Abstract
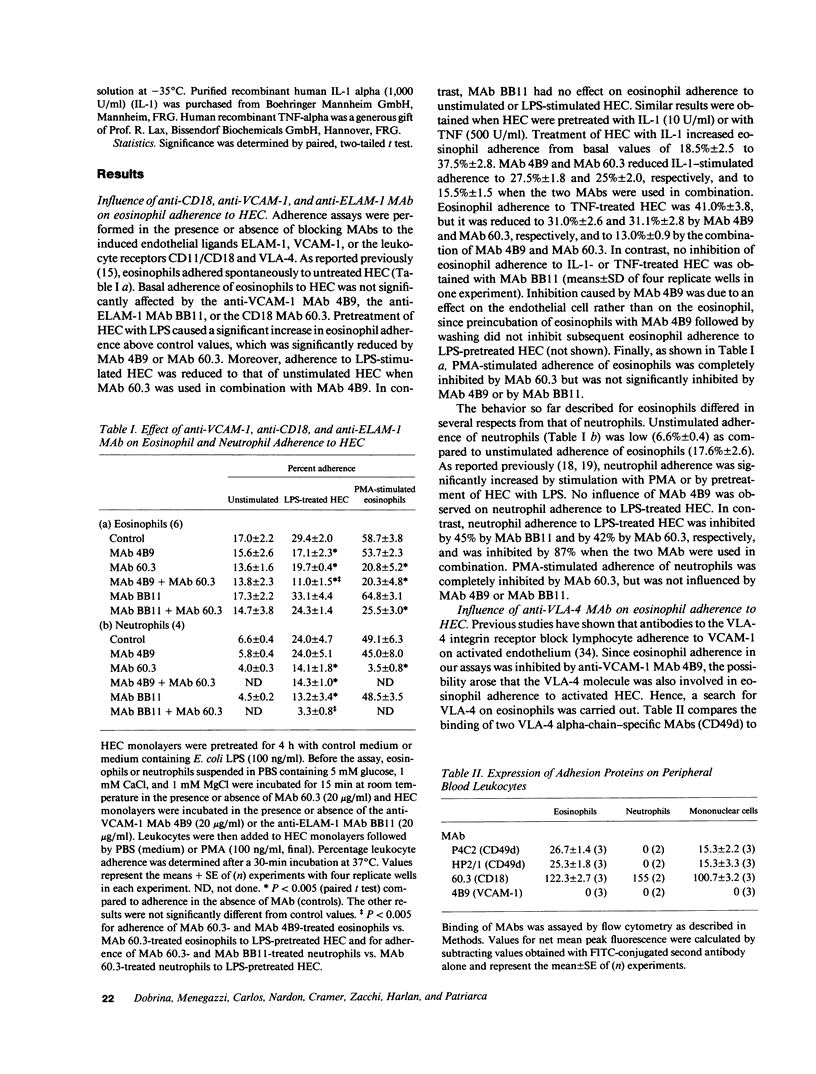
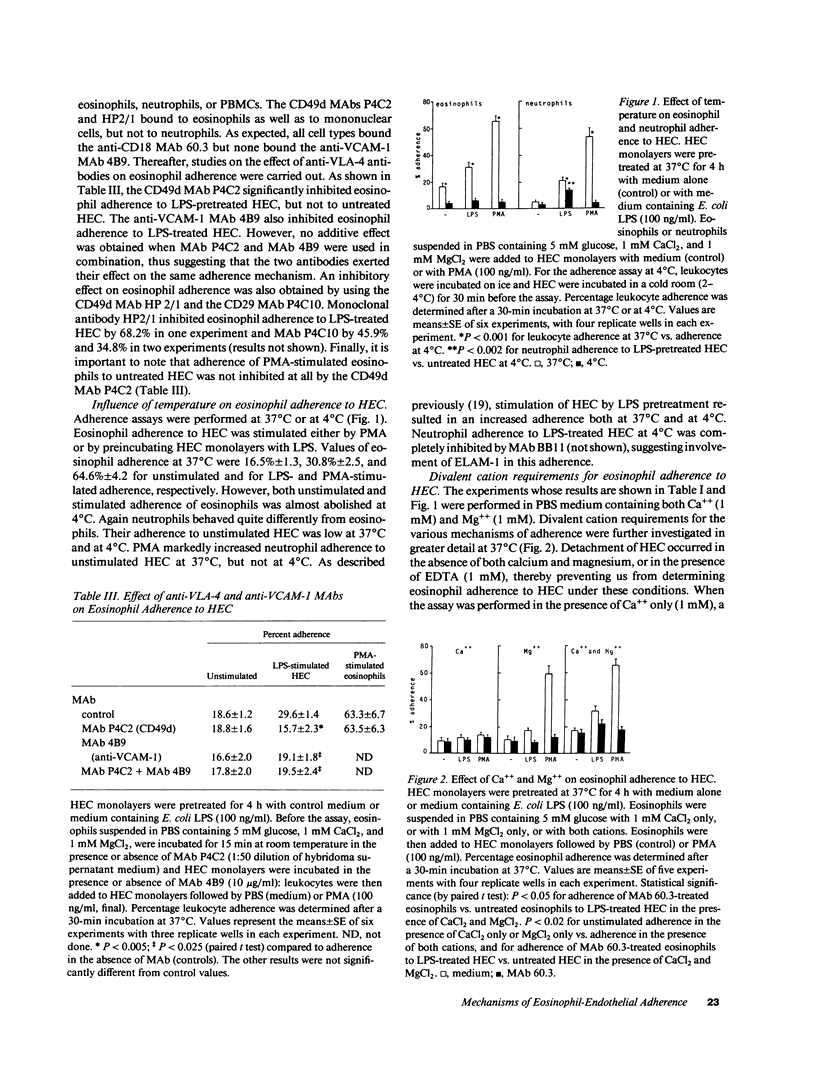
We have examined the mechanisms involved in the adherence of normal peripheral blood eosinophils to cultured human umbilical vein endothelial cells (HEC) under three conditions: (a) adherence in the absence of treatment of HEC or eosinophils with activating agents (basal adherence); (b) adherence induced by stimulation of eosinophils with phorbol ester (eosinophil-dependent adherence); and (c) adherence induced by pretreatment of HEC with LPS, tumor necrosis factor (TNF), or IL-1 (endothelial-dependent adherence). A mechanism was identified that was equally active in basal, eosinophil-dependent, and endothelial-dependent adherence. This mechanism was optimally active in the presence of both Ca++ and Mg++, and reduced in the presence of Ca++ only or Mg++ only. Furthermore, like the other mechanisms of eosinophil adherence, it was active at 37 degrees C but not at 4 degrees C. A second mechanism of adherence was involved in eosinophil- and in endothelial-dependent adherence. This mechanism was dependent on the CD11/CD18 adhesion complex of eosinophils (i.e., inhibited by anti-CD18 MAb) and it was active in the presence of Ca++ and Mg++ or Mg++ only, but not Ca++ only. The third mechanism of adherence was specific for endothelial-dependent adherence. It involved the endothelial ligand vascular cell adhesion molecule-1 (VCAM-1) and the eosinophil receptor very late activation antigen-4 (VLA-4, CD49d/CD29, i.e., inhibited by anti-VCAM-1 MAb or anti-VLA-4 MAb). This mechanism was active in the presence of Ca++ and Mg++ but not of Ca++ only or Mg++ only, and was not up- or downregulated when eosinophils were stimulated with phorbol ester. In contrast, the endothelial leukocyte adhesion molecule-1 (ELAM-1), that binds neutrophils and monocytes, was not involved in eosinophil adherence to LPS-, TNF-, or IL-1-stimulated HEC (i.e., not inhibited by anti-ELAM-1 MAb). We conclude that eosinophils, like monocytes and lymphocytes, bind to the cytokine-induced endothelial ligand VCAM-1 via the integrin receptor VLA-4.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. C., Schmalsteig F. C., Finegold M. J., Hughes B. J., Rothlein R., Miller L. J., Kohl S., Tosi M. F., Jacobs R. L., Waldrop T. C. The severe and moderate phenotypes of heritable Mac-1, LFA-1 deficiency: their quantitative definition and relation to leukocyte dysfunction and clinical features. J Infect Dis. 1985 Oct;152(4):668–689. doi: 10.1093/infdis/152.4.668. [DOI] [PubMed] [Google Scholar]
- Andrews P. C., Krinsky N. I. The reductive cleavage of myeloperoxidase in half, producing enzymically active hemi-myeloperoxidase. J Biol Chem. 1981 May 10;256(9):4211–4218. [PubMed] [Google Scholar]
- Beatty P. G., Ledbetter J. A., Martin P. J., Price T. H., Hansen J. A. Definition of a common leukocyte cell-surface antigen (Lp95-150) associated with diverse cell-mediated immune functions. J Immunol. 1983 Dec;131(6):2913–2918. [PubMed] [Google Scholar]
- Benjamin C., Dougas I., Chi-Rosso G., Luhowskyj S., Rosa M., Newman B., Osborn L., Vassallo C., Hession C., Goelz S. A blocking monoclonal antibody to endothelial-leukocyte adhesion molecule-1 (ELAM1). Biochem Biophys Res Commun. 1990 Aug 31;171(1):348–353. doi: 10.1016/0006-291x(90)91400-m. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Mendrick D. L., Cotran R. S., Gimbrone M. A., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990 Jan;136(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Carlos T. M., Harlan J. M. Membrane proteins involved in phagocyte adherence to endothelium. Immunol Rev. 1990 Apr;114:5–28. doi: 10.1111/j.1600-065x.1990.tb00559.x. [DOI] [PubMed] [Google Scholar]
- Carlos T. M., Schwartz B. R., Kovach N. L., Yee E., Rosa M., Osborn L., Chi-Rosso G., Newman B., Lobb R., Rosso M. Vascular cell adhesion molecule-1 mediates lymphocyte adherence to cytokine-activated cultured human endothelial cells. Blood. 1990 Sep 1;76(5):965–970. [PubMed] [Google Scholar]
- Cramer R., Soranzo M. R., Dri P., Menegazzi R., Pitotti A., Zabucchi G., Patriarca P. A simple reliable assay for myeloperoxidase activity in mixed neutrophil-eosinophil cell suspensions: application to detection of myeloperoxidase deficiency. J Immunol Methods. 1984 May 11;70(1):119–125. doi: 10.1016/0022-1759(84)90396-x. [DOI] [PubMed] [Google Scholar]
- Dobrina A., Carlos T. M., Schwartz B. R., Beatty P. G., Ochs H. D., Harlan J. M. Phorbol ester causes down-regulation of CD11/CD18-independent neutrophil adherence to endothelium. Immunology. 1990 Mar;69(3):429–434. [PMC free article] [PubMed] [Google Scholar]
- Dobrina A., Schwartz B. R., Carlos T. M., Ochs H. D., Beatty P. G., Harlan J. M. CD11/CD18-independent neutrophil adherence to inducible endothelial-leucocyte adhesion molecules (E-LAM) in vitro. Immunology. 1989 Aug;67(4):502–508. [PMC free article] [PubMed] [Google Scholar]
- Elices M. J., Osborn L., Takada Y., Crouse C., Luhowskyj S., Hemler M. E., Lobb R. R. VCAM-1 on activated endothelium interacts with the leukocyte integrin VLA-4 at a site distinct from the VLA-4/fibronectin binding site. Cell. 1990 Feb 23;60(4):577–584. doi: 10.1016/0092-8674(90)90661-w. [DOI] [PubMed] [Google Scholar]
- Gleich G. J., Frigas E., Loegering D. A., Wassom D. L., Steinmuller D. Cytotoxic properties of the eosinophil major basic protein. J Immunol. 1979 Dec;123(6):2925–2927. [PubMed] [Google Scholar]
- Hemler M. E., Huang C., Takada Y., Schwarz L., Strominger J. L., Clabby M. L. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987 Aug 25;262(24):11478–11485. [PubMed] [Google Scholar]
- Hudson G. Quantitative study of the eosinophil granulocytes. Semin Hematol. 1968 Apr;5(2):166–186. [PubMed] [Google Scholar]
- Jaffe E. A., Nachman R. L., Becker C. G., Minick C. R. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973 Nov;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jong E. C., Henderson W. R., Klebanoff S. J. Bactericidal activity of eosinophil peroxidase. J Immunol. 1980 Mar;124(3):1378–1382. [PubMed] [Google Scholar]
- Kimani G., Tonnesen M. G., Henson P. M. Stimulation of eosinophil adherence to human vascular endothelial cells in vitro by platelet-activating factor. J Immunol. 1988 May 1;140(9):3161–3166. [PubMed] [Google Scholar]
- Kroegel C., Costabel U., Matthys H. Mechanism of membrane damage mediated by eosinophil major basic protein. Lancet. 1987 Jun 13;1(8546):1380–1381. doi: 10.1016/s0140-6736(87)90686-6. [DOI] [PubMed] [Google Scholar]
- Lamas A. M., Mulroney C. M., Schleimer R. P. Studies on the adhesive interaction between purified human eosinophils and cultured vascular endothelial cells. J Immunol. 1988 Mar 1;140(5):1500–1505. [PubMed] [Google Scholar]
- Maciag T., Cerundolo J., Ilsley S., Kelley P. R., Forand R. An endothelial cell growth factor from bovine hypothalamus: identification and partial characterization. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5674–5678. doi: 10.1073/pnas.76.11.5674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Migler R., DeChatelet L. R., Bass D. A. Human eosinophilic peroxidase: role in bactericidal activity. Blood. 1978 Mar;51(3):445–456. [PubMed] [Google Scholar]
- Nutman T. B., Ottesen E. A., Cohen S. G. The eosinophil, eosinophilia, and eosinophil-related disorders. III. Clinical assessments and eosinophil related disorders. Allergy Proc. 1989 Jan-Feb;10(1):33–46. doi: 10.2500/108854189778968560. [DOI] [PubMed] [Google Scholar]
- Nutman T. B., Ottesen E. A., Cohen S. G. The eosinophil, eosinophilia, and eosinophil-related disorders. IV. Eosinophil related disorders (continued). Allergy Proc. 1989 Jan-Feb;10(1):47–62. doi: 10.2500/108854189778968579. [DOI] [PubMed] [Google Scholar]
- Osborn L., Hession C., Tizard R., Vassallo C., Luhowskyj S., Chi-Rosso G., Lobb R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989 Dec 22;59(6):1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Rice G. E., Munro J. M., Bevilacqua M. P. Inducible cell adhesion molecule 110 (INCAM-110) is an endothelial receptor for lymphocytes. A CD11/CD18-independent adhesion mechanism. J Exp Med. 1990 Apr 1;171(4):1369–1374. doi: 10.1084/jem.171.4.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. R., Wayner E. A., Carlos T. M., Ochs H. D., Harlan J. M. Identification of surface proteins mediating adherence of CD11/CD18-deficient lymphoblastoid cells to cultured human endothelium. J Clin Invest. 1990 Jun;85(6):2019–2022. doi: 10.1172/JCI114668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal C. E., Valone F. H., Holtzman M. J., Goetzl E. J. Preferential human eosinophil chemotactic activity of the platelet-activating factor (PAF) 1-0-hexadecyl-2-acetyl-sn-glyceryl-3-phosphocholine (AGEPC). J Clin Immunol. 1987 Mar;7(2):179–184. doi: 10.1007/BF00916012. [DOI] [PubMed] [Google Scholar]
- Smith C. W., Rothlein R., Hughes B. J., Mariscalco M. M., Rudloff H. E., Schmalstieg F. C., Anderson D. C. Recognition of an endothelial determinant for CD 18-dependent human neutrophil adherence and transendothelial migration. J Clin Invest. 1988 Nov;82(5):1746–1756. doi: 10.1172/JCI113788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Madrid F., De Landázuri M. O., Morago G., Cebrián M., Acevedo A., Bernabeu C. VLA-3: a novel polypeptide association within the VLA molecular complex: cell distribution and biochemical characterization. Eur J Immunol. 1986 Nov;16(11):1343–1349. doi: 10.1002/eji.1830161106. [DOI] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Wardlaw A. J., Moqbel R., Cromwell O., Kay A. B. Platelet-activating factor. A potent chemotactic and chemokinetic factor for human eosinophils. J Clin Invest. 1986 Dec;78(6):1701–1706. doi: 10.1172/JCI112765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. J., Test S. T., Eckmann C. M., Roos D., Regiani S. Brominating oxidants generated by human eosinophils. Science. 1986 Oct 10;234(4773):200–203. doi: 10.1126/science.3018933. [DOI] [PubMed] [Google Scholar]



