Abstract
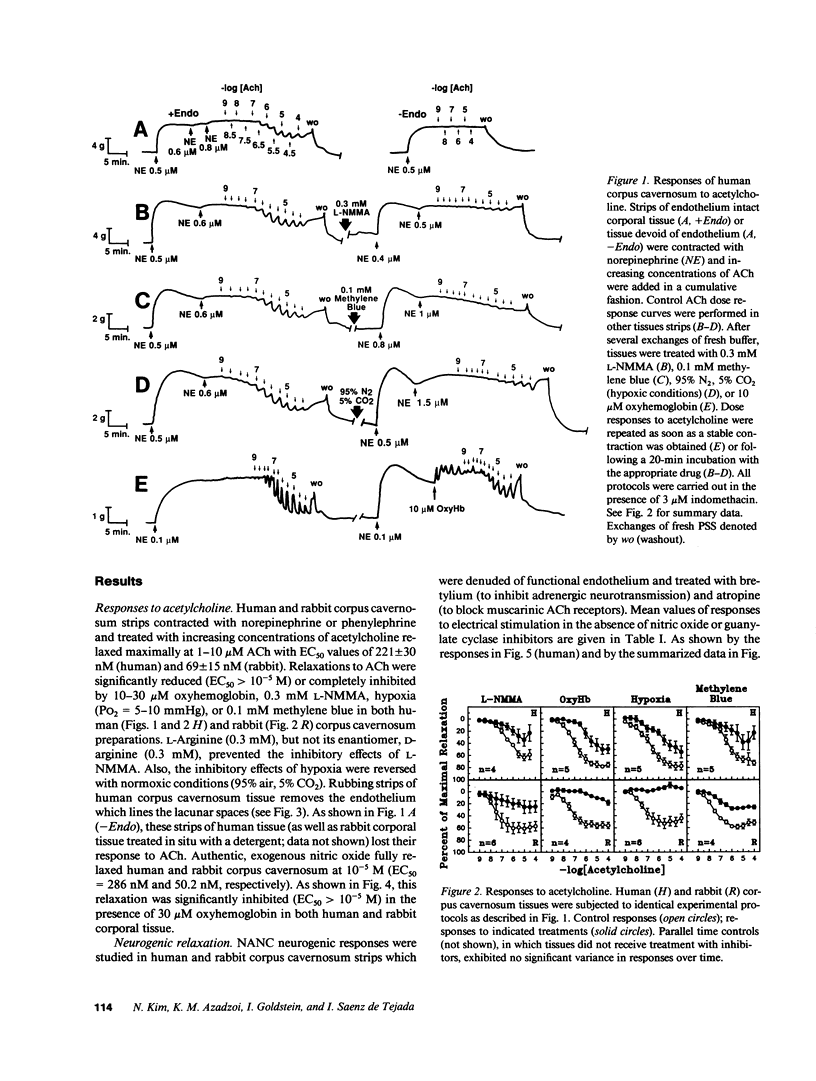
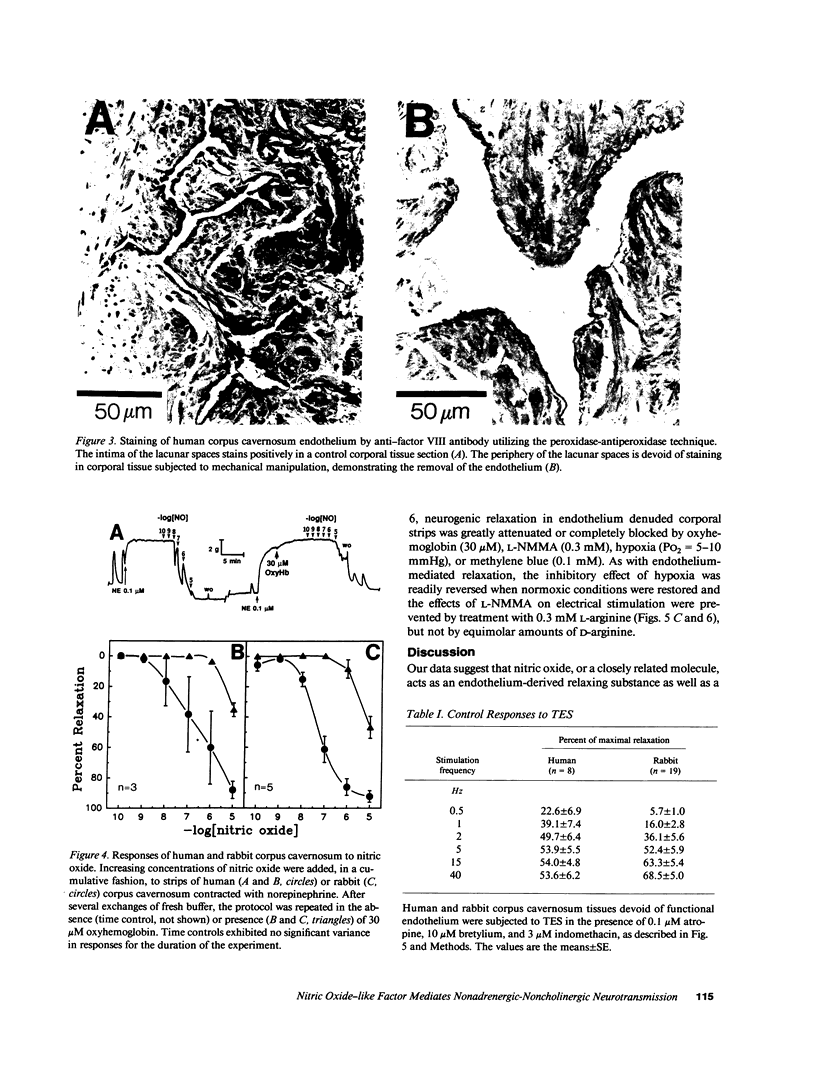
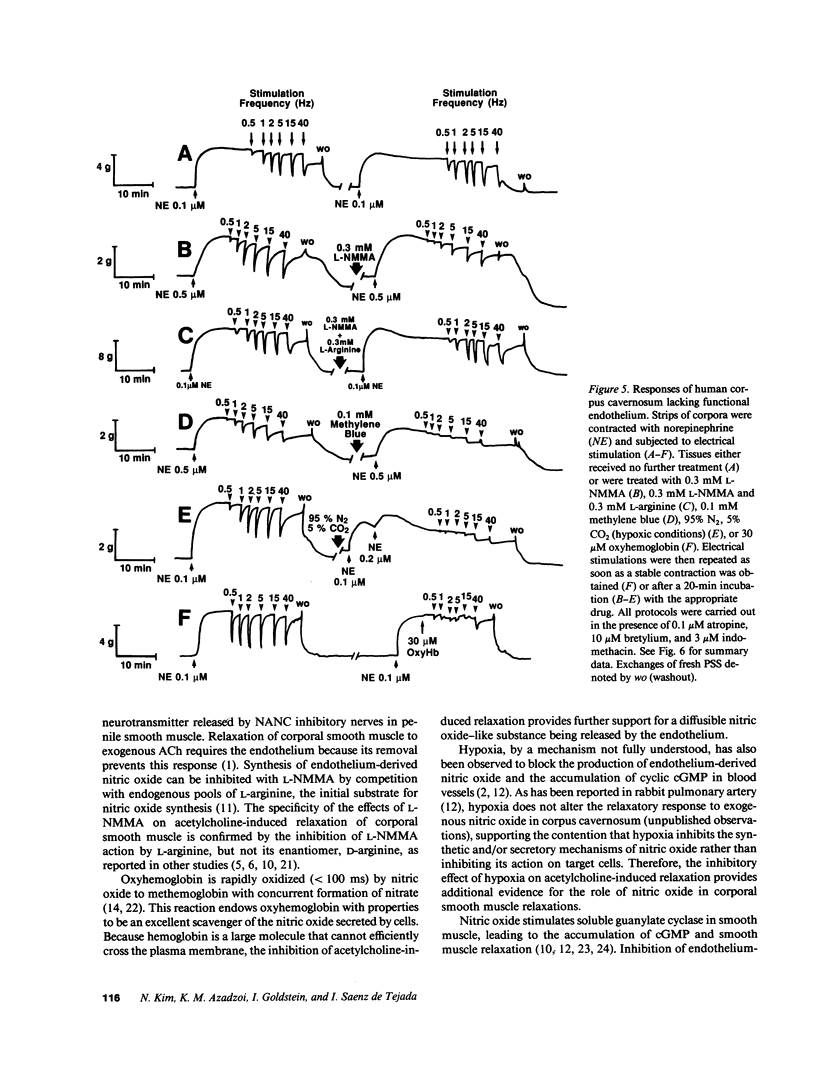
This study was initiated to characterize nonadrenergic-noncholinergic (NANC) inhibitory neurotransmission in penile corpus cavernosum. Using organ baths, isometric tension measurements were made in strips of human and rabbit corpus cavernosum. In examining endothelium-mediated responses, cumulative additions of exogenous acetylcholine elicited dose-dependent relaxations which were significantly reduced or completely inhibited in tissues treated with NG-monomethyl L-arginine (L-NMMA; an inhibitor of nitric oxide synthesis), oxyhemoglobin (a nitric oxide scavenger), or methylene blue (a guanylate cyclase blocker). Tissues exposed to hypoxic conditions (PO2 = 5-10 mmHg) also did not respond to exogenous acetylcholine. Mechanical removal of the endothelium in human corporal strips or in situ treatment of rabbit corpora with detergent blocked the relaxation to acetylcholine. Transmural electrical stimulation of corporal tissue strips denuded of functional endothelium, in the presence of adrenergic blockade with bretylium and muscarinic receptor blockade with atropine, caused frequency-dependent relaxation. This neurogenic relaxation was reduced or prevented by L-NMMA, oxyhemoglobin, methylene blue, and hypoxia. The effects of L-NMMA were reversed by L-arginine and the effects of hypoxia were readily reversed by normoxic conditions. Authentic, exogenous nitric oxide relaxed corporal strips which were contracted with adrenergic agonists and this effect was significantly inhibited by oxyhemoglobin. It is concluded that (a) endothelium-mediated responses of corpus cavernosum smooth muscle are mediated by a diffusible nitric oxide-like substance; (b) NANC neurogenic inhibitory responses do not require functional endothelium, and (c) nitric oxide, or a closely related substance, may act as an inhibitory neurotransmitter in penile corpus cavernosum smooth muscle.
Full text
PDF



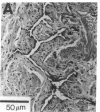
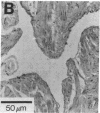
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnette M., Torphy T. J., Grous M., Fine C., Ormsbee H. S., 3rd Cyclic GMP: a potential mediator of neurally- and drug-induced relaxation of opossum lower esophageal sphincter. J Pharmacol Exp Ther. 1989 May;249(2):524–528. [PubMed] [Google Scholar]
- Bredt D. S., Hwang P. M., Snyder S. H. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990 Oct 25;347(6295):768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- Bult H., Boeckxstaens G. E., Pelckmans P. A., Jordaens F. H., Van Maercke Y. M., Herman A. G. Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature. 1990 May 24;345(6273):346–347. doi: 10.1038/345346a0. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garthwaite J., Charles S. L., Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988 Nov 24;336(6197):385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- Gibson A., Mirzazadeh S., Hobbs A. J., Moore P. K. L-NG-monomethyl arginine and L-NG-nitro arginine inhibit non-adrenergic, non-cholinergic relaxation of the mouse anococcygeus muscle. Br J Pharmacol. 1990 Mar;99(3):602–606. doi: 10.1111/j.1476-5381.1990.tb12976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Liu X. R., Martin W. The effects of L-arginine and NG-monomethyl L-arginine on the response of the rat anococcygeus muscle to NANC nerve stimulation. Br J Pharmacol. 1989 Dec;98(4):1080–1082. doi: 10.1111/j.1476-5381.1989.tb12650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorsky L. D., Förstermann U., Ishii K., Murad F. Production of an EDRF-like activity in the cytosol of N1E-115 neuroblastoma cells. FASEB J. 1990 Mar;4(5):1494–1500. doi: 10.1096/fasebj.4.5.2155150. [DOI] [PubMed] [Google Scholar]
- Gruetter C. A., Gruetter D. Y., Lyon J. E., Kadowitz P. J., Ignarro L. J. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981 Oct;219(1):181–186. [PubMed] [Google Scholar]
- Gruetter C. A., Kadowitz P. J., Ignarro L. J. Methylene blue inhibits coronary arterial relaxation and guanylate cyclase activation by nitroglycerin, sodium nitrite, and amyl nitrite. Can J Physiol Pharmacol. 1981 Feb;59(2):150–156. doi: 10.1139/y81-025. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Bush P. A., Buga G. M., Wood K. S., Fukuto J. M., Rajfer J. Nitric oxide and cyclic GMP formation upon electrical field stimulation cause relaxation of corpus cavernosum smooth muscle. Biochem Biophys Res Commun. 1990 Jul 31;170(2):843–850. doi: 10.1016/0006-291x(90)92168-y. [DOI] [PubMed] [Google Scholar]
- Johns R. A., Linden J. M., Peach M. J. Endothelium-dependent relaxation and cyclic GMP accumulation in rabbit pulmonary artery are selectively impaired by moderate hypoxia. Circ Res. 1989 Dec;65(6):1508–1515. doi: 10.1161/01.res.65.6.1508. [DOI] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Koutts J., Howard M. A., Firkin B. G. Factor VIII physiology and pathology in man. Prog Hematol. 1979;11:115–145. [PubMed] [Google Scholar]
- Mukai K., Rosai J., Burgdorf W. H. Localization of factor VIII-related antigen in vascular endothelial cells using an immunoperoxidase method. Am J Surg Pathol. 1980 Jun;4(3):273–276. doi: 10.1097/00000478-198006000-00008. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Hodson H. F., Moncada S. A specific inhibitor of nitric oxide formation from L-arginine attenuates endothelium-dependent relaxation. Br J Pharmacol. 1989 Feb;96(2):418–424. doi: 10.1111/j.1476-5381.1989.tb11833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saenz de Tejada I., Blanco R., Goldstein I., Azadzoi K., de las Morenas A., Krane R. J., Cohen R. A. Cholinergic neurotransmission in human corpus cavernosum. I. Responses of isolated tissue. Am J Physiol. 1988 Mar;254(3 Pt 2):H459–H467. doi: 10.1152/ajpheart.1988.254.3.H459. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Goldstein I., Azadzoi K., Krane R. J., Cohen R. A. Impaired neurogenic and endothelium-mediated relaxation of penile smooth muscle from diabetic men with impotence. N Engl J Med. 1989 Apr 20;320(16):1025–1030. doi: 10.1056/NEJM198904203201601. [DOI] [PubMed] [Google Scholar]
- Saenz de Tejada I., Kim N., Lagan I., Krane R. J., Goldstein I. Regulation of adrenergic activity in penile corpus cavernosum. J Urol. 1989 Oct;142(4):1117–1121. doi: 10.1016/s0022-5347(17)39009-2. [DOI] [PubMed] [Google Scholar]
- Simons E. R., Hartzband P., Whitin J., Chapman C. Circular Dichroism Studies of Cyanate-Induced Conformational Changes in Hemoglobins A and S. Biochemistry. 1976 Sep 7;15(18):4059–4064. doi: 10.1021/bi00663a022. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfamariam B., Halpern W., Osol G. Effects of perfusion and endothelium on the reactivity of isolated resistance arteries. Blood Vessels. 1985;22(6):301–305. doi: 10.1159/000158616. [DOI] [PubMed] [Google Scholar]
- Vane J. R., Anggård E. E., Botting R. M. Regulatory functions of the vascular endothelium. N Engl J Med. 1990 Jul 5;323(1):27–36. doi: 10.1056/NEJM199007053230106. [DOI] [PubMed] [Google Scholar]
- Wright C. D., Mülsch A., Busse R., Osswald H. Generation of nitric oxide by human neutrophils. Biochem Biophys Res Commun. 1989 Apr 28;160(2):813–819. doi: 10.1016/0006-291x(89)92506-0. [DOI] [PubMed] [Google Scholar]