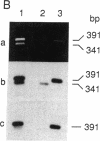
Abstract
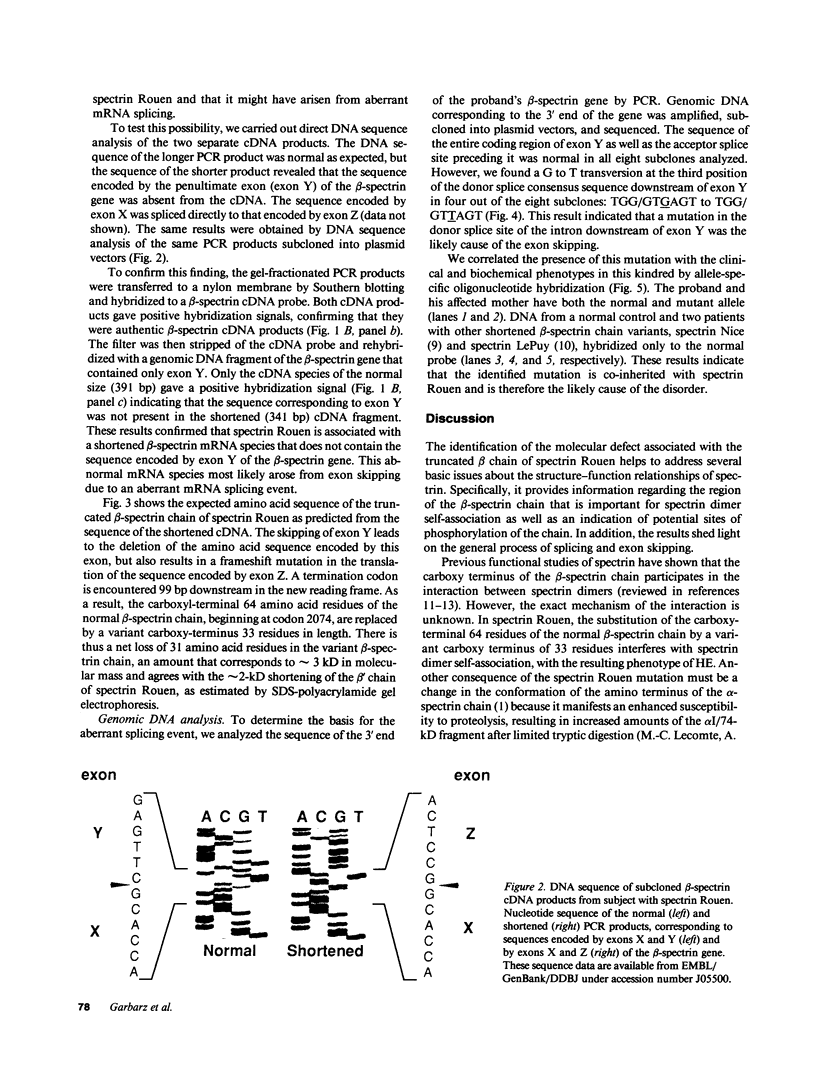
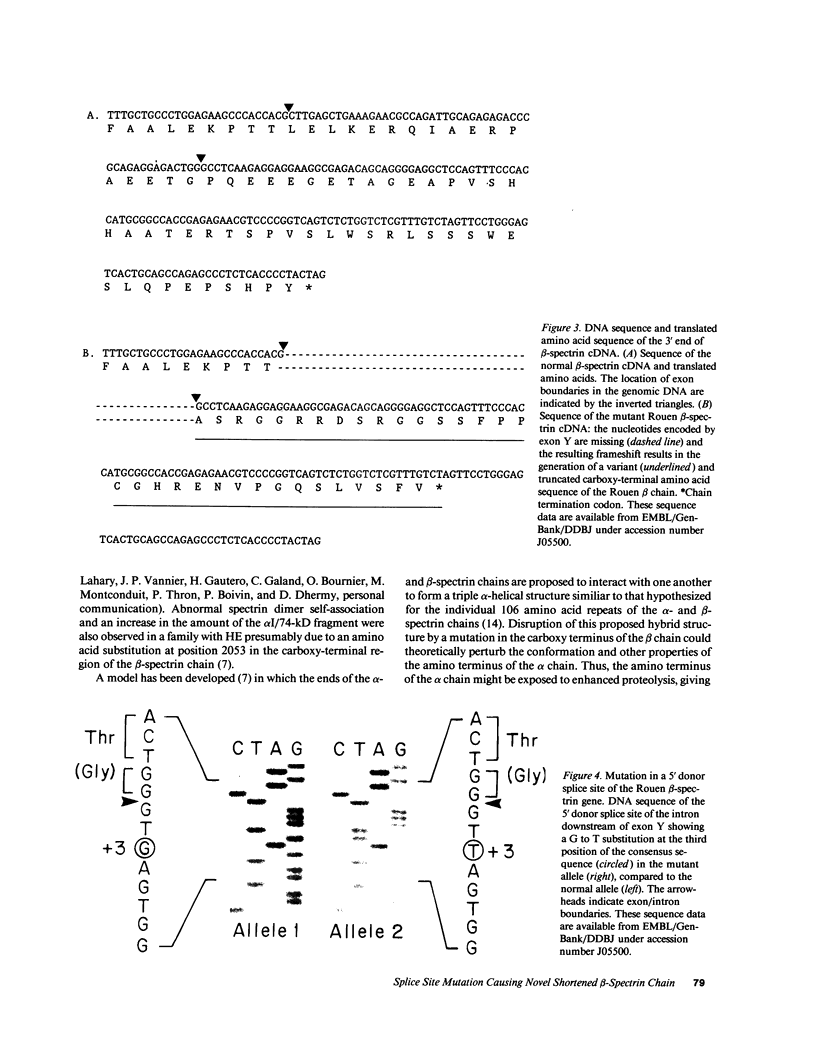
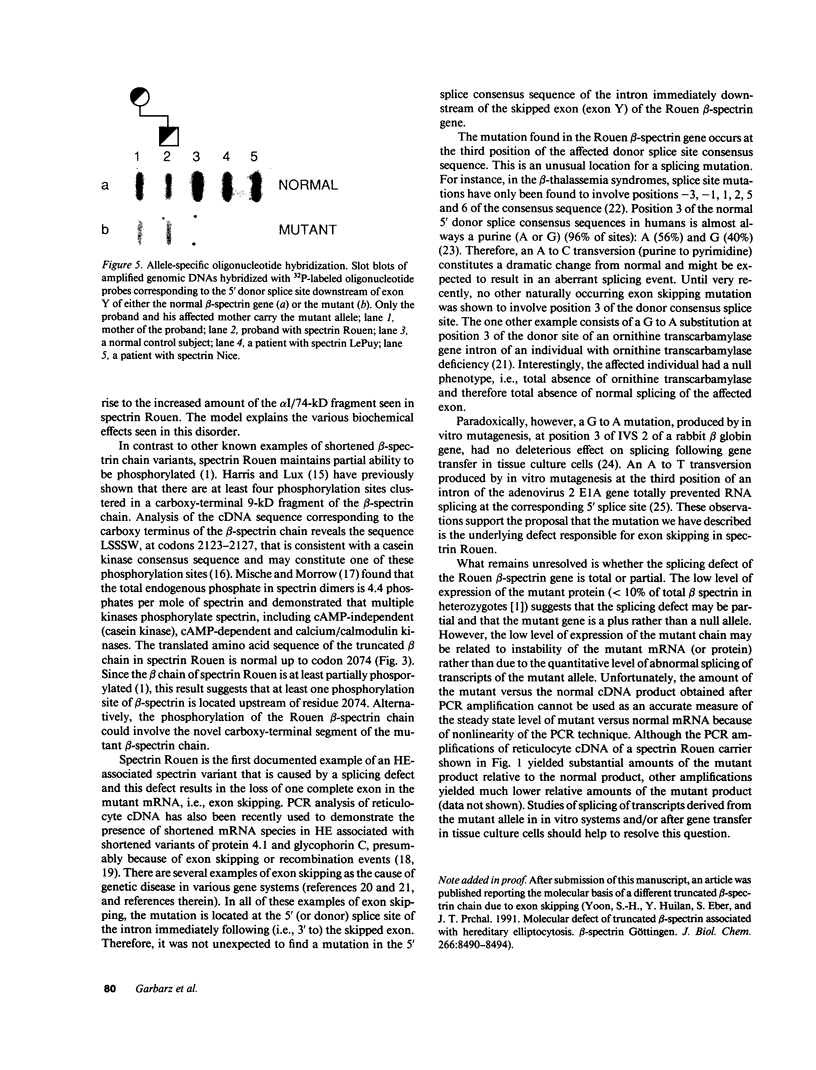
The molecular defect responsible for the shortened beta-spectrin chain variant, spectrin Rouen, was identified by analysis of cDNA and genomic DNA of affected individuals after amplification by the polymerase chain reaction. Peripheral blood reticulocyte RNA was transcribed into cDNA and amplified using primers corresponding to the 3' end of beta-spectrin cDNA. Agarose gel electrophoresis of cDNA amplification products from affected individuals revealed the expected band of 391 bp as well as a shortened band of 341 bp. Nucleotide sequencing of the shortened cDNA amplification product revealed that the sequences corresponding to the penultimate exon of the beta-spectrin gene (exon Y) were absent. This result was confirmed by hybridization of a Southern blot of amplification products with a labeled probe specific for exon Y. Nucleotide sequencing of the proband's amplified genomic DNA corresponding to this region of the beta-spectrin gene revealed a mutation in the 5' donor consensus splice site of the intron downstream of the Y exon, TGG/GTGAGT to TGG/GTTAGT, in one allele. We postulate that this mutation leads to the splicing out or skipping of exon Y, thus producing a shortened beta-spectrin chain. To our knowledge, this is the first documented example of exon skipping as the cause of a shortened beta-spectrin chain in a case of hereditary elliptocytosis. The exon skip results in the loss of the 17 amino acids of exon Y and creates a frameshift with the synthesis of 33 novel amino acids prior to premature chain termination 14 residues upstream of the normal carboxy terminus of the beta-spectrin chain, giving a mutant beta-spectrin chain that is 31 amino acids shorter than the normal chain.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Chang S., Reid M. E., Conboy J., Kan Y. W., Mohandas N. Molecular characterization of erythrocyte glycophorin C variants. Blood. 1991 Feb 1;77(3):644–648. [PubMed] [Google Scholar]
- Conboy J., Marchesi S., Kim R., Agre P., Kan Y. W., Mohandas N. Molecular analysis of insertion/deletion mutations in protein 4.1 in elliptocytosis. II. Determination of molecular genetic origins of rearrangements. J Clin Invest. 1990 Aug;86(2):524–530. doi: 10.1172/JCI114739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaunay J., Alloisio N., Morlé L., Pothier B. The red cell skeleton and its genetic disorders. Mol Aspects Med. 1990;11(3):161–241. doi: 10.1016/0098-2997(90)90001-i. [DOI] [PubMed] [Google Scholar]
- Dhermy D., Lecomte M. C., Garbarz M., Bournier O., Galand C., Gautero H., Feo C., Alloisio N., Delaunay J., Boivin P. Spectrin beta-chain variant associated with hereditary elliptocytosis. J Clin Invest. 1982 Oct;70(4):707–715. doi: 10.1172/JCI110666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey J. R., Harrison L. M., Franklin K. F., Metcalf K. M., Radisky E. S., Kushner J. P. Uroporphyrinogen decarboxylase: a splice site mutation causes the deletion of exon 6 in multiple families with porphyria cutanea tarda. J Clin Invest. 1990 Nov;86(5):1416–1422. doi: 10.1172/JCI114856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris H. W., Jr, Lux S. E. Structural characterization of the phosphorylation sites of human erythrocyte spectrin. J Biol Chem. 1980 Dec 10;255(23):11512–11520. [PubMed] [Google Scholar]
- Kan Y. W., Holland J. P., Dozy A. M., Varmus H. E. Demonstration of non-functional beta-globin mRNA in homozygous beta (0) thalassemia. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5140–5144. doi: 10.1073/pnas.72.12.5140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr The thalassemia syndromes: molecular basis and prenatal diagnosis in 1990. Semin Hematol. 1990 Jul;27(3):209–228. [PubMed] [Google Scholar]
- Montell C., Berk A. J. Elimination of mRNA splicing by a point mutation outside the conserved GU at 5' splice sites. Nucleic Acids Res. 1984 May 11;12(9):3821–3827. doi: 10.1093/nar/12.9.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohshima Y., Gotoh Y. Signals for the selection of a splice site in pre-mRNA. Computer analysis of splice junction sequences and like sequences. J Mol Biol. 1987 May 20;195(2):247–259. doi: 10.1016/0022-2836(87)90647-4. [DOI] [PubMed] [Google Scholar]
- Pothier B., Morlé L., Alloisio N., Ducluzeau M. T., Caldani C., Féo C., Garbarz M., Chaveroche I., Dhermy D., Lecomte M. C. Spectrin Nice (beta 220/216): a shortened beta-chain variant associated with an increase of the alpha I/74 fragment in a case of elliptocytosis. Blood. 1987 Jun;69(6):1759–1765. [PubMed] [Google Scholar]
- Saiki R. K., Bugawan T. L., Horn G. T., Mullis K. B., Erlich H. A. Analysis of enzymatically amplified beta-globin and HLA-DQ alpha DNA with allele-specific oligonucleotide probes. Nature. 1986 Nov 13;324(6093):163–166. doi: 10.1038/324163a0. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Tse W. T., Lecomte M. C., Costa F. F., Garbarz M., Feo C., Boivin P., Dhermy D., Forget B. G. Point mutation in the beta-spectrin gene associated with alpha I/74 hereditary elliptocytosis. Implications for the mechanism of spectrin dimer self-association. J Clin Invest. 1990 Sep;86(3):909–916. doi: 10.1172/JCI114792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelmann J. C., Chang J. G., Tse W. T., Scarpa A. L., Marchesi V. T., Forget B. G. Full-length sequence of the cDNA for human erythroid beta-spectrin. J Biol Chem. 1990 Jul 15;265(20):11827–11832. [PubMed] [Google Scholar]
- Winkelmann J. C., Leto T. L., Watkins P. C., Eddy R., Shows T. B., Linnenbach A. J., Sahr K. E., Kathuria N., Marchesi V. T., Forget B. G. Molecular cloning of the cDNA for human erythrocyte beta-spectrin. Blood. 1988 Jul;72(1):328–334. [PubMed] [Google Scholar]
- Yoon S. H., Yu H., Eber S., Prchal J. T. Molecular defect of truncated beta-spectrin associated with hereditary elliptocytosis. Beta-spectrin Gottingen. J Biol Chem. 1991 May 5;266(13):8490–8494. [PubMed] [Google Scholar]