| H11B | 0.4142 | 0.5500 | 0.8333 | 0.307* | |
| H11C | 0.3847 | 0.4500 | 0.8455 | 0.307* | |
| C12 | 0.5830 (6) | 0.4609 (5) | 0.7853 (3) | 0.136 (3) | |
| H12A | 0.6164 | 0.5190 | 0.7906 | 0.204* | |
| H12B | 0.6256 | 0.4293 | 0.7559 | 0.204* | |
| H12C | 0.5879 | 0.4289 | 0.8220 | 0.204* | |
| C13 | 0.2279 (4) | 0.5113 (3) | 0.65150 (15) | 0.0518 (10) | |
| H13 | 0.1642 | 0.4836 | 0.6688 | 0.062* | |
| C14 | 0.2094 (3) | 0.5947 (2) | 0.62079 (15) | 0.0494 (9) | |
| C15 | 0.3027 (4) | 0.6388 (2) | 0.59373 (16) | 0.0522 (10) | |
| C16 | 0.2787 (4) | 0.7172 (3) | 0.56345 (17) | 0.0627 (11) | |
| C17 | 0.1650 (5) | 0.7516 (3) | 0.55948 (18) | 0.0703 (13) | |
| H17 | 0.1500 | 0.8044 | 0.5393 | 0.084* | |
| C18 | 0.0757 (4) | 0.7064 (3) | 0.58573 (19) | 0.0657 (12) | |
| C19 | 0.0958 (4) | 0.6301 (3) | 0.61582 (17) | 0.0579 (10) | |
| H19 | 0.0336 | 0.6010 | 0.6334 | 0.070* | |
| C20 | 0.8777 (5) | 0.5253 (3) | 0.7251 (2) | 0.0784 (14) | |
| H20 | 0.8885 | 0.5804 | 0.7430 | 0.094* | |
| C21 | 0.9117 (4) | 0.4493 (4) | 0.7540 (2) | 0.0772 (14) | |
| H21 | 0.9476 | 0.4540 | 0.7912 | 0.093* | |
| C22 | 0.8946 (4) | 0.3656 (3) | 0.72966 (18) | 0.0672 (12) | |
| C23 | 0.8386 (3) | 0.3623 (3) | 0.67371 (17) | 0.0538 (10) | |
| C24 | 0.8085 (3) | 0.4385 (3) | 0.64220 (17) | 0.0542 (10) | |
| C25 | 0.8273 (4) | 0.5199 (3) | 0.66907 (18) | 0.0664 (12) | |
| H25 | 0.8057 | 0.5717 | 0.6492 | 0.080* | |
| C26 | 0.7578 (4) | 0.4342 (3) | 0.57945 (18) | 0.0682 (12) | |
| H26 | 0.7622 | 0.3720 | 0.5668 | 0.082* | |
| C27 | 0.6302 (5) | 0.4608 (5) | 0.5760 (3) | 0.135 (3) | |
| H27A | 0.5876 | 0.4291 | 0.6055 | 0.202* | |
| H27B | 0.5980 | 0.4467 | 0.5376 | 0.202* | |
| H27C | 0.6235 | 0.5235 | 0.5828 | 0.202* | |
| C28 | 0.8313 (7) | 0.4877 (5) | 0.5378 (2) | 0.123 (2) | |
| H28A | 0.8103 | 0.4723 | 0.4978 | 0.185* | |
| H28B | 0.9132 | 0.4750 | 0.5451 | 0.185* | |
| H28C | 0.8172 | 0.5499 | 0.5439 | 0.185* | |
| C29 | 0.9318 (5) | 0.2826 (4) | 0.7619 (2) | 0.0933 (17) | |
| H29 | 0.9285 | 0.2336 | 0.7334 | 0.112* | |
| C30 | 0.8473 (7) | 0.2609 (6) | 0.8100 (4) | 0.168 (4) | |
| H30A | 0.8482 | 0.3080 | 0.8386 | 0.253* | |
| H30B | 0.8707 | 0.2063 | 0.8288 | 0.253* | |
| H30C | 0.7693 | 0.2546 | 0.7933 | 0.253* | |
| C31 | 1.0574 (6) | 0.2868 (5) | 0.7873 (3) | 0.129 (2) | |
| H31A | 1.0612 | 0.3293 | 0.8188 | 0.193* | |
| H31B | 1.1101 | 0.3043 | 0.7569 | 0.193* | |
| H31C | 1.0800 | 0.2293 | 0.8021 | 0.193* | |
| C32 | 0.7163 (4) | 0.2424 (3) | 0.64959 (16) | 0.0545 (10) | |
| H32 | 0.6581 | 0.2726 | 0.6696 | 0.065* | |
| C33 | 0.6875 (3) | 0.1580 (3) | 0.62199 (15) | 0.0487 (9) | |
| C34 | 0.7715 (3) | 0.1114 (3) | 0.58969 (17) | 0.0523 (10) | |
| C35 | 0.7374 (4) | 0.0327 (3) | 0.56155 (18) | 0.0582 (10) | |
| C36 | 0.6244 (4) | 0.0016 (3) | 0.56471 (18) | 0.0599 (11) | |
| H36 | 0.6026 | −0.0508 | 0.5456 | 0.072* | |
| C37 | 0.5435 (4) | 0.0485 (3) | 0.59632 (17) | 0.0577 (11) | |
| C38 | 0.5731 (3) | 0.1258 (3) | 0.62503 (16) | 0.0535 (10) | |
| H38 | 0.5171 | 0.1564 | 0.6464 | 0.064* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.1269 (5) | 0.0750 (4) | 0.0857 (4) | −0.0169 (3) | 0.0053 (3) | 0.0256 (3) |
| Br2 | 0.0774 (4) | 0.0823 (4) | 0.1261 (5) | 0.0228 (3) | −0.0081 (3) | −0.0355 (3) |
| Cl1 | 0.0945 (9) | 0.0948 (10) | 0.0926 (9) | 0.0458 (8) | −0.0318 (7) | −0.0111 (7) |
| Cl2 | 0.0632 (7) | 0.0844 (8) | 0.0938 (9) | −0.0237 (6) | −0.0056 (6) | 0.0003 (7) |
| O1 | 0.0665 (18) | 0.0584 (18) | 0.080 (2) | 0.0030 (14) | 0.0035 (15) | 0.0180 (15) |
| O2 | 0.0524 (17) | 0.0645 (19) | 0.084 (2) | −0.0023 (14) | −0.0048 (14) | −0.0114 (15) |
| N1 | 0.058 (2) | 0.049 (2) | 0.0534 (19) | 0.0039 (16) | −0.0065 (15) | 0.0063 (15) |
| N2 | 0.059 (2) | 0.055 (2) | 0.055 (2) | −0.0039 (16) | −0.0047 (16) | −0.0025 (16) |
| C1 | 0.071 (3) | 0.052 (3) | 0.071 (3) | 0.000 (2) | 0.011 (2) | 0.009 (2) |
| C2 | 0.092 (3) | 0.065 (3) | 0.067 (3) | 0.017 (3) | 0.011 (3) | 0.025 (3) |
| C3 | 0.093 (3) | 0.079 (4) | 0.053 (3) | 0.015 (3) | −0.008 (2) | 0.018 (3) |
| C4 | 0.073 (3) | 0.065 (3) | 0.048 (2) | 0.008 (2) | −0.007 (2) | 0.007 (2) |
| C5 | 0.050 (2) | 0.048 (2) | 0.051 (2) | 0.0043 (18) | 0.0009 (17) | 0.0100 (19) |
| C6 | 0.050 (2) | 0.054 (2) | 0.050 (2) | 0.0012 (18) | 0.0025 (17) | 0.006 (2) |
| C7 | 0.070 (3) | 0.058 (3) | 0.069 (3) | 0.002 (2) | −0.009 (2) | −0.004 (2) |
| C8 | 0.102 (4) | 0.190 (7) | 0.064 (3) | 0.030 (4) | 0.000 (3) | −0.010 (4) |
| C9 | 0.068 (4) | 0.263 (10) | 0.104 (5) | −0.039 (5) | −0.011 (3) | −0.006 (5) |
| C10 | 0.109 (4) | 0.084 (4) | 0.072 (3) | 0.007 (3) | −0.039 (3) | −0.003 (3) |
| C11 | 0.127 (6) | 0.229 (10) | 0.262 (11) | −0.018 (6) | 0.058 (7) | −0.171 (9) |
| C12 | 0.104 (5) | 0.158 (7) | 0.146 (6) | −0.026 (4) | 0.000 (4) | −0.046 (5) |
| C13 | 0.062 (3) | 0.053 (2) | 0.041 (2) | 0.000 (2) | 0.0007 (18) | 0.0027 (18) |
| C14 | 0.065 (2) | 0.045 (2) | 0.038 (2) | 0.0080 (19) | −0.0076 (18) | −0.0048 (17) |
| C15 | 0.072 (3) | 0.043 (2) | 0.041 (2) | 0.003 (2) | −0.0069 (19) | −0.0025 (18) |
| C16 | 0.094 (3) | 0.050 (3) | 0.044 (2) | −0.001 (2) | −0.007 (2) | 0.001 (2) |
| C17 | 0.109 (4) | 0.049 (3) | 0.052 (3) | 0.018 (3) | −0.023 (3) | 0.000 (2) |
| C18 | 0.083 (3) | 0.059 (3) | 0.054 (3) | 0.023 (2) | −0.022 (2) | −0.007 (2) |
| C19 | 0.062 (2) | 0.062 (3) | 0.049 (2) | 0.012 (2) | −0.0101 (19) | −0.008 (2) |
| C20 | 0.097 (4) | 0.075 (3) | 0.063 (3) | −0.025 (3) | 0.005 (3) | −0.022 (3) |
| C21 | 0.094 (3) | 0.087 (4) | 0.050 (3) | −0.013 (3) | −0.009 (2) | −0.013 (3) |
| C22 | 0.077 (3) | 0.072 (3) | 0.052 (3) | −0.008 (2) | −0.004 (2) | −0.007 (2) |
| C23 | 0.053 (2) | 0.057 (3) | 0.052 (2) | −0.0062 (19) | 0.0045 (19) | −0.011 (2) |
| C24 | 0.057 (2) | 0.056 (3) | 0.049 (2) | −0.0057 (19) | 0.0018 (18) | −0.009 (2) |
| C25 | 0.080 (3) | 0.059 (3) | 0.060 (3) | −0.007 (2) | 0.007 (2) | −0.007 (2) |
| C26 | 0.086 (3) | 0.060 (3) | 0.058 (3) | 0.002 (2) | −0.014 (2) | −0.009 (2) |
| C27 | 0.100 (5) | 0.185 (8) | 0.117 (5) | 0.036 (5) | −0.044 (4) | −0.016 (5) |
| C28 | 0.183 (6) | 0.135 (5) | 0.051 (3) | −0.051 (5) | −0.004 (3) | 0.000 (3) |
| C29 | 0.125 (5) | 0.091 (4) | 0.062 (3) | −0.005 (3) | −0.023 (3) | 0.004 (3) |
| C30 | 0.131 (6) | 0.188 (8) | 0.186 (8) | −0.012 (6) | 0.008 (6) | 0.109 (7) |
| C31 | 0.123 (5) | 0.164 (7) | 0.099 (5) | 0.027 (5) | −0.016 (4) | 0.031 (4) |
| C32 | 0.057 (3) | 0.061 (3) | 0.045 (2) | 0.002 (2) | −0.0028 (18) | 0.0005 (19) |
| C33 | 0.053 (2) | 0.052 (2) | 0.040 (2) | −0.0047 (18) | −0.0110 (17) | 0.0057 (18) |
| C34 | 0.050 (2) | 0.051 (2) | 0.055 (2) | 0.0019 (19) | −0.0112 (18) | 0.007 (2) |
| C35 | 0.062 (3) | 0.048 (2) | 0.064 (3) | 0.011 (2) | −0.015 (2) | 0.001 (2) |
| C36 | 0.070 (3) | 0.046 (2) | 0.063 (3) | 0.002 (2) | −0.017 (2) | 0.007 (2) |
| C37 | 0.059 (2) | 0.062 (3) | 0.051 (2) | −0.011 (2) | −0.011 (2) | 0.013 (2) |
| C38 | 0.056 (2) | 0.057 (3) | 0.047 (2) | −0.0009 (19) | −0.0038 (18) | 0.0064 (19) |
Geometric parameters (Å, °)
| Br1—C16 | 1.880 (5) | C15—C16 | 1.388 (5) |
| Br2—C35 | 1.878 (4) | C16—C17 | 1.392 (6) |
| Cl1—C18 | 1.748 (5) | C17—C18 | 1.369 (6) |
| Cl2—C37 | 1.751 (4) | C17—H17 | 0.9300 |
| O1—C15 | 1.339 (5) | C18—C19 | 1.351 (6) |
| O1—H1A | 0.8200 | C19—H19 | 0.9300 |
| O2—C34 | 1.339 (4) | C20—C21 | 1.368 (7) |
| O2—H2A | 0.8200 | C20—C25 | 1.382 (6) |
| N1—C13 | 1.273 (5) | C20—H20 | 0.9300 |
| N1—C5 | 1.427 (5) | C21—C22 | 1.387 (6) |
| N2—C32 | 1.276 (5) | C21—H21 | 0.9300 |
| N2—C23 | 1.434 (5) | C22—C23 | 1.406 (5) |
| C1—C2 | 1.377 (6) | C22—C29 | 1.503 (7) |
| C1—C6 | 1.389 (5) | C23—C24 | 1.390 (5) |
| C1—H1 | 0.9300 | C24—C25 | 1.381 (6) |
| C2—C3 | 1.370 (7) | C24—C26 | 1.523 (5) |
| C2—H2 | 0.9300 | C25—H25 | 0.9300 |
| C3—C4 | 1.380 (6) | C26—C27 | 1.504 (7) |
| C3—H3 | 0.9300 | C26—C28 | 1.507 (7) |
| C4—C5 | 1.402 (5) | C26—H26 | 0.9800 |
| C4—C10 | 1.510 (6) | C27—H27A | 0.9600 |
| C5—C6 | 1.393 (5) | C27—H27B | 0.9600 |
| C6—C7 | 1.521 (5) | C27—H27C | 0.9600 |
| C7—C9 | 1.503 (7) | C28—H28A | 0.9600 |
| C7—C8 | 1.507 (7) | C28—H28B | 0.9600 |
| C7—H7 | 0.9800 | C28—H28C | 0.9600 |
| C8—H8A | 0.9600 | C29—C30 | 1.505 (8) |
| C8—H8B | 0.9600 | C29—C31 | 1.527 (8) |
| C8—H8C | 0.9600 | C29—H29 | 0.9800 |
| C9—H9A | 0.9600 | C30—H30A | 0.9600 |
| C9—H9B | 0.9600 | C30—H30B | 0.9600 |
| C9—H9C | 0.9600 | C30—H30C | 0.9600 |
| C10—C11 | 1.482 (8) | C31—H31A | 0.9600 |
| C10—C12 | 1.522 (8) | C31—H31B | 0.9600 |
| C10—H10 | 0.9800 | C31—H31C | 0.9600 |
| C11—H11A | 0.9600 | C32—C33 | 1.450 (5) |
| C11—H11B | 0.9600 | C32—H32 | 0.9300 |
| C11—H11C | 0.9600 | C33—C38 | 1.390 (5) |
| C12—H12A | 0.9600 | C33—C34 | 1.404 (5) |
| C12—H12B | 0.9600 | C34—C35 | 1.395 (5) |
| C12—H12C | 0.9600 | C35—C36 | 1.371 (6) |
| C13—C14 | 1.448 (5) | C36—C37 | 1.373 (6) |
| C13—H13 | 0.9300 | C36—H36 | 0.9300 |
| C14—C19 | 1.398 (5) | C37—C38 | 1.370 (5) |
| C14—C15 | 1.403 (5) | C38—H38 | 0.9300 |
| C15—O1—H1A | 109.5 | C18—C19—H19 | 119.7 |
| C34—O2—H2A | 109.5 | C14—C19—H19 | 119.7 |
| C13—N1—C5 | 119.9 (3) | C21—C20—C25 | 119.7 (4) |
| C32—N2—C23 | 118.7 (3) | C21—C20—H20 | 120.1 |
| C2—C1—C6 | 120.7 (4) | C25—C20—H20 | 120.1 |
| C2—C1—H1 | 119.7 | C20—C21—C22 | 122.2 (4) |
| C6—C1—H1 | 119.7 | C20—C21—H21 | 118.9 |
| C3—C2—C1 | 120.7 (4) | C22—C21—H21 | 118.9 |
| C3—C2—H2 | 119.7 | C21—C22—C23 | 116.5 (4) |
| C1—C2—H2 | 119.7 | C21—C22—C29 | 121.8 (4) |
| C2—C3—C4 | 121.4 (4) | C23—C22—C29 | 121.6 (4) |
| C2—C3—H3 | 119.3 | C24—C23—C22 | 122.3 (4) |
| C4—C3—H3 | 119.3 | C24—C23—N2 | 119.5 (3) |
| C3—C4—C5 | 117.0 (4) | C22—C23—N2 | 118.1 (4) |
| C3—C4—C10 | 121.7 (4) | C25—C24—C23 | 118.1 (4) |
| C5—C4—C10 | 121.3 (4) | C25—C24—C26 | 120.0 (4) |
| C6—C5—C4 | 122.8 (4) | C23—C24—C26 | 121.8 (4) |
| C6—C5—N1 | 119.6 (3) | C24—C25—C20 | 120.9 (4) |
| C4—C5—N1 | 117.5 (4) | C24—C25—H25 | 119.6 |
| C1—C6—C5 | 117.3 (4) | C20—C25—H25 | 119.6 |
| C1—C6—C7 | 119.6 (4) | C27—C26—C28 | 111.9 (5) |
| C5—C6—C7 | 123.0 (4) | C27—C26—C24 | 112.3 (4) |
| C9—C7—C8 | 111.1 (5) | C28—C26—C24 | 111.1 (4) |
| C9—C7—C6 | 113.3 (4) | C27—C26—H26 | 107.1 |
| C8—C7—C6 | 111.0 (4) | C28—C26—H26 | 107.1 |
| C9—C7—H7 | 107.0 | C24—C26—H26 | 107.1 |
| C8—C7—H7 | 107.0 | C26—C27—H27A | 109.5 |
| C6—C7—H7 | 107.0 | C26—C27—H27B | 109.5 |
| C7—C8—H8A | 109.5 | H27A—C27—H27B | 109.5 |
| C7—C8—H8B | 109.5 | C26—C27—H27C | 109.5 |
| H8A—C8—H8B | 109.5 | H27A—C27—H27C | 109.5 |
| C7—C8—H8C | 109.5 | H27B—C27—H27C | 109.5 |
| H8A—C8—H8C | 109.5 | C26—C28—H28A | 109.5 |
| H8B—C8—H8C | 109.5 | C26—C28—H28B | 109.5 |
| C7—C9—H9A | 109.5 | H28A—C28—H28B | 109.5 |
| C7—C9—H9B | 109.5 | C26—C28—H28C | 109.5 |
| H9A—C9—H9B | 109.5 | H28A—C28—H28C | 109.5 |
| C7—C9—H9C | 109.5 | H28B—C28—H28C | 109.5 |
| H9A—C9—H9C | 109.5 | C22—C29—C30 | 110.8 (5) |
| H9B—C9—H9C | 109.5 | C22—C29—C31 | 113.4 (5) |
| C11—C10—C4 | 110.7 (5) | C30—C29—C31 | 110.0 (5) |
| C11—C10—C12 | 109.6 (5) | C22—C29—H29 | 107.4 |
| C4—C10—C12 | 113.6 (5) | C30—C29—H29 | 107.4 |
| C11—C10—H10 | 107.6 | C31—C29—H29 | 107.4 |
| C4—C10—H10 | 107.6 | C29—C30—H30A | 109.5 |
| C12—C10—H10 | 107.6 | C29—C30—H30B | 109.5 |
| C10—C11—H11A | 109.5 | H30A—C30—H30B | 109.5 |
| C10—C11—H11B | 109.5 | C29—C30—H30C | 109.5 |
| H11A—C11—H11B | 109.5 | H30A—C30—H30C | 109.5 |
| C10—C11—H11C | 109.5 | H30B—C30—H30C | 109.5 |
| H11A—C11—H11C | 109.5 | C29—C31—H31A | 109.5 |
| H11B—C11—H11C | 109.5 | C29—C31—H31B | 109.5 |
| C10—C12—H12A | 109.5 | H31A—C31—H31B | 109.5 |
| C10—C12—H12B | 109.5 | C29—C31—H31C | 109.5 |
| H12A—C12—H12B | 109.5 | H31A—C31—H31C | 109.5 |
| C10—C12—H12C | 109.5 | H31B—C31—H31C | 109.5 |
| H12A—C12—H12C | 109.5 | N2—C32—C33 | 122.3 (4) |
| H12B—C12—H12C | 109.5 | N2—C32—H32 | 118.8 |
| N1—C13—C14 | 121.9 (4) | C33—C32—H32 | 118.8 |
| N1—C13—H13 | 119.1 | C38—C33—C34 | 120.0 (4) |
| C14—C13—H13 | 119.1 | C38—C33—C32 | 119.1 (4) |
| C19—C14—C15 | 119.3 (4) | C34—C33—C32 | 120.8 (3) |
| C19—C14—C13 | 119.5 (4) | O2—C34—C35 | 119.1 (4) |
| C15—C14—C13 | 121.2 (3) | O2—C34—C33 | 122.4 (3) |
| O1—C15—C16 | 119.5 (4) | C35—C34—C33 | 118.5 (4) |
| O1—C15—C14 | 122.0 (3) | C36—C35—C34 | 121.1 (4) |
| C16—C15—C14 | 118.5 (4) | C36—C35—Br2 | 120.3 (3) |
| C15—C16—C17 | 121.2 (4) | C34—C35—Br2 | 118.5 (3) |
| C15—C16—Br1 | 118.4 (3) | C35—C36—C37 | 119.4 (4) |
| C17—C16—Br1 | 120.4 (3) | C35—C36—H36 | 120.3 |
| C18—C17—C16 | 118.8 (4) | C37—C36—H36 | 120.3 |
| C18—C17—H17 | 120.6 | C38—C37—C36 | 121.6 (4) |
| C16—C17—H17 | 120.6 | C38—C37—Cl2 | 119.0 (3) |
| C19—C18—C17 | 121.6 (4) | C36—C37—Cl2 | 119.4 (3) |
| C19—C18—Cl1 | 119.9 (4) | C37—C38—C33 | 119.4 (4) |
| C17—C18—Cl1 | 118.5 (3) | C37—C38—H38 | 120.3 |
| C18—C19—C14 | 120.6 (4) | C33—C38—H38 | 120.3 |
| C6—C1—C2—C3 | −1.5 (7) | C25—C20—C21—C22 | −1.8 (8) |
| C1—C2—C3—C4 | 2.1 (7) | C20—C21—C22—C23 | −0.9 (7) |
| C2—C3—C4—C5 | −0.1 (7) | C20—C21—C22—C29 | −179.7 (5) |
| C2—C3—C4—C10 | 179.0 (5) | C21—C22—C23—C24 | 4.1 (6) |
| C3—C4—C5—C6 | −2.6 (6) | C29—C22—C23—C24 | −177.0 (4) |
| C10—C4—C5—C6 | 178.4 (4) | C21—C22—C23—N2 | −179.7 (4) |
| C3—C4—C5—N1 | −179.0 (4) | C29—C22—C23—N2 | −0.9 (6) |
| C10—C4—C5—N1 | 2.0 (6) | C32—N2—C23—C24 | −82.8 (5) |
| C13—N1—C5—C6 | 71.3 (5) | C32—N2—C23—C22 | 100.9 (4) |
| C13—N1—C5—C4 | −112.2 (4) | C22—C23—C24—C25 | −4.6 (6) |
| C2—C1—C6—C5 | −1.0 (6) | N2—C23—C24—C25 | 179.3 (3) |
| C2—C1—C6—C7 | 176.6 (4) | C22—C23—C24—C26 | 174.5 (4) |
| C4—C5—C6—C1 | 3.1 (6) | N2—C23—C24—C26 | −1.7 (6) |
| N1—C5—C6—C1 | 179.4 (3) | C23—C24—C25—C20 | 1.8 (6) |
| C4—C5—C6—C7 | −174.4 (4) | C26—C24—C25—C20 | −177.3 (4) |
| N1—C5—C6—C7 | 2.0 (6) | C21—C20—C25—C24 | 1.3 (7) |
| C1—C6—C7—C9 | 60.1 (6) | C25—C24—C26—C27 | −70.8 (6) |
| C5—C6—C7—C9 | −122.5 (5) | C23—C24—C26—C27 | 110.2 (5) |
| C1—C6—C7—C8 | −65.7 (6) | C25—C24—C26—C28 | 55.4 (6) |
| C5—C6—C7—C8 | 111.7 (5) | C23—C24—C26—C28 | −123.7 (5) |
| C3—C4—C10—C11 | −74.4 (7) | C21—C22—C29—C30 | 75.9 (7) |
| C5—C4—C10—C11 | 104.6 (6) | C23—C22—C29—C30 | −102.9 (6) |
| C3—C4—C10—C12 | 49.4 (7) | C21—C22—C29—C31 | −48.4 (7) |
| C5—C4—C10—C12 | −131.6 (5) | C23—C22—C29—C31 | 132.9 (5) |
| C5—N1—C13—C14 | −176.3 (3) | C23—N2—C32—C33 | 177.4 (3) |
| N1—C13—C14—C19 | 177.5 (4) | N2—C32—C33—C38 | −178.4 (4) |
| N1—C13—C14—C15 | 0.3 (5) | N2—C32—C33—C34 | −2.0 (6) |
| C19—C14—C15—O1 | −178.6 (3) | C38—C33—C34—O2 | 178.5 (3) |
| C13—C14—C15—O1 | −1.4 (5) | C32—C33—C34—O2 | 2.2 (5) |
| C19—C14—C15—C16 | 0.8 (5) | C38—C33—C34—C35 | −0.5 (5) |
| C13—C14—C15—C16 | 178.1 (3) | C32—C33—C34—C35 | −176.8 (3) |
| O1—C15—C16—C17 | 179.2 (4) | O2—C34—C35—C36 | −178.4 (4) |
| C14—C15—C16—C17 | −0.3 (6) | C33—C34—C35—C36 | 0.6 (6) |
| O1—C15—C16—Br1 | 0.3 (5) | O2—C34—C35—Br2 | −0.3 (5) |
| C14—C15—C16—Br1 | −179.2 (3) | C33—C34—C35—Br2 | 178.7 (3) |
| C15—C16—C17—C18 | −0.5 (6) | C34—C35—C36—C37 | −0.3 (6) |
| Br1—C16—C17—C18 | 178.3 (3) | Br2—C35—C36—C37 | −178.3 (3) |
| C16—C17—C18—C19 | 0.8 (6) | C35—C36—C37—C38 | −0.2 (6) |
| C16—C17—C18—Cl1 | 179.3 (3) | C35—C36—C37—Cl2 | 179.8 (3) |
| C17—C18—C19—C14 | −0.3 (6) | C36—C37—C38—C33 | 0.3 (6) |
| Cl1—C18—C19—C14 | −178.7 (3) | Cl2—C37—C38—C33 | −179.6 (3) |
| C15—C14—C19—C18 | −0.5 (6) | C34—C33—C38—C37 | 0.0 (5) |
| C13—C14—C19—C18 | −177.8 (3) | C32—C33—C38—C37 | 176.4 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1A···N1 | 0.82 | 1.87 | 2.598 (4) | 147 |
| O2—H2A···N2 | 0.82 | 1.88 | 2.610 (4) | 147 |
| C28—H28A···Cg1i | 0.96 | 2.96 | 3.773 (6) | 144 |
| C16—Br1···Cg4i | 1.880 | 3.53 | 4.75 (2) | 120 |
Symmetry codes: (i) −x+1, −y+1, −z+1.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LX2070).
References
- Bruker (2004). APEX2 and SAINT. Bruker AXS Inc., Madison, Wisconsin, USA.
- Chang, S., Jones, L. II, Wang, C., Henling, L. M. & Grubbs, R. H. (1998). Organometallics, 17, 3460–3465.
- Chen, F. & Ye, H.-Y. (2008). Acta Cryst. E64, o1757. [DOI] [PMC free article] [PubMed]
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
- Figuet, M., Averbuch-Pouchot, M. T., du Moulinet d’Hardemare, A. & Jarjayes, O. (2001). Eur. J. Inorg. Chem. pp. 2089–2096.
- Kennedy, A. R. & Reglinski, J. (2001). Acta Cryst. E57, o1027–o1028.
- Lin, J., Cui, G.-H., Li, J.-R. & Xu, S.-S. (2005). Acta Cryst. E61, o627–o628.
- Pu, X.-H. (2008). Acta Cryst. E64, o1734. [DOI] [PMC free article] [PubMed]
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Thamotharan, S., Parthasarathi, V., Anitha, S. M., Prasad, A., Rao, T. R. & Linden, A. (2003). Acta Cryst. E59, o1856–o1857.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536808035071/lx2070sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536808035071/lx2070Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report