Abstract
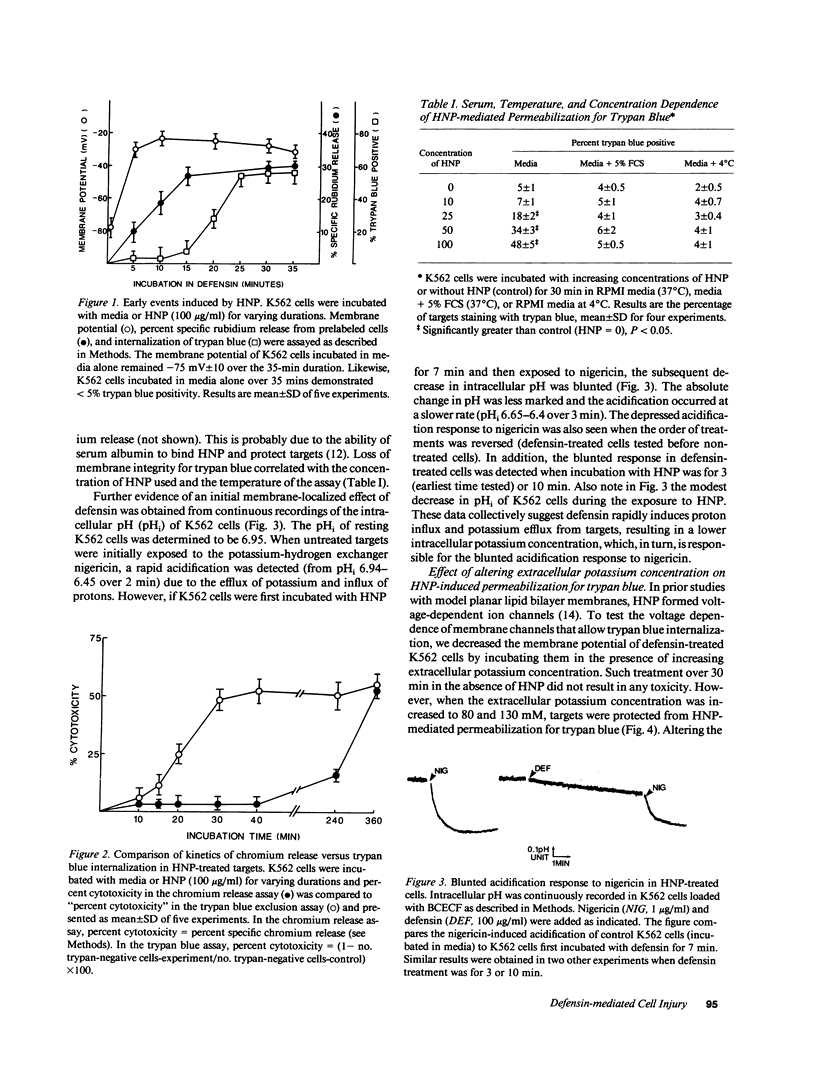
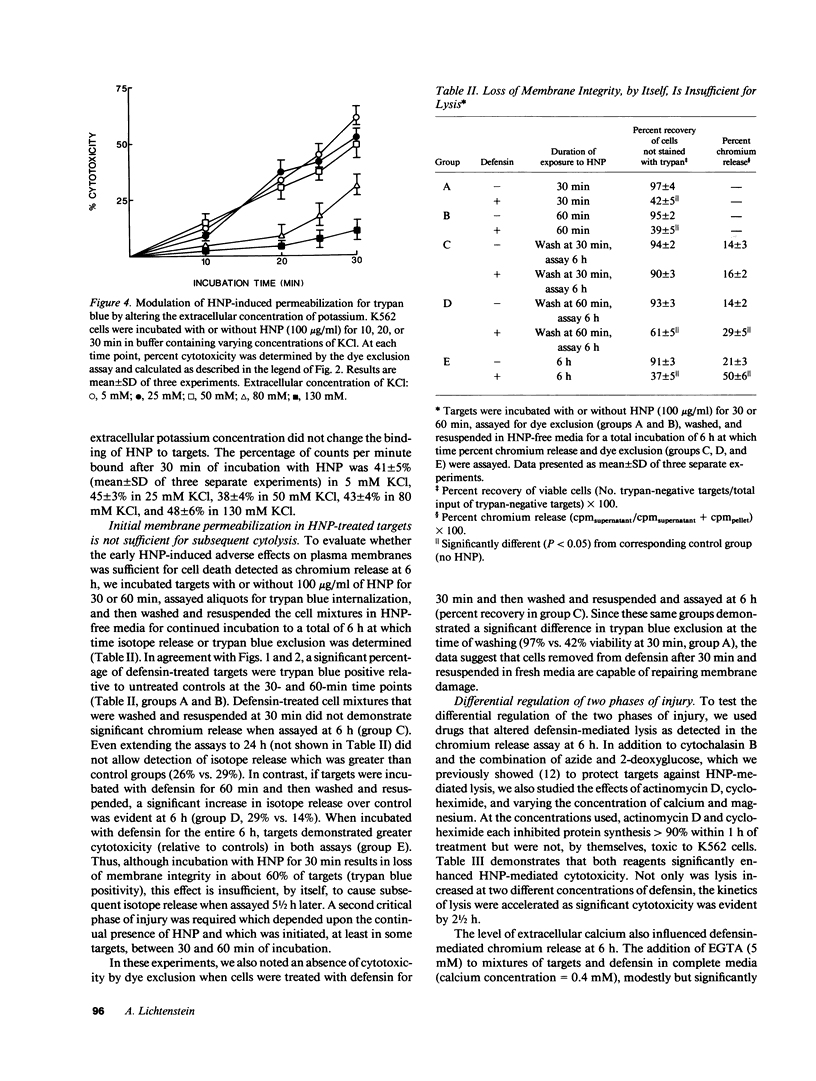
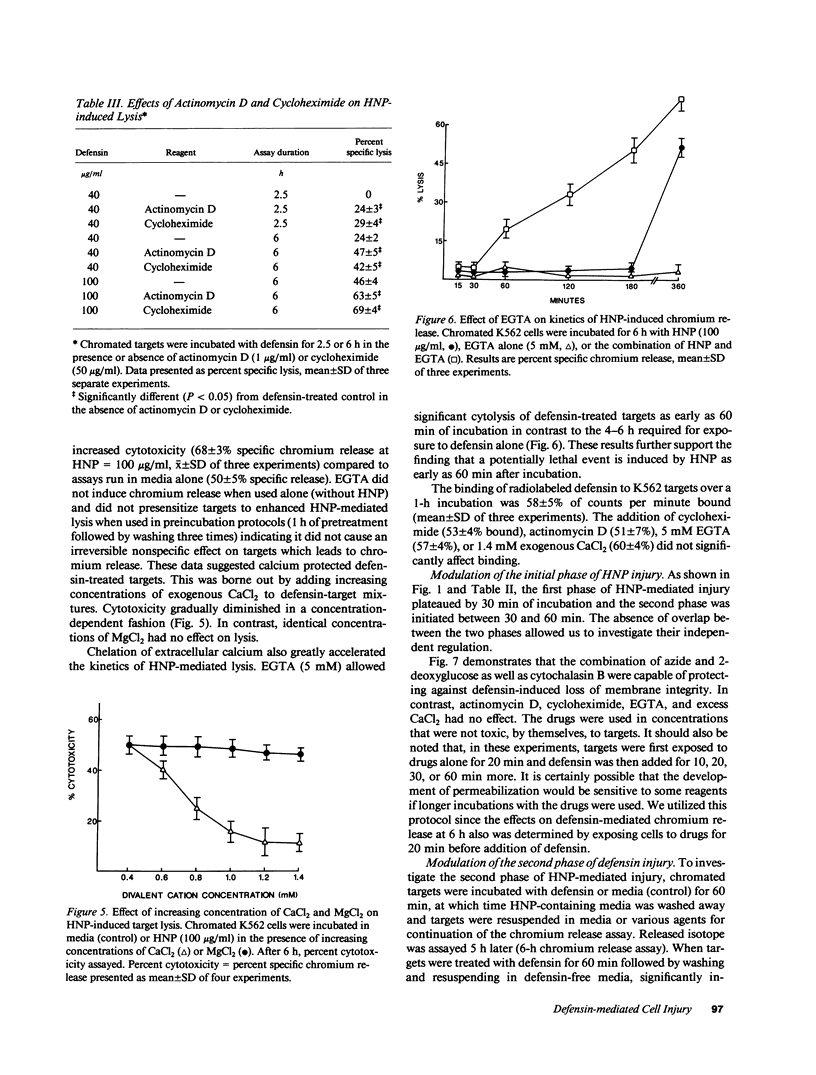
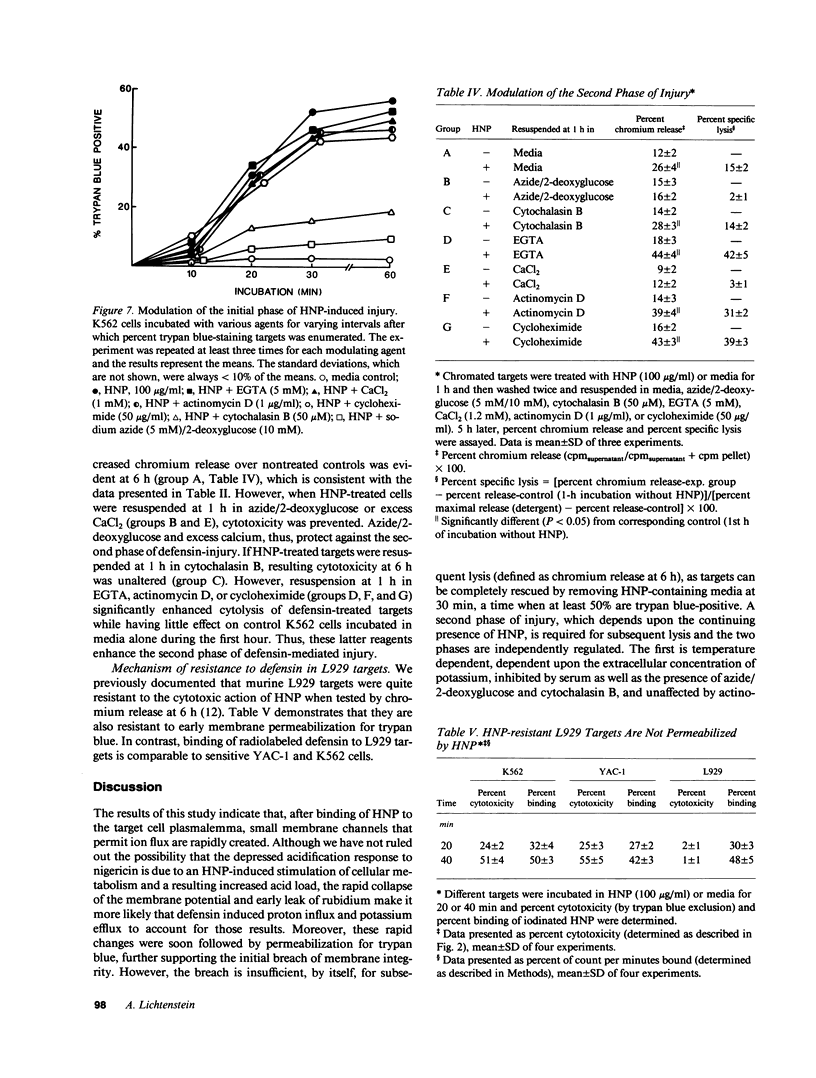
Defensins induce ion channels in model lipid bilayers and permeabilize the membranes of Escherichia coli. We investigated whether similar membrane-active events occur during defensin-mediated cytolysis of tumor cells. Although defensin-treated K562 targets did not release chromium-labeled cytoplasmic components for 5-6 h, they experienced a rapid collapse (within minutes) of the membrane potential, efflux of rubidium, and influx of trypan blue. Defensin treatment also blunted the subsequent acidification response induced by nigericin, thereby further supporting the notion of enhanced transmembrane ion flow during exposure. These initial effects on the plasma membrane were not sufficient for subsequent lysis; a second phase of injury was required which involved the continued presence of defensin. The rapid membrane permeabilization phase was inhibited by azide/2-deoxyglucose, cytochalasin B, and increased concentrations of extracellular potassium and was unaffected by actinomycin-D, cycloheximide, and varying the calcium concentration. In contrast, the second phase was unaffected by cytochalasin B, inhibited by azide/2-deoxyglucose, enhanced by actinomycin D and cycloheximide, and varied with calcium concentration. These results indicate the initial adverse effect of defensins on mammalian cells occurs at the cell membrane. It is possible that the second phase of injury is mediated intracellularly by defensin that has been internalized through this leaky membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhakdi S., Tranum-Jensen J. Molecular nature of the complement lesion. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5655–5659. doi: 10.1073/pnas.75.11.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. F., Koski C. L., Shin M. L. Elimination of terminal complement intermediates from the plasma membrane of nucleated cells: the rate of disappearance differs for cells carrying C5b-7 or C5b-8 or a mixture of C5b-8 with a limited number of C5b-9. J Immunol. 1985 Mar;134(3):1804–1809. [PubMed] [Google Scholar]
- Dallegri F., Frumento G., Ballestrero A., Goretti R., Patrone F. Relationship between antibody-dependent tumour cell lysis and primary granule exocytosis by human neutrophils. Clin Exp Immunol. 1987 Nov;70(2):479–483. [PMC free article] [PubMed] [Google Scholar]
- Dallegri F., Patrone F., Frumento G., Sacchetti C. Antibody-dependent killing of tumor cells by polymorphonuclear leukocytes. Involvement of oxidative and nonoxidative mechanisms. J Natl Cancer Inst. 1984 Aug;73(2):331–339. doi: 10.1093/jnci/73.2.331. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Terwilliger T. C., Tsui F. Structural studies of bee melittin. Biophys J. 1980 Oct;32(1):252–254. doi: 10.1016/S0006-3495(80)84953-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English D., Lukens J. N. Regulation of neutrophil inflammatory mediator release: chemotactic peptide activation of stimulus-dependent cytotoxicity. J Immunol. 1983 Feb;130(2):850–856. [PubMed] [Google Scholar]
- Füssle R., Bhakdi S., Sziegoleit A., Tranum-Jensen J., Kranz T., Wellensiek H. J. On the mechanism of membrane damage by Staphylococcus aureus alpha-toxin. J Cell Biol. 1981 Oct;91(1):83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987 Mar;55(3):568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Yamaguchi D. T., Kleeman C. R., Muallem S. Cytosolic pH regulation in osteoblasts. Interaction of Na+ and H+ with the extracellular and intracellular faces of the Na+/H+ exchanger. J Gen Physiol. 1988 Aug;92(2):239–261. doi: 10.1085/jgp.92.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A., Olsen K. J., Lee M. K., Lichtenheld M. G., Podack E. R. Cytolysis by Ca-permeable transmembrane channels. Pore formation causes extensive DNA degradation and cell lysis. J Exp Med. 1989 Mar 1;169(3):765–777. doi: 10.1084/jem.169.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan B. L., Selsted M. E., Ganz T., Lehrer R. I. Antimicrobial defensin peptides form voltage-dependent ion-permeable channels in planar lipid bilayer membranes. Proc Natl Acad Sci U S A. 1990 Jan;87(1):210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz P., Simone C. B., Henkart P. A., Fauci A. S. Mechanisms of antibody-dependent cellular cytotoxicity: the use of effector cells from chronic granulomatous disease patients as investigative probes. J Clin Invest. 1980 Jan;65(1):55–63. doi: 10.1172/JCI109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Barton A., Daher K. A., Harwig S. S., Ganz T., Selsted M. E. Interaction of human defensins with Escherichia coli. Mechanism of bactericidal activity. J Clin Invest. 1989 Aug;84(2):553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T., Szklarek D., Selsted M. E. Modulation of the in vitro candidacidal activity of human neutrophil defensins by target cell metabolism and divalent cations. J Clin Invest. 1988 Jun;81(6):1829–1835. doi: 10.1172/JCI113527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenstein A. K., Ganz T., Nguyen T. M., Selsted M. E., Lehrer R. I. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J Immunol. 1988 Apr 15;140(8):2686–2694. [PubMed] [Google Scholar]
- Lichtenstein A. K., Ganz T., Selsted M. E., Lehrer R. I. Synergistic cytolysis mediated by hydrogen peroxide combined with peptide defensins. Cell Immunol. 1988 Jun;114(1):104–116. doi: 10.1016/0008-8749(88)90258-4. [DOI] [PubMed] [Google Scholar]
- Lichtenstein A., Ganz T., Selsted M. E., Lehrer R. I. In vitro tumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986 Dec;68(6):1407–1410. [PubMed] [Google Scholar]
- Lichtenstein A. Neutrophil-mediated nonoxidative tumor lysis stimulated by high concentrations of phorbol myristate acetate. Clin Immunol Immunopathol. 1988 Jun;47(3):296–309. doi: 10.1016/s0090-1229(88)80008-4. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Brukner L. H., Silverstein S. C., Cohn Z. A. Extracellular cytolysis by activated macrophages and granulocytes. I. Pharmacologic triggering of effector cells and the release of hydrogen peroxide. J Exp Med. 1979 Jan 1;149(1):84–99. doi: 10.1084/jem.149.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okrent D. G., Lichtenstein A. K., Ganz T. Direct cytotoxicity of polymorphonuclear leukocyte granule proteins to human lung-derived cells and endothelial cells. Am Rev Respir Dis. 1990 Jan;141(1):179–185. doi: 10.1164/ajrccm/141.1.179. [DOI] [PubMed] [Google Scholar]
- Podack E. R., Dennert G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. 1983 Mar 31-Apr 6Nature. 302(5907):442–445. doi: 10.1038/302442a0. [DOI] [PubMed] [Google Scholar]
- Siebens H., Tevethia S. S., Babior B. M. Neutrophil-mediated antibody-dependent killing of herpes-simplex-virus-infected cells. Blood. 1979 Jul;54(1):88–94. [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A. Cell-mediated killing: a common mechanism? Cell. 1986 Aug 29;46(5):641–642. doi: 10.1016/0092-8674(86)90336-3. [DOI] [PubMed] [Google Scholar]
- Young J. D., Unkeless J. C., Kaback H. R., Cohn Z. A. Mouse macrophage Fc receptor for IgG gamma 2b/gamma 1 in artificial and plasma membrane vesicles functions as a ligand-dependent ionophore. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1636–1640. doi: 10.1073/pnas.80.6.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young J. D., Young T. M., Lu L. P., Unkeless J. C., Cohn Z. A. Characterization of a membrane pore-forming protein from Entamoeba histolytica. J Exp Med. 1982 Dec 1;156(6):1677–1690. doi: 10.1084/jem.156.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M. Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5449–5453. doi: 10.1073/pnas.84.15.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]



