Abstract
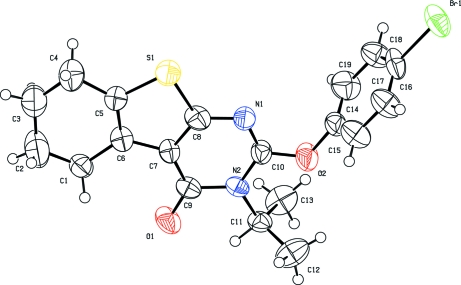
In the title compound, C19H19BrN2O2S, the central thienopyrimidine ring system is essentially planar, with a maximum displacement of 0.068 (3) Å. The attached cyclohexene ring adopts a half-chair conformation. The molecular conformation and crystal packing are stabilized by three intramolecular C—H⋯O hydrogen bonds and two C—H⋯π interactions.
Related literature
For background to the use of pyrimidine derivatives as drugs, see: Ding et al. (2004 ▶). For a description of the Cambridge Structural Database, see: Allen (2002 ▶). For a related structure, see: Zeng et al. (2006 ▶).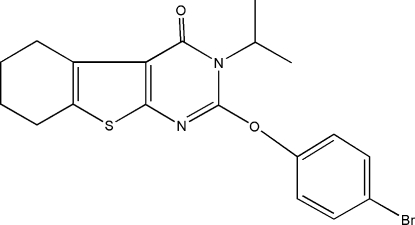
Experimental
Crystal data
C19H19BrN2O2S
M r = 418.32
Monoclinic,
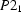
a = 13.3957 (7) Å
b = 5.7366 (3) Å
c = 13.3956 (7) Å
β = 115.5410 (10)°
V = 928.81 (8) Å3
Z = 2
Mo Kα radiation
μ = 2.34 mm−1
T = 298 (2) K
0.20 × 0.10 × 0.10 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.652, T max = 0.800
5798 measured reflections
3228 independent reflections
2346 reflections with I > 2σ(I)
R int = 0.106
Refinement
R[F 2 > 2σ(F 2)] = 0.067
wR(F 2) = 0.204
S = 1.07
3228 reflections
228 parameters
1 restraint
H-atom parameters constrained
Δρmax = 7.69 e Å−3
Δρmin = −2.63 e Å−3
Absolute structure: Flack (1983 ▶), 1424 Freidel pairs
Flack parameter: 0.00 (8)
Data collection: SMART (Bruker, 2001 ▶); cell refinement: SAINT (Bruker, 2001 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: PLATON (Spek, 2003 ▶); software used to prepare material for publication: SHELXTL (Sheldrick, 2008 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680803732X/at2674sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680803732X/at2674Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C11—H11⋯O1 | 0.98 | 2.20 | 2.726 (10) | 112 |
| C12—H12B⋯O2 | 0.96 | 2.43 | 2.915 (13) | 111 |
| C13—H13A⋯O2 | 0.96 | 2.38 | 2.951 (10) | 117 |
| C12—H12A⋯Cg1i | 0.96 | 2.92 | 3.854 (11) | 165 |
| C12—H12A⋯Cg2i | 0.96 | 2.71 | 3.434 (11) | 133 |
Symmetry code: (i) 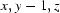 . Cg1 and Cg2 are the centroids of the thiophene (S1/C5–C8) and pyrimidine (N1/N2/C7–C10) rings, respectively.
. Cg1 and Cg2 are the centroids of the thiophene (S1/C5–C8) and pyrimidine (N1/N2/C7–C10) rings, respectively.
Acknowledgments
We gratefully acknowledge financial support of this work by the Research Foundation for Students and Teachers of Yunyang Medical College (grant Nos. 2007QDJ15, 2007ZQB19, 2007ZQB20).
supplementary crystallographic information
Comment
Pyrimidine derivatives are attracting the increasing attention of synthetic community because of the important role played by such systems in many natural products, antibiotics and drugs (Ding et al., 2004). In recent years, we have been engaged in the preparation of the derivatives of heterocycles via aza-Wittig reaction. The title compound, (I), was synthesized and structurally characterized in this context.
The molecular structure indicates that the thieno[2,3-d]pyrimidine moiety is a conjugated system (Fig. 1). All ring atoms in thieno[2,3-d]pyrimidine are essentially coplanar (Zeng et al., 2006). The bond lengths and angles are within experimental error, in the ranges of values in previously reported structures in the Cambridge Structural Database (Version 5.26; Allen, 2002).
The cyclohexene ring adopts a half-chair conformation. The crystal packing is stabilized by three intramolecular C—H···O hydrogen bonds and two C—H···π interactions (Table 1). There exist no intermolecular hydrogen bonding interactions and no π-π stackings.
Experimental
To a solution of iminophosphorane (1.45 g, 3 mmol) in anhydrous dichloromethane (15 ml) was added iso-propyl isocyanate (3 mmol) under dry nitrogen at room temperature. After the reaction mixture was left unstirred for 48 h at room temperature, the solvent was removed off under reduced pressure and ether/petroleum ether (1:2 v/v, 20 ml) was added to precipitate triphenylphosphine oxide. After filtration, the solvent was removed, and the residue was dissolved in CH3CN (15 ml). After adding 4-Br-PhOH (3.1 mmol) and excess K2CO3 to the solution of carbodiimide, The mixture was stirred for 24 h at room temperature, the solution was condensed and the residue was recrystallized by EtOH to give the title compound, (I), in yield of 80% (m.p. 478 K). Elemental analysis calculated for C19H19BrN2O2S: C 54.42, H 4.57, N 6.68. Found: C 54.56, H 4.42, N 6.53. Crystals suitable for single crystal X-ray diffraction were obtained by vapor diffusion of hexane and dichloromethane (1:3 v/v) at room temperature.
Refinement
H atoms were placed at calculated positions and treated as riding atoms, with C—H = 0.93–0.98 Å, and Uiso(H) = 1.2Ueq(C) for CH or 1.5Ueq(C) for CH3.
Figures
Fig. 1.
View of the molecule of (I) showing the atom-labeling scheme. Displacement ellipsoids are drawn at 50% probability level. H-atoms are represented by circles of arbitrary size.
Crystal data
| C19H19BrN2O2S | F000 = 428 |
| Mr = 419.33 | Dx = 1.499 Mg m−3 |
| Monoclinic, P21 | Melting point: 478K K |
| Hall symbol: P 2yb | Mo Kα radiation λ = 0.71073 Å |
| a = 13.3957 (7) Å | Cell parameters from 2048 reflections |
| b = 5.7366 (3) Å | θ = 2.9–24.5º |
| c = 13.3956 (7) Å | µ = 2.34 mm−1 |
| β = 115.5410 (10)º | T = 298 (2) K |
| V = 928.81 (8) Å3 | Block, colorless |
| Z = 2 | 0.20 × 0.10 × 0.10 mm |
Data collection
| Bruker SMART CCD area-detector diffractometer | 3228 independent reflections |
| Radiation source: fine-focus sealed tube | 2346 reflections with I > 2σ(I) |
| Monochromator: graphite | Rint = 0.106 |
| T = 298(2) K | θmax = 25.0º |
| φ and ω scans | θmin = 1.7º |
| Absorption correction: multi-scan(SADABS; Sheldrick, 1996) | h = −13→15 |
| Tmin = 0.652, Tmax = 0.800 | k = −6→6 |
| 5798 measured reflections | l = −15→15 |
Refinement
| Refinement on F2 | Hydrogen site location: inferred from neighbouring sites |
| Least-squares matrix: full | H-atom parameters constrained |
| R[F2 > 2σ(F2)] = 0.067 | w = 1/[σ2(Fo2) + (0.1151P)2] where P = (Fo2 + 2Fc2)/3 |
| wR(F2) = 0.204 | (Δ/σ)max = 0.001 |
| S = 1.07 | Δρmax = 0.67 e Å−3 |
| 3228 reflections | Δρmin = −0.62 e Å−3 |
| 228 parameters | Extinction correction: none |
| 1 restraint | Absolute structure: Flack (1983), 1424 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.00 (8) |
| Secondary atom site location: difference Fourier map |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br1 | 0.84533 (6) | 0.7555 (2) | 0.71718 (7) | 0.0762 (4) | |
| C1 | 0.0942 (6) | 1.2447 (18) | 0.8813 (6) | 0.0534 (18) | |
| H1A | 0.0444 | 1.3157 | 0.8118 | 0.064* | |
| H1B | 0.0650 | 1.0921 | 0.8853 | 0.064* | |
| C2 | 0.0967 (9) | 1.390 (3) | 0.9738 (10) | 0.103 (5) | |
| H2A | 0.0231 | 1.4514 | 0.9536 | 0.124* | |
| H2B | 0.1152 | 1.2908 | 1.0380 | 0.124* | |
| C3 | 0.1719 (9) | 1.578 (2) | 1.0038 (11) | 0.093 (4) | |
| H3A | 0.1730 | 1.6464 | 1.0704 | 0.111* | |
| H3B | 0.1416 | 1.6942 | 0.9459 | 0.111* | |
| C4 | 0.2917 (7) | 1.537 (2) | 1.0246 (7) | 0.067 (3) | |
| H4A | 0.3234 | 1.6797 | 1.0119 | 0.081* | |
| H4B | 0.3351 | 1.4885 | 1.1006 | 0.081* | |
| C5 | 0.2930 (6) | 1.3513 (13) | 0.9472 (6) | 0.0460 (19) | |
| C6 | 0.2071 (5) | 1.2157 (15) | 0.8824 (5) | 0.046 (2) | |
| C7 | 0.2363 (6) | 1.0548 (14) | 0.8163 (6) | 0.0405 (16) | |
| C8 | 0.3446 (6) | 1.0816 (16) | 0.8335 (6) | 0.052 (2) | |
| C9 | 0.1666 (6) | 0.9073 (15) | 0.7312 (6) | 0.0457 (18) | |
| C10 | 0.3302 (6) | 0.8295 (14) | 0.7015 (6) | 0.047 (2) | |
| C11 | 0.1472 (6) | 0.6545 (14) | 0.5719 (6) | 0.0459 (18) | |
| H11 | 0.0726 | 0.6617 | 0.5683 | 0.055* | |
| C12 | 0.1754 (9) | 0.3997 (19) | 0.5775 (8) | 0.079 (3) | |
| H12A | 0.1906 | 0.3406 | 0.6497 | 0.119* | |
| H12B | 0.2395 | 0.3796 | 0.5638 | 0.119* | |
| H12C | 0.1142 | 0.3161 | 0.5228 | 0.119* | |
| C13 | 0.1386 (8) | 0.767 (2) | 0.4657 (6) | 0.071 (2) | |
| H13A | 0.2104 | 0.7707 | 0.4661 | 0.107* | |
| H13B | 0.1112 | 0.9233 | 0.4608 | 0.107* | |
| H13C | 0.0889 | 0.6784 | 0.4033 | 0.107* | |
| C14 | 0.4794 (6) | 0.7242 (18) | 0.6612 (7) | 0.055 (2) | |
| C15 | 0.5512 (8) | 0.553 (2) | 0.7233 (8) | 0.073 (3) | |
| H15 | 0.5268 | 0.4320 | 0.7536 | 0.087* | |
| C16 | 0.6596 (8) | 0.563 (2) | 0.7394 (9) | 0.074 (3) | |
| H16 | 0.7096 | 0.4498 | 0.7815 | 0.088* | |
| C17 | 0.6938 (5) | 0.741 (2) | 0.6934 (6) | 0.061 (2) | |
| C18 | 0.6221 (7) | 0.9049 (19) | 0.6317 (8) | 0.066 (2) | |
| H18 | 0.6458 | 1.0245 | 0.6002 | 0.079* | |
| C19 | 0.5134 (8) | 0.895 (2) | 0.6152 (9) | 0.073 (3) | |
| H19 | 0.4634 | 1.0073 | 0.5720 | 0.088* | |
| N1 | 0.3955 (5) | 0.9634 (13) | 0.7793 (6) | 0.0527 (17) | |
| N2 | 0.2193 (4) | 0.7931 (13) | 0.6717 (4) | 0.0415 (13) | |
| O1 | 0.0683 (4) | 0.8637 (12) | 0.7061 (5) | 0.0664 (19) | |
| O2 | 0.3666 (4) | 0.7053 (13) | 0.6384 (5) | 0.072 (2) | |
| S1 | 0.41333 (15) | 1.2916 (5) | 0.93045 (17) | 0.0604 (6) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br1 | 0.0420 (4) | 0.1275 (10) | 0.0622 (5) | −0.0010 (6) | 0.0253 (4) | −0.0105 (6) |
| C1 | 0.048 (4) | 0.055 (5) | 0.061 (4) | −0.001 (5) | 0.027 (3) | 0.001 (5) |
| C2 | 0.063 (6) | 0.162 (13) | 0.089 (8) | −0.006 (7) | 0.037 (6) | −0.052 (8) |
| C3 | 0.079 (7) | 0.105 (10) | 0.106 (9) | −0.008 (7) | 0.051 (7) | −0.036 (8) |
| C4 | 0.059 (5) | 0.090 (7) | 0.043 (4) | 0.009 (5) | 0.013 (4) | −0.009 (5) |
| C5 | 0.045 (4) | 0.050 (5) | 0.038 (4) | 0.004 (3) | 0.014 (3) | 0.001 (3) |
| C6 | 0.039 (4) | 0.057 (6) | 0.036 (3) | 0.007 (4) | 0.011 (3) | 0.004 (4) |
| C7 | 0.032 (3) | 0.052 (4) | 0.033 (3) | 0.007 (3) | 0.010 (3) | 0.002 (3) |
| C8 | 0.033 (4) | 0.070 (6) | 0.043 (4) | −0.004 (4) | 0.006 (3) | −0.002 (4) |
| C9 | 0.037 (4) | 0.055 (5) | 0.040 (4) | −0.004 (4) | 0.012 (3) | 0.006 (3) |
| C10 | 0.038 (4) | 0.053 (5) | 0.049 (4) | 0.004 (3) | 0.018 (3) | −0.006 (3) |
| C11 | 0.037 (4) | 0.053 (5) | 0.044 (4) | −0.009 (3) | 0.013 (3) | −0.004 (3) |
| C12 | 0.100 (8) | 0.058 (6) | 0.061 (6) | −0.002 (6) | 0.016 (6) | 0.001 (5) |
| C13 | 0.083 (6) | 0.069 (6) | 0.044 (4) | 0.001 (6) | 0.011 (4) | 0.007 (5) |
| C14 | 0.039 (4) | 0.068 (6) | 0.061 (4) | −0.009 (4) | 0.024 (3) | −0.022 (5) |
| C15 | 0.054 (5) | 0.088 (8) | 0.076 (7) | −0.002 (5) | 0.028 (5) | 0.010 (6) |
| C16 | 0.050 (5) | 0.093 (8) | 0.076 (6) | 0.004 (5) | 0.027 (5) | 0.019 (6) |
| C17 | 0.034 (3) | 0.106 (7) | 0.043 (4) | 0.005 (6) | 0.016 (3) | −0.019 (6) |
| C18 | 0.049 (5) | 0.072 (6) | 0.074 (6) | 0.000 (5) | 0.023 (5) | 0.016 (5) |
| C19 | 0.048 (5) | 0.077 (7) | 0.084 (7) | 0.011 (5) | 0.019 (5) | 0.009 (5) |
| N1 | 0.034 (3) | 0.063 (5) | 0.057 (4) | −0.007 (3) | 0.016 (3) | −0.022 (4) |
| N2 | 0.031 (3) | 0.049 (4) | 0.043 (3) | −0.005 (3) | 0.013 (2) | 0.002 (3) |
| O1 | 0.033 (3) | 0.103 (6) | 0.062 (3) | −0.012 (3) | 0.018 (2) | −0.019 (3) |
| O2 | 0.039 (3) | 0.098 (6) | 0.079 (4) | −0.011 (3) | 0.025 (3) | −0.041 (4) |
| S1 | 0.0373 (9) | 0.0736 (16) | 0.0600 (11) | −0.0053 (11) | 0.0114 (8) | −0.0194 (12) |
Geometric parameters (Å, °)
| Br1—C17 | 1.916 (7) | C10—O2 | 1.347 (9) |
| C1—C2 | 1.481 (14) | C10—N2 | 1.378 (8) |
| C1—C6 | 1.515 (9) | C11—N2 | 1.497 (9) |
| C1—H1A | 0.9700 | C11—C12 | 1.504 (14) |
| C1—H1B | 0.9700 | C11—C13 | 1.521 (12) |
| C2—C3 | 1.410 (18) | C11—H11 | 0.9800 |
| C2—H2A | 0.9700 | C12—H12A | 0.9600 |
| C2—H2B | 0.9700 | C12—H12B | 0.9600 |
| C3—C4 | 1.523 (14) | C12—H12C | 0.9600 |
| C3—H3A | 0.9700 | C13—H13A | 0.9600 |
| C3—H3B | 0.9700 | C13—H13B | 0.9600 |
| C4—C5 | 1.492 (12) | C13—H13C | 0.9600 |
| C4—H4A | 0.9700 | C14—C19 | 1.336 (15) |
| C4—H4B | 0.9700 | C14—C15 | 1.377 (15) |
| C5—C6 | 1.350 (11) | C14—O2 | 1.412 (9) |
| C5—S1 | 1.754 (8) | C15—C16 | 1.374 (13) |
| C6—C7 | 1.445 (10) | C15—H15 | 0.9300 |
| C7—C8 | 1.375 (10) | C16—C17 | 1.370 (15) |
| C7—C9 | 1.404 (11) | C16—H16 | 0.9300 |
| C8—N1 | 1.370 (10) | C17—C18 | 1.342 (14) |
| C8—S1 | 1.719 (9) | C18—C19 | 1.377 (12) |
| C9—O1 | 1.236 (9) | C18—H18 | 0.9300 |
| C9—N2 | 1.431 (10) | C19—H19 | 0.9300 |
| C10—N1 | 1.287 (10) | ||
| C2—C1—C6 | 113.0 (7) | N2—C11—C12 | 114.8 (7) |
| C2—C1—H1A | 109.0 | N2—C11—C13 | 111.6 (7) |
| C6—C1—H1A | 109.0 | C12—C11—C13 | 112.1 (8) |
| C2—C1—H1B | 109.0 | N2—C11—H11 | 105.8 |
| C6—C1—H1B | 109.0 | C12—C11—H11 | 105.8 |
| H1A—C1—H1B | 107.8 | C13—C11—H11 | 105.8 |
| C3—C2—C1 | 115.1 (10) | C11—C12—H12A | 109.5 |
| C3—C2—H2A | 108.5 | C11—C12—H12B | 109.5 |
| C1—C2—H2A | 108.5 | H12A—C12—H12B | 109.5 |
| C3—C2—H2B | 108.5 | C11—C12—H12C | 109.5 |
| C1—C2—H2B | 108.5 | H12A—C12—H12C | 109.5 |
| H2A—C2—H2B | 107.5 | H12B—C12—H12C | 109.5 |
| C2—C3—C4 | 120.1 (11) | C11—C13—H13A | 109.5 |
| C2—C3—H3A | 107.3 | C11—C13—H13B | 109.5 |
| C4—C3—H3A | 107.3 | H13A—C13—H13B | 109.5 |
| C2—C3—H3B | 107.3 | C11—C13—H13C | 109.5 |
| C4—C3—H3B | 107.3 | H13A—C13—H13C | 109.5 |
| H3A—C3—H3B | 106.9 | H13B—C13—H13C | 109.5 |
| C5—C4—C3 | 108.0 (8) | C19—C14—C15 | 121.0 (8) |
| C5—C4—H4A | 110.1 | C19—C14—O2 | 120.1 (9) |
| C3—C4—H4A | 110.1 | C15—C14—O2 | 118.6 (9) |
| C5—C4—H4B | 110.1 | C16—C15—C14 | 118.6 (10) |
| C3—C4—H4B | 110.1 | C16—C15—H15 | 120.7 |
| H4A—C4—H4B | 108.4 | C14—C15—H15 | 120.7 |
| C6—C5—C4 | 126.6 (7) | C17—C16—C15 | 119.9 (10) |
| C6—C5—S1 | 112.4 (5) | C17—C16—H16 | 120.1 |
| C4—C5—S1 | 121.0 (6) | C15—C16—H16 | 120.1 |
| C5—C6—C7 | 112.3 (6) | C18—C17—C16 | 120.6 (7) |
| C5—C6—C1 | 120.9 (7) | C18—C17—Br1 | 119.9 (8) |
| C7—C6—C1 | 126.7 (7) | C16—C17—Br1 | 119.5 (8) |
| C8—C7—C9 | 119.2 (7) | C17—C18—C19 | 119.7 (10) |
| C8—C7—C6 | 111.8 (7) | C17—C18—H18 | 120.1 |
| C9—C7—C6 | 128.4 (6) | C19—C18—H18 | 120.1 |
| N1—C8—C7 | 125.8 (7) | C14—C19—C18 | 120.2 (9) |
| N1—C8—S1 | 121.3 (5) | C14—C19—H19 | 119.9 |
| C7—C8—S1 | 112.8 (6) | C18—C19—H19 | 119.9 |
| O1—C9—C7 | 126.9 (7) | C10—N1—C8 | 113.9 (6) |
| O1—C9—N2 | 118.7 (7) | C10—N2—C9 | 119.9 (6) |
| C7—C9—N2 | 114.4 (6) | C10—N2—C11 | 122.7 (6) |
| N1—C10—O2 | 121.4 (6) | C9—N2—C11 | 117.2 (5) |
| N1—C10—N2 | 126.6 (7) | C10—O2—C14 | 117.8 (6) |
| O2—C10—N2 | 112.0 (6) | C8—S1—C5 | 90.6 (4) |
| C6—C1—C2—C3 | 36.3 (16) | Br1—C17—C18—C19 | −179.7 (8) |
| C1—C2—C3—C4 | −50.1 (18) | C15—C14—C19—C18 | −1.6 (16) |
| C2—C3—C4—C5 | 34.6 (16) | O2—C14—C19—C18 | −175.9 (9) |
| C3—C4—C5—C6 | −9.8 (13) | C17—C18—C19—C14 | 0.5 (16) |
| C3—C4—C5—S1 | 169.1 (8) | O2—C10—N1—C8 | 178.4 (7) |
| C4—C5—C6—C7 | 179.3 (8) | N2—C10—N1—C8 | −1.7 (13) |
| S1—C5—C6—C7 | 0.4 (8) | C7—C8—N1—C10 | 4.8 (13) |
| C4—C5—C6—C1 | 1.0 (12) | S1—C8—N1—C10 | −173.3 (6) |
| S1—C5—C6—C1 | −177.9 (6) | N1—C10—N2—C9 | −1.2 (12) |
| C2—C1—C6—C5 | −13.3 (13) | O2—C10—N2—C9 | 178.8 (7) |
| C2—C1—C6—C7 | 168.6 (10) | N1—C10—N2—C11 | 173.0 (8) |
| C5—C6—C7—C8 | −1.3 (10) | O2—C10—N2—C11 | −7.0 (10) |
| C1—C6—C7—C8 | 176.9 (7) | O1—C9—N2—C10 | −176.8 (7) |
| C5—C6—C7—C9 | −172.1 (8) | C7—C9—N2—C10 | 1.2 (10) |
| C1—C6—C7—C9 | 6.1 (13) | O1—C9—N2—C11 | 8.7 (10) |
| C9—C7—C8—N1 | −4.8 (13) | C7—C9—N2—C11 | −173.3 (7) |
| C6—C7—C8—N1 | −176.6 (8) | C12—C11—N2—C10 | 66.0 (10) |
| C9—C7—C8—S1 | 173.4 (6) | C13—C11—N2—C10 | −63.0 (10) |
| C6—C7—C8—S1 | 1.6 (9) | C12—C11—N2—C9 | −119.6 (9) |
| C8—C7—C9—O1 | 179.4 (8) | C13—C11—N2—C9 | 111.4 (8) |
| C6—C7—C9—O1 | −10.4 (14) | N1—C10—O2—C14 | 0.8 (13) |
| C8—C7—C9—N2 | 1.5 (11) | N2—C10—O2—C14 | −179.2 (7) |
| C6—C7—C9—N2 | 171.7 (7) | C19—C14—O2—C10 | −86.5 (11) |
| C19—C14—C15—C16 | 1.5 (15) | C15—C14—O2—C10 | 99.1 (10) |
| O2—C14—C15—C16 | 175.9 (9) | N1—C8—S1—C5 | 177.1 (8) |
| C14—C15—C16—C17 | −0.4 (16) | C7—C8—S1—C5 | −1.2 (7) |
| C15—C16—C17—C18 | −0.6 (16) | C6—C5—S1—C8 | 0.4 (6) |
| C15—C16—C17—Br1 | 179.7 (8) | C4—C5—S1—C8 | −178.6 (7) |
| C16—C17—C18—C19 | 0.6 (15) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C11—H11···O1 | 0.98 | 2.20 | 2.726 (10) | 112 |
| C12—H12B···O2 | 0.96 | 2.43 | 2.915 (13) | 111 |
| C13—H13A···O2 | 0.96 | 2.38 | 2.951 (10) | 117 |
| C12—H12B···Cg1 | 0.96 | 2.92 | 3.854 (11) | 165 |
| C12—H12B···Cg2 | 0.96 | 2.71 | 3.434 (11) | 133 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: AT2674).
References
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. [DOI] [PubMed]
- Bruker (2001). SMART and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Ding, M. W., Xu, S. Z. & Zhao, J. F. (2004). J. Org. Chem.69, 8366–8371. [DOI] [PubMed]
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2003). J. Appl. Cryst.36, 7–13.
- Zeng, X.-H., Ding, M.-W. & He, H.-W. (2006). Acta Cryst. E62, o731–o732.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S160053680803732X/at2674sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S160053680803732X/at2674Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report