Abstract
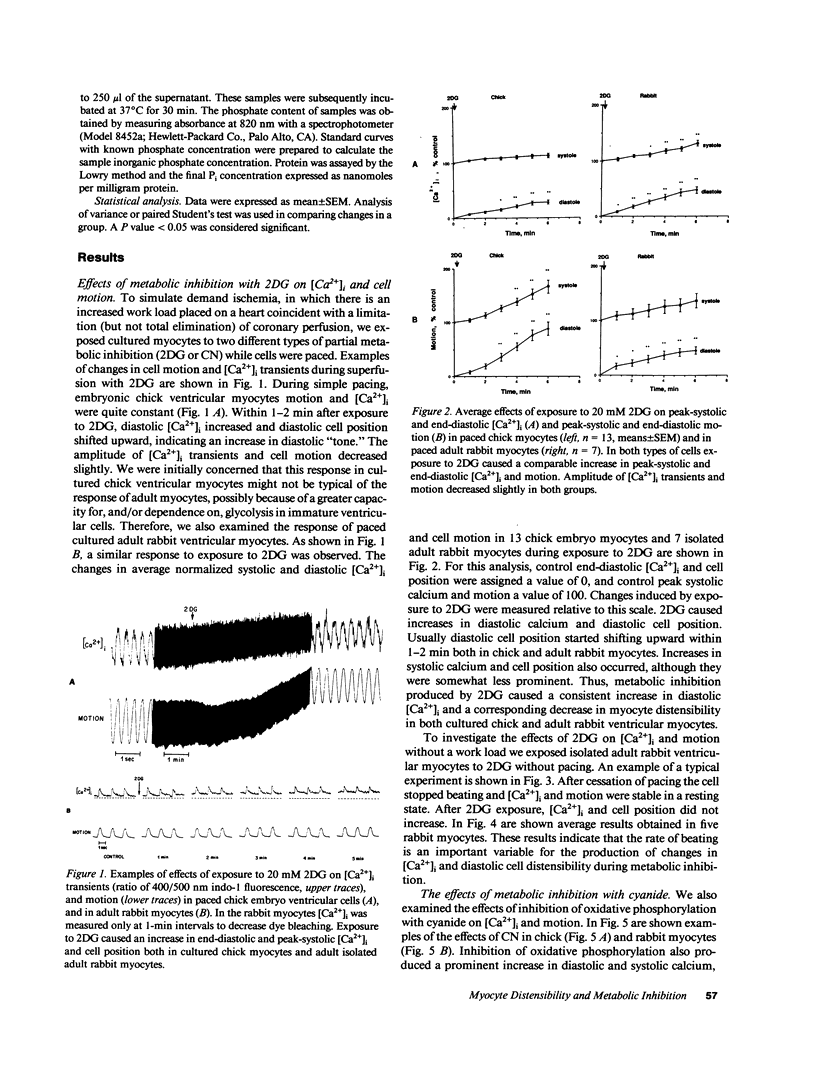
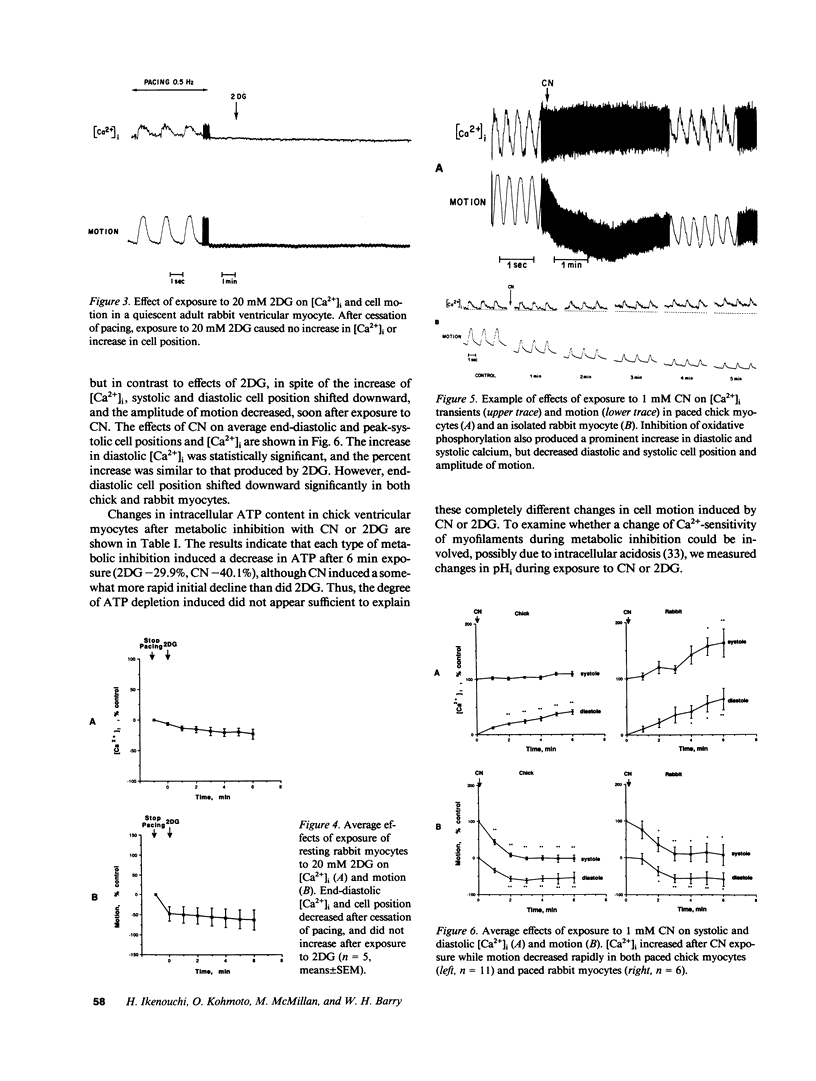
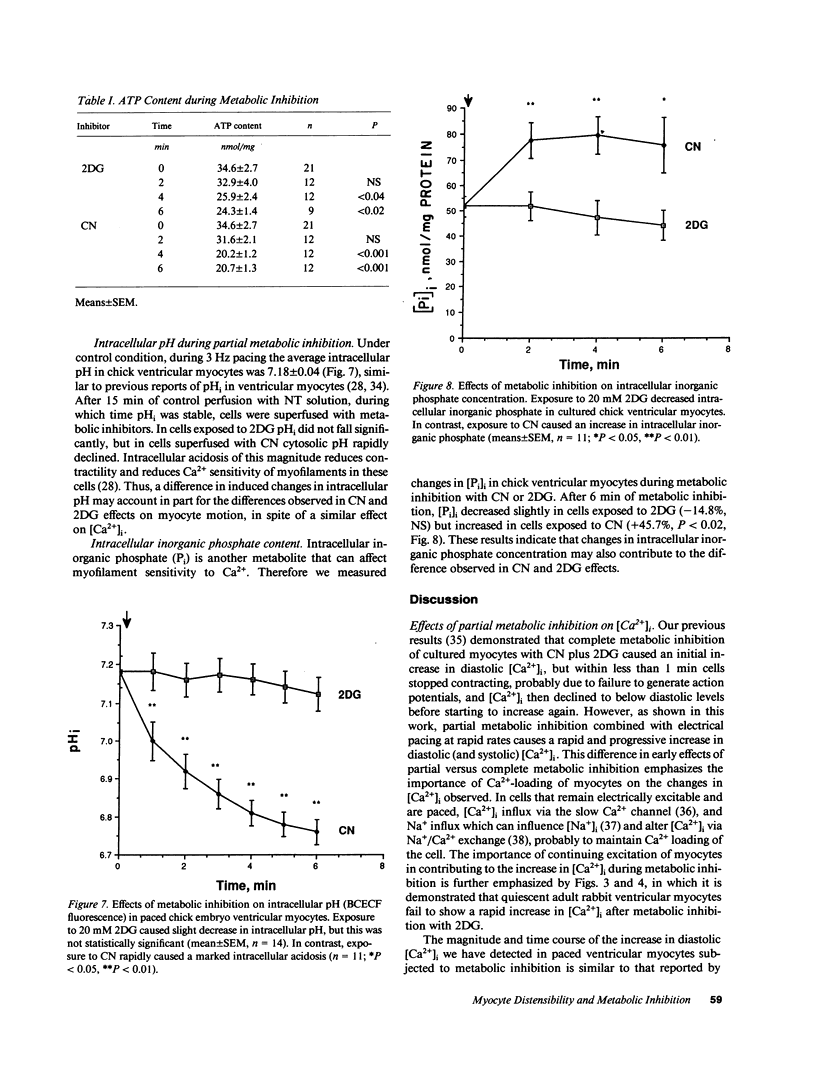
Ischemia may cause increased or decreased distensibility of the left ventricle, but the cellular mechanisms involved have not been clarified. We examined the possible contributions of changes in intracellular inorganic phosphate, pH, and Ca2+ concentrations to altered diastolic function in cultured myocytes subjected to partial metabolic inhibition. Paced cultured embryonic chick and adult rabbit ventricular myocytes superfused with 20 mM 2-deoxyglucose (2DG) exhibited an increase in end-diastolic intracellular free calcium concentration ([Ca2+]i) and an upward shift in end-diastolic cell position. These results indicate that glycolytic blockade increases diastolic and systolic calcium in paced ventricular myocytes, and that this elevated diastolic calcium influences the extent of diastolic relaxation. In contrast, paced ventricular myocytes superfused with 1 mM cyanide (CN) exhibited a similar increase in end-diastolic [Ca2+]i but a decrease in end-diastolic cell position and amplitude of motion. Although changes in ATP contents were similar in both groups (2DG, -29.9%; CN, -40.1%), alterations of intracellular pH and inorganic phosphate concentrations were different. In 2DG-treated cells, pHi did not decrease significantly (7.18 +/- 0.04 to 7.12 +/- 0.11, n = 14) but in the CN group it decreased markedly within 6 min (7.18 +/- 0.04 to 6.76 +/- 0.11, n = 11, P less than 0.01). Intracellular inorganic phosphate decreased slightly in the 2DG group (-14.8%, NS) but increased in cells exposed to CN (45.7%, P less than 0.02). We conclude that while a prominent increase in diastolic [Ca2+]i occurs in rapidly paced ventricular myocytes exposed to either inhibitors of glycolysis or oxidative phosphorylation, the effects of this increase in [Ca2+]i on diastolic distensibility may be influenced by intracellular accumulation of metabolites that decrease the sensitivity of myofilament to [Ca2+]i.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D. G., Morris P. G., Orchard C. H., Pirolo J. S. A nuclear magnetic resonance study of metabolism in the ferret heart during hypoxia and inhibition of glycolysis. J Physiol. 1985 Apr;361:185–204. doi: 10.1113/jphysiol.1985.sp015640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyagi T., Iizuka M., Takahashi T., Ohya T., Serizawa T., Momomura S., Sato H., Mochizuki T., Matsui H., Ikenouchi H. Wall motion asynchrony prolongs time constant of left ventricular relaxation. Am J Physiol. 1989 Sep;257(3 Pt 2):H883–H890. doi: 10.1152/ajpheart.1989.257.3.H883. [DOI] [PubMed] [Google Scholar]
- Apstein C. S., Grossman W. Opposite initial effects of supply and demand ischemia on left ventricular diastolic compliance: the ischemia-diastolic paradox. J Mol Cell Cardiol. 1987 Jan;19(1):119–128. doi: 10.1016/s0022-2828(87)80551-5. [DOI] [PubMed] [Google Scholar]
- Ashraf M., Rahamathulla P. M. Cardiac injury in short duration anoxia and modification by diltiazem, a calcium channel blocking agent. J Am Coll Cardiol. 1984 May;3(5):1237–1244. doi: 10.1016/s0735-1097(84)80182-5. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Brooker J. Z., Alderman E. L., Harrison D. C. Changes in diastolic stiffness and tone of the left ventricle during angina pectoris. Circulation. 1974 Feb;49(2):255–263. doi: 10.1161/01.cir.49.2.255. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Peeters G. A., Rasmussen C. A., Jr, Cunningham M. J. Role of changes in [Ca2+]i in energy deprivation contracture. Circ Res. 1987 Nov;61(5):726–734. doi: 10.1161/01.res.61.5.726. [DOI] [PubMed] [Google Scholar]
- Barry W. H., Rasmussen C. A., Jr, Ishida H., Bridge J. H. External Na-independent Ca extrusion in cultured ventricular cells. Magnitude and functional significance. J Gen Physiol. 1986 Sep;88(3):393–411. doi: 10.1085/jgp.88.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill D. M., Fozzard H. A., Makielski J. C., Wasserstrom J. A. Effect of prolonged depolarizations on twitch tension and intracellular sodium activity in sheep cardiac Purkinje fibres. J Physiol. 1987 Mar;384:355–375. doi: 10.1113/jphysiol.1987.sp016459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brutsaert D. L., Rademakers F. E., Sys S. U. Triple control of relaxation: implications in cardiac disease. Circulation. 1984 Jan;69(1):190–196. doi: 10.1161/01.cir.69.1.190. [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Fozzard H. A., Sheu S. S. Increase in intracellular sodium ion activity during stimulation in mammalian cardiac muscle. Circ Res. 1982 May;50(5):651–662. doi: 10.1161/01.res.50.5.651. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Elliott A. C., Smith G. L. The contribution of intracellular acidosis to the decline of developed pressure in ferret hearts exposed to cyanide. J Physiol. 1987 Oct;391:99–108. doi: 10.1113/jphysiol.1987.sp016728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Nichols C. G., O'Neill S. C., Smith G. L., Valdeolmillos M. The effects of metabolic inhibition on intracellular calcium and pH in isolated rat ventricular cells. J Physiol. 1989 Apr;411:393–418. doi: 10.1113/jphysiol.1989.sp017580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of pH on the myofilaments and the sarcoplasmic reticulum of skinned cells from cardiace and skeletal muscles. J Physiol. 1978 Mar;276:233–255. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Haddad J., Decker M. L., Hsieh L. C., Lesch M., Samarel A. M., Decker R. S. Attachment and maintenance of adult rabbit cardiac myocytes in primary cell culture. Am J Physiol. 1988 Jul;255(1 Pt 1):C19–C27. doi: 10.1152/ajpcell.1988.255.1.C19. [DOI] [PubMed] [Google Scholar]
- Henry P. D., Schuchleib R., Davis J., Weiss E. S., Sobel B. E. Myocardial contracture and accumulation of mitochondrial calcium in ischemic rabbit heart. Am J Physiol. 1977 Dec;233(6):H677–H684. doi: 10.1152/ajpheart.1977.233.6.H677. [DOI] [PubMed] [Google Scholar]
- Ishida H., Kohmoto O., Bridge J. H., Barry W. H. Alterations in cation homeostasis in cultured chick ventricular cells during and after recovery from adenosine triphosphate depletion. J Clin Invest. 1988 Apr;81(4):1173–1181. doi: 10.1172/JCI113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihara Y., Grossman W., Morgan J. P. Direct measurement of changes in intracellular calcium transients during hypoxia, ischemia, and reperfusion of the intact mammalian heart. Circ Res. 1989 Oct;65(4):1029–1044. doi: 10.1161/01.res.65.4.1029. [DOI] [PubMed] [Google Scholar]
- Kohmoto O., Barry W. H. Mechanism of protective effects of Ca++ channel blockers on energy deprivation contracture in cultured ventricular myocytes. J Pharmacol Exp Ther. 1989 Feb;248(2):871–878. [PubMed] [Google Scholar]
- Kohmoto O., Spitzer K. W., Movsesian M. A., Barry W. H. Effects of intracellular acidosis on [Ca2+]i transients, transsarcolemmal Ca2+ fluxes, and contraction in ventricular myocytes. Circ Res. 1990 Mar;66(3):622–632. doi: 10.1161/01.res.66.3.622. [DOI] [PubMed] [Google Scholar]
- Koretsune Y., Marban E. Relative roles of Ca2(+)-dependent and Ca2(+)-independent mechanisms in hypoxic contractile dysfunction. Circulation. 1990 Aug;82(2):528–535. doi: 10.1161/01.cir.82.2.528. [DOI] [PubMed] [Google Scholar]
- Kusuoka H., Weisfeldt M. L., Zweier J. L., Jacobus W. E., Marban E. Mechanism of early contractile failure during hypoxia in intact ferret heart: evidence for modulation of maximal Ca2+-activated force by inorganic phosphate. Circ Res. 1986 Sep;59(3):270–282. doi: 10.1161/01.res.59.3.270. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lavanchy N., Martin J., Rossi A. Effects of diltiazem on the energy metabolism of the isolated rat heart submitted to ischaemia: a 31P NMR study. J Mol Cell Cardiol. 1986 Sep;18(9):931–941. doi: 10.1016/s0022-2828(86)80007-4. [DOI] [PubMed] [Google Scholar]
- Lee H. C., Mohabir R., Smith N., Franz M. R., Clusin W. T. Effect of ischemia on calcium-dependent fluorescence transients in rabbit hearts containing indo 1. Correlation with monophasic action potentials and contraction. Circulation. 1988 Oct;78(4):1047–1059. doi: 10.1161/01.cir.78.4.1047. [DOI] [PubMed] [Google Scholar]
- Lorell B. H., Apstein C. S., Cunningham M. J., Schoen F. J., Weinberg E. O., Peeters G. A., Barry W. H. Contribution of endothelial cells to calcium-dependent fluorescence transients in rabbit hearts loaded with indo 1. Circ Res. 1990 Aug;67(2):415–425. doi: 10.1161/01.res.67.2.415. [DOI] [PubMed] [Google Scholar]
- Lorell B. H., Palacios I., Daggett W. M., Jacobs M. L., Fowler B. N., Newell J. B. Right ventricular distension and left ventricular compliance. Am J Physiol. 1981 Jan;240(1):H87–H98. doi: 10.1152/ajpheart.1981.240.1.H87. [DOI] [PubMed] [Google Scholar]
- Mann T., Brodie B. R., Grossman W., McLaurin L. P. Effect of angina on the left ventricular diastolic pressure-volume relationship. Circulation. 1977 May;55(5):761–766. doi: 10.1161/01.cir.55.5.761. [DOI] [PubMed] [Google Scholar]
- Maruyama Y., Ashikawa K., Isoyama S., Kanatsuka H., Ino-Oka E., Takishima T. Mechanical interactions between four heart chambers with and without the pericardium in canine hearts. Circ Res. 1982 Jan;50(1):86–100. doi: 10.1161/01.res.50.1.86. [DOI] [PubMed] [Google Scholar]
- Momomura S., Bradley A. B., Grossman W. Left ventricular diastolic pressure-segment length relations and end-diastolic distensibility in dogs with coronary stenoses. An angina physiology model. Circ Res. 1984 Aug;55(2):203–214. doi: 10.1161/01.res.55.2.203. [DOI] [PubMed] [Google Scholar]
- Momomura S., Ingwall J. S., Parker J. A., Sahagian P., Ferguson J. J., Grossman W. The relationships of high energy phosphates, tissue pH, and regional blood flow to diastolic distensibility in the ischemic dog myocardium. Circ Res. 1985 Dec;57(6):822–835. doi: 10.1161/01.res.57.6.822. [DOI] [PubMed] [Google Scholar]
- Paulus W. J., Serizawa T., Grossman W. Altered left ventricular diastolic properties during pacing-induced ischemia in dogs with coronary stenoses. Potentiation by caffeine. Circ Res. 1982 Feb;50(2):218–227. doi: 10.1161/01.res.50.2.218. [DOI] [PubMed] [Google Scholar]
- Paulus W. J. Upward shift and outward bulge. Divergent myocardial effects of pacing angina and brief coronary occlusion. Circulation. 1990 Apr;81(4):1436–1439. doi: 10.1161/01.cir.81.4.1436. [DOI] [PubMed] [Google Scholar]
- Peeters G. A., Hlady V., Bridge J. H., Barry W. H. Simultaneous measurement of calcium transients and motion in cultured heart cells. Am J Physiol. 1987 Dec;253(6 Pt 2):H1400–H1408. doi: 10.1152/ajpheart.1987.253.6.H1400. [DOI] [PubMed] [Google Scholar]
- Pirolo J. S., Allen D. G. Assessment of techniques for preventing glycolysis in cardiac muscle. Cardiovasc Res. 1986 Nov;20(11):837–844. doi: 10.1093/cvr/20.11.837. [DOI] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross J., Jr Acute displacement of the diastolic pressure-volume curve of the left ventricle: role of the pericardium and the right ventricle. Circulation. 1979 Jan;59(1):32–37. doi: 10.1161/01.cir.59.1.32. [DOI] [PubMed] [Google Scholar]
- Serizawa T., Carabello B. A., Grossman W. Effect of pacing-induced ischemia on left ventricular diastolic pressure-volume relations in dogs with coronary stenoses. Circ Res. 1980 Mar;46(3):430–439. doi: 10.1161/01.res.46.3.430. [DOI] [PubMed] [Google Scholar]
- Serizawa T., Vogel W. M., Apstein C. S., Grossman W. Comparison of acute alterations in left ventricular relaxation and diastolic chamber stiffness induced by hypoxia and ischemia. Role of myocardial oxygen supply-demand imbalance. J Clin Invest. 1981 Jul;68(1):91–102. doi: 10.1172/JCI110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Waters D. D., Da Luz P., Wyatt H. L., Swan H. J., Forrester J. S. Early changes in regional and global left ventricular function induced by graded reductions in regional coronary perfusion. Am J Cardiol. 1977 Apr;39(4):537–543. doi: 10.1016/s0002-9149(77)80163-x. [DOI] [PubMed] [Google Scholar]
- Wiegner A. W., Allen G. J., Bing O. H. Weak and strong myocardium in series: implications for segmental dysfunction. Am J Physiol. 1978 Dec;235(6):H776–H783. doi: 10.1152/ajpheart.1978.235.6.H776. [DOI] [PubMed] [Google Scholar]
- duBell W. H., Houser S. R. Voltage and beat dependence of Ca2+ transient in feline ventricular myocytes. Am J Physiol. 1989 Sep;257(3 Pt 2):H746–H759. doi: 10.1152/ajpheart.1989.257.3.H746. [DOI] [PubMed] [Google Scholar]



