Abstract
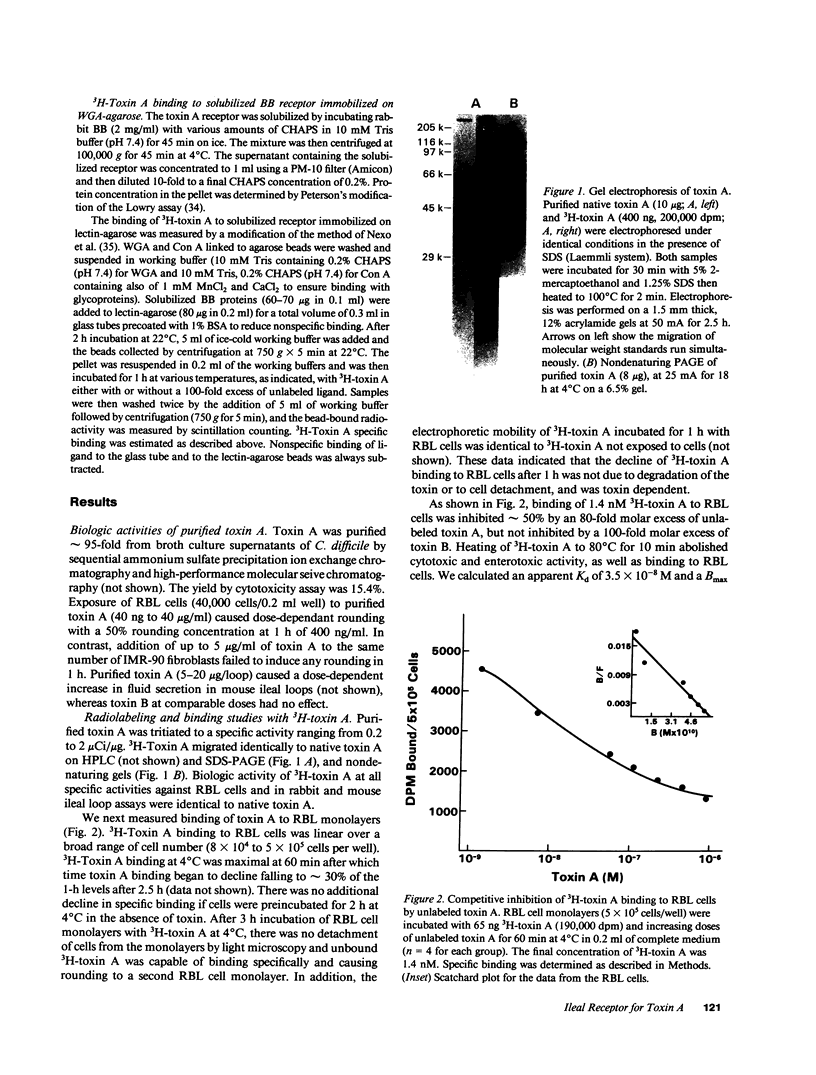
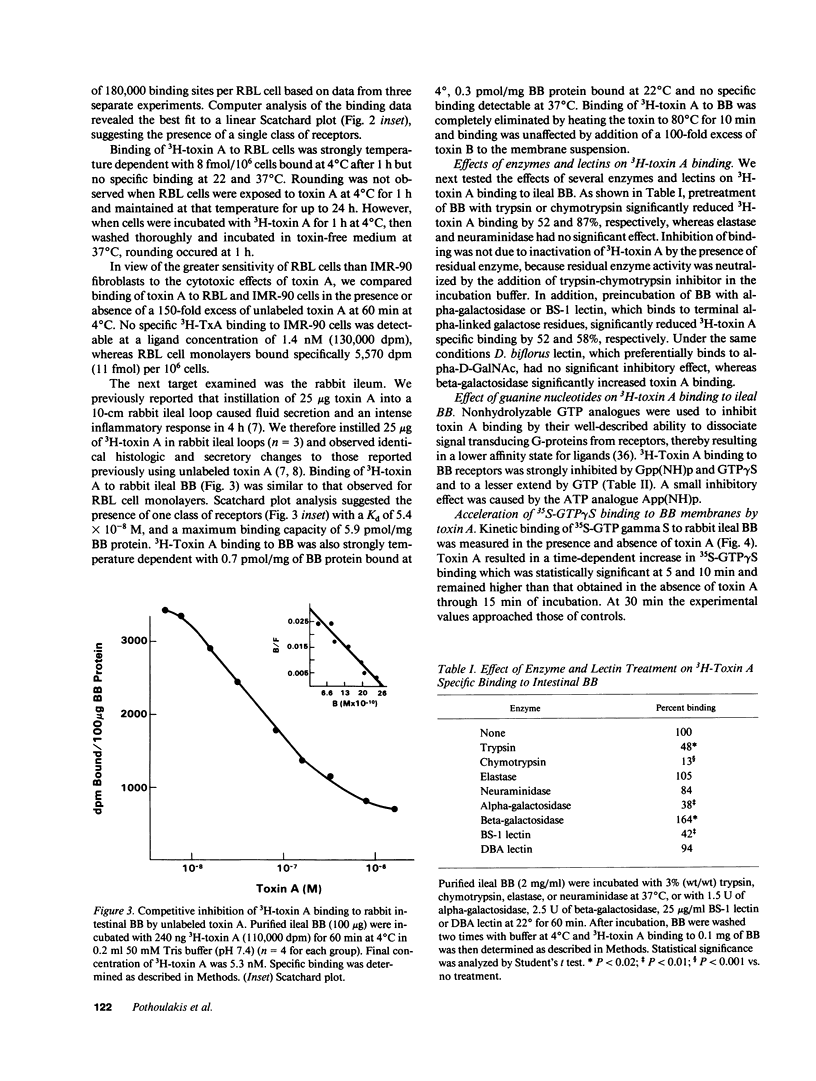
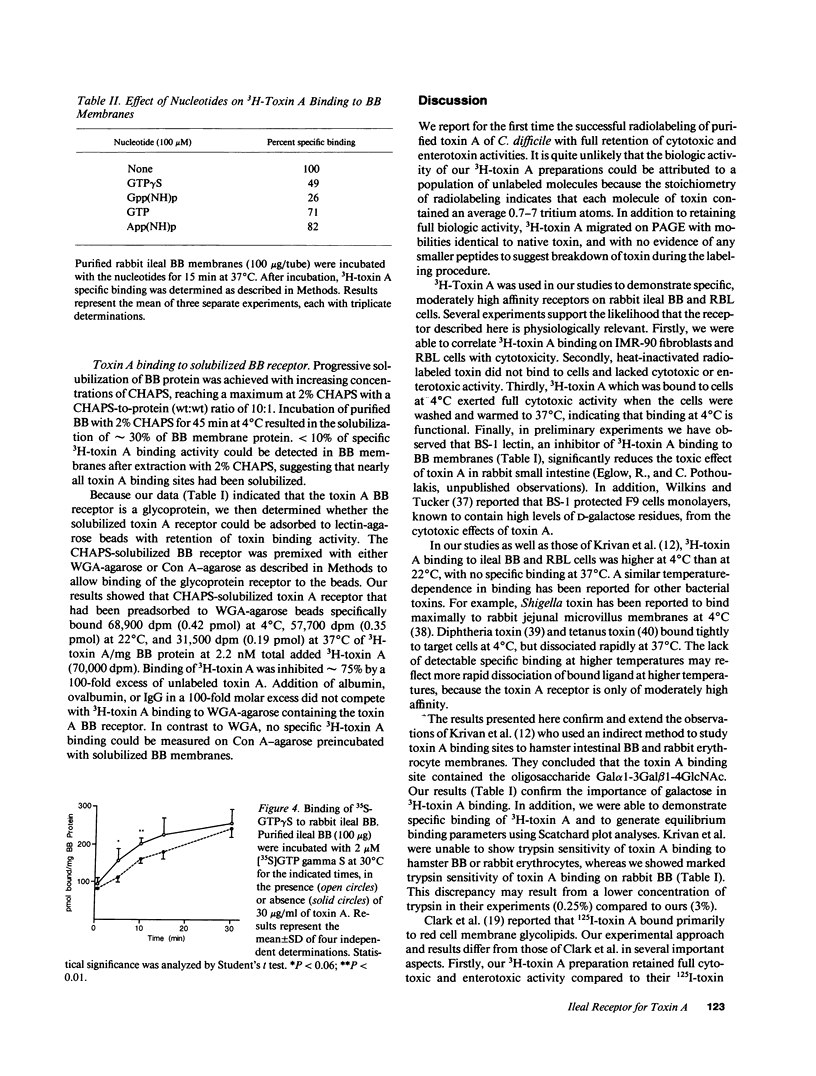
The purpose of this study was to characterize the surface receptor for toxin A, the enterotoxin from Clostridium difficile, on rabbit intestinal brush borders (BB) and on rat basophilic leukemia (RBL) cells. Purified toxin A was radiolabeled using a modified Bolton-Hunter method to sp act 2 microCi/micrograms, with retention of full biologic activity. 3H-Toxin A bound specifically to a single class of receptors on rabbit BB and on RBL cells with dissociation constants of 5.4 x 10(-8) and 3.5 x 10(-8) M, respectively. RBL cells were highly sensitive to toxin A (cell rounding) and had 180,000 specific binding sites per cell, whereas IMR-90 fibroblasts were far less sensitive to toxin A and lacked detectable specific binding sites. Exposure of BB to trypsin or chymotrypsin significantly reduced 3H-toxin A specific binding. Preincubation of BB with Bandeirea simplicifolia (BS-1) lectin also reduced specific binding, and CHAPS-solubilized receptors could be immobilized with WGA-agarose. The addition of 100 nM toxin A accelerated the association of 35S-GTP gamma S with rabbit ileal BB, and preincubation of BB with the GTP analogues GTP gamma S or Gpp(NH)p, significantly reduced 3H-toxin A specific binding. Our data indicate that the membrane receptor for toxin A is a galactose and N-acetyl-glucosamine-containing glycoprotein which appears to be coupled to a G protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams G. D., Allo M., Rifkin G. D., Fekety R., Silva J., Jr Mucosal damage mediated by clostridial toxin in experimental clindamycin-associated colitis. Gut. 1980 Jun;21(6):493–499. doi: 10.1136/gut.21.6.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett J. G., Chang T. W., Gurwith M., Gorbach S. L., Onderdonk A. B. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N Engl J Med. 1978 Mar 9;298(10):531–534. doi: 10.1056/NEJM197803092981003. [DOI] [PubMed] [Google Scholar]
- Bartlett J. G., Onderdonk A. B., Cisneros R. L., Kasper D. L. Clindamycin-associated colitis due to a toxin-producing species of Clostridium in hamsters. J Infect Dis. 1977 Nov;136(5):701–705. doi: 10.1093/infdis/136.5.701. [DOI] [PubMed] [Google Scholar]
- Blomqvist L., Appelgren L. E., Thelestam M. Distribution of 3H-labeled staphylococcal alpha-toxin and a toxin fragment in mice. Infect Immun. 1987 Aug;55(8):1906–1913. doi: 10.1128/iai.55.8.1906-1913.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark G. F., Krivan H. C., Wilkins T. D., Smith D. F. Toxin A from Clostridium difficile binds to rabbit erythrocyte glycolipids with terminal Gal alpha 1-3Gal beta 1-4GlcNAc sequences. Arch Biochem Biophys. 1987 Aug 15;257(1):217–229. doi: 10.1016/0003-9861(87)90561-3. [DOI] [PubMed] [Google Scholar]
- Critchley D. R., Nelson P. G., Habig W. H., Fishman P. H. Fate of tetanus toxin bound to the surface of primary neurons in culture: evidence for rapid internalization. J Cell Biol. 1985 May;100(5):1499–1507. doi: 10.1083/jcb.100.5.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. METHOD FOR ASSAY OF INTESTINAL DISACCHARIDASES. Anal Biochem. 1964 Jan;7:18–25. doi: 10.1016/0003-2697(64)90115-0. [DOI] [PubMed] [Google Scholar]
- DE S. N., CHATTERJE D. N. An experimental study of the mechanism of action of Vibriod cholerae on the intestinal mucous membrane. J Pathol Bacteriol. 1953 Oct;66(2):559–562. doi: 10.1002/path.1700660228. [DOI] [PubMed] [Google Scholar]
- Dove C. H., Wang S. Z., Price S. B., Phelps C. J., Lyerly D. M., Wilkins T. D., Johnson J. L. Molecular characterization of the Clostridium difficile toxin A gene. Infect Immun. 1990 Feb;58(2):480–488. doi: 10.1128/iai.58.2.480-488.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. D. High-affinity binding of staphylococcal enterotoxins A and B to HLA-DR. Nature. 1989 May 18;339(6221):221–223. doi: 10.1038/339221a0. [DOI] [PubMed] [Google Scholar]
- George W. L., Rolfe R. D., Finegold S. M. Clostridium difficile and its cytotoxin in feces of patients with antimicrobial agent-associated diarrhea and miscellaneous conditions. J Clin Microbiol. 1982 Jun;15(6):1049–1053. doi: 10.1128/jcm.15.6.1049-1053.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert R. J., Triadafilopoulos G., Pothoulakis C., Giampaolo C., LaMont J. T. Effect of purified Clostridium difficile toxins on intestinal smooth muscle. I. Toxin A. Am J Physiol. 1989 Apr;256(4 Pt 1):G759–G766. doi: 10.1152/ajpgi.1989.256.4.G759. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Goldsmith P., Gierschik P., Milligan G., Unson C. G., Vinitsky R., Malech H. L., Spiegel A. M. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987 Oct 25;262(30):14683–14688. [PubMed] [Google Scholar]
- Gray L. S., Huber K. S., Gray M. C., Hewlett E. L., Engelhard V. H. Pertussis toxin effects on T lymphocytes are mediated through CD3 and not by pertussis toxin catalyzed modification of a G protein. J Immunol. 1989 Mar 1;142(5):1631–1638. [PubMed] [Google Scholar]
- Hecht G., Pothoulakis C., LaMont J. T., Madara J. L. Clostridium difficile toxin A perturbs cytoskeletal structure and tight junction permeability of cultured human intestinal epithelial monolayers. J Clin Invest. 1988 Nov;82(5):1516–1524. doi: 10.1172/JCI113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer U., Nelson K., Perrotto J., Isselbacher K. J. Glucose transport in isolated brush border membrane from rat small intestine. J Biol Chem. 1973 Jan 10;248(1):25–32. [PubMed] [Google Scholar]
- Kornfeld H., Cruikshank W. W., Pyle S. W., Berman J. S., Center D. M. Lymphocyte activation by HIV-1 envelope glycoprotein. Nature. 1988 Sep 29;335(6189):445–448. doi: 10.1038/335445a0. [DOI] [PubMed] [Google Scholar]
- Krivan H. C., Clark G. F., Smith D. F., Wilkins T. D. Cell surface binding site for Clostridium difficile enterotoxin: evidence for a glycoconjugate containing the sequence Gal alpha 1-3Gal beta 1-4GlcNAc. Infect Immun. 1986 Sep;53(3):573–581. doi: 10.1128/iai.53.3.573-581.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marrack P., Kappler J. The staphylococcal enterotoxins and their relatives. Science. 1990 May 11;248(4956):705–711. doi: 10.1126/science.2185544. [DOI] [PubMed] [Google Scholar]
- Miller P. D., Pothoulakis C., Baeker T. R., LaMont J. T., Rothstein T. L. Macrophage-dependent stimulation of T cell-depleted spleen cells by Clostridium difficile toxin A and calcium ionophore. Cell Immunol. 1990 Mar;126(1):155–163. doi: 10.1016/0008-8749(90)90308-e. [DOI] [PubMed] [Google Scholar]
- Mitchell T. J., Ketley J. M., Haslam S. C., Stephen J., Burdon D. W., Candy D. C., Daniel R. Effect of toxin A and B of Clostridium difficile on rabbit ileum and colon. Gut. 1986 Jan;27(1):78–85. doi: 10.1136/gut.27.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobassaleh M., Donohue-Rolfe A., Jacewicz M., Grand R. J., Keusch G. T. Pathogenesis of shigella diarrhea: evidence for a developmentally regulated glycolipid receptor for shigella toxin involved in the fluid secretory response of rabbit small intestine. J Infect Dis. 1988 May;157(5):1023–1031. doi: 10.1093/infdis/157.5.1023. [DOI] [PubMed] [Google Scholar]
- Moore R., Pothoulakis C., LaMont J. T., Carlson S., Madara J. L. C. difficile toxin A increases intestinal permeability and induces Cl- secretion. Am J Physiol. 1990 Aug;259(2 Pt 1):G165–G172. doi: 10.1152/ajpgi.1990.259.2.G165. [DOI] [PubMed] [Google Scholar]
- Morris R. E., Gerstein A. S., Bonventre P. F., Saelinger C. B. Receptor-mediated entry of diphtheria toxin into monkey kidney (Vero) cells: electron microscopic evaluation. Infect Immun. 1985 Dec;50(3):721–727. doi: 10.1128/iai.50.3.721-727.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munson P. J. LIGAND: a computerized analysis of ligand binding data. Methods Enzymol. 1983;92:543–576. doi: 10.1016/0076-6879(83)92044-x. [DOI] [PubMed] [Google Scholar]
- Nexł E., Hock R. A., Hollenberg M. D. Lectin-agarose immobilization, a new method for detecting soluble membrane receptors. Application to studies with epidermal growth factor-urogastrone and transcobalamin-II. J Biol Chem. 1979 Sep 25;254(18):8740–8743. [PubMed] [Google Scholar]
- Ohishi I. Response of mouse intestinal loop to botulinum C2 toxin: enterotoxic activity induced by cooperation of nonlinked protein components. Infect Immun. 1983 May;40(2):691–695. doi: 10.1128/iai.40.2.691-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi W., Levine H. G., LaMont J. T., Clark R. A. Inactivation of Clostridium difficile cytotoxin by the neutrophil myeloperoxidase system. J Infect Dis. 1984 Feb;149(2):215–219. doi: 10.1093/infdis/149.2.215. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pothoulakis C., Barone L. M., Ely R., Faris B., Clark M. E., Franzblau C., LaMont J. T. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986 Jan 25;261(3):1316–1321. [PubMed] [Google Scholar]
- Pothoulakis C., Sullivan R., Melnick D. A., Triadafilopoulos G., Gadenne A. S., Meshulam T., LaMont J. T. Clostridium difficile toxin A stimulates intracellular calcium release and chemotactic response in human granulocytes. J Clin Invest. 1988 Jun;81(6):1741–1745. doi: 10.1172/JCI113514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C., Triadafilopoulos G., Clark M., Franzblau C., LaMont J. T. Clostridium difficile cytotoxin inhibits protein synthesis in fibroblasts and intestinal mucosa. Gastroenterology. 1986 Nov;91(5):1147–1153. doi: 10.1016/s0016-5085(86)80010-5. [DOI] [PubMed] [Google Scholar]
- Rosoff P. M., Walker R., Winberry L. Pertussis toxin triggers rapid second messenger production in human T lymphocytes. J Immunol. 1987 Oct 1;139(7):2419–2423. [PubMed] [Google Scholar]
- Rothman S. W., Brown J. E., Diecidue A., Foret D. A. Differential cytotoxic effects of toxins A and B isolated from Clostridium difficile. Infect Immun. 1984 Nov;46(2):324–331. doi: 10.1128/iai.46.2.324-331.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldin D. C., Adelman S., Austen K. F., Stevens R. L., Hein A., Caulfield J. P., Woodbury R. G. Homology of the rat basophilic leukemia cell and the rat mucosal mast cell. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3871–3875. doi: 10.1073/pnas.82.11.3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. J., Prpic V., Johns J. A., Powers F. S., Graber S. E., Forbes J. T., Exton J. H. Bacterial toxins affect early events of T lymphocyte activation. J Clin Invest. 1989 Jan;83(1):234–242. doi: 10.1172/JCI113865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir H., Theoharides T. C., Gershon M. D., Askenase P. W. Serotonin storage pools in basophil leukemia and mast cells: characterization of two types of serotonin binding protein and radioautographic analysis of the intracellular distribution of [3H]serotonin. J Cell Biol. 1982 Jun;93(3):638–647. doi: 10.1083/jcb.93.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., O'Brien M. J., LaMont J. T. Differential effects of Clostridium difficile toxins A and B on rabbit ileum. Gastroenterology. 1987 Aug;93(2):273–279. doi: 10.1016/0016-5085(87)91014-6. [DOI] [PubMed] [Google Scholar]
- Triadafilopoulos G., Pothoulakis C., Weiss R., Giampaolo C., Lamont J. T. Comparative study of Clostridium difficile toxin A and cholera toxin in rabbit ileum. Gastroenterology. 1989 Nov;97(5):1186–1192. doi: 10.1016/0016-5085(89)91689-2. [DOI] [PubMed] [Google Scholar]
- Wedel N., Toselli P., Pothoulakis C., Faris B., Oliver P., Franzblau C., LaMont T. Ultrastructural effects of Clostridium difficile toxin B on smooth muscle cells and fibroblasts. Exp Cell Res. 1983 Oct 15;148(2):413–422. doi: 10.1016/0014-4827(83)90163-5. [DOI] [PubMed] [Google Scholar]




